Abstract
Mutation in B-Raf leads to gain of function in melanoma and causes aggressive behavior for proliferation. Most of the therapeutics are ineffective in this scenario. However, regulation of this aggressive behavior by targeting the key molecules would be viable strategy to develop novel and effective therapeutics. In this report we provide evidences that the resveratrol is potent to regulate melanoma cell growth than other inducers of apoptosis. Resveratrol inhibits pronounced cell proliferation in melanoma than other tumor cell types. Cell cycle analysis using flow cytometry shows that the treatment with resveratrol results in S phase arrest. Resveratrol inhibits microphthalmia-associated transcription factor (MITF) and its dependent genes without interfering the MITF DNA binding in vitro. Resveratrol-mediated cell death is protected in MITF overexpressed cells and it is aggravated in MITF knocked down cells. These suggest the resveratrol-mediated decrease in MITF is the possible cause of melanoma cell death. Though resveratrol-mediated downregulation of NF-κB is responsible for cell apoptosis, but the downregulation of MITF is the main reason for melanoma-specific cell death. Thus, resveratrol can be effective chemotherapeutic agent against rapid proliferative melanoma cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Considering ‘bench to bedside’ concept to design any therapeutics, the mechanism of action is not carried out properly in recent days. Even, chemotherapy becomes ineffective whenever cells become resistant against these drugs. Thus, understanding the mechanism of action would be helpful in multiple ways - to design many drugs in combination chemotherapy; low concentration of drugs that give better results with less side-effects; to administer alternative drugs for chemotherapy. Such drugs inhibit the growth of a variety of cancer cells by utilizing diverse mechanisms that include cell cycle arrest, induction of apoptosis, disruption of microtubules, inhibition of angiogenesis, and/or increasing oxidative damage [1]. Molecules involve in the specific tumor growth would be useful to target for better and specific therapy. Understanding the tumor-specific rate-limiting markers and designing targeted therapy would be viable strategy in the current therapeutic regime.
Melanoma is a form of skin cancer. Although its occurrence is only 2 % of all skin cancers, it is responsible for most of the skin cancer deaths. The survival rate is only 16 % at advanced stage of the disease [2]. Melanoma develops from nevi, which are benign tumors. Mutations in the molecules on Ras-Raf-MEK-ERK pathway are the main reasons for the onset of melanoma [3]. Especially somatic mutations in B-Raf are associated with 66 % of the melanoma cases. Many of the mutations are in kinase domain leading to its activation independent of signal from Ras. V600EB-Raf accounts for 80 % of the B-Raf mutations [4, 5]. The downstream molecules, like mitogen activated protein kinases are often activated upon B-Raf mutation and resulted gain of function for melanoma cell proliferation [6, 7]. Vemurafenib, known to inhibit the mutant form of B-Raf, has shown to inhibit melanoma cell growth in combination with alkylating agent, temozolomide [8]. Melanoma frequently develops resistance against vemurafenib after intial responses [9, 10]. Micropthalmia associated transcription factor (MITF), a basic helix-loop-helix leucine zipper transcription factor is very important for survival, proliferation and differentiation of melanocytes [11]. It also controls cell cycle proliferative genes like CDK2, CDK4, Cyclin D1 and anti apoptotic genes like Bcl2 and melanoma inhibitor of apoptosis (ML-IAP) [12–16]. Besides many proliferative and pro-survival genes, MITF also regulates cell cycle inhibitors p21 and p16 via interaction with Rb protein [17–19]. MITF is activated by ERK2 through S73 phosphorylation followed by its degradation via proteasome [20, 21]. Hyper activation of MITF in V600EB-Raf mutated melanoma leads to differentiation and downregulation of it leads to apoptosis [22].
Resveratrol (3,5,4′-trihydroxystilbene), a naturally occurring compound present in grapes and red wine. It has been shown to exert chemopreventive effects against several cancers. It acts as antioxidant and inhibits tumor cell growth [23, 24]. It also induces cell death via activation of p53 through activation of ERK and p38 MAPK [25]. Resveratrol is known to suppress NF-κB induced by various agents [26–28] and thereby acts as anti-tumorigenic, anti-inflammatory, and ant-arthritic agent.
In the current study, we report that resveratrol is a potent antimitotic agent and inducer of apoptosis. We report here, for the first time, that this polyphenol efficiently arrests melanoma cells at S phase of the cell cycle and induces apoptosis, thus inhibiting cell growth. Resveratrol is less potent to induce cell death in other tumor cells and it inhibits melanoma cells’ specific transcription factor, MITF and its dependent genes and inhibits melanoma cell proliferation. Resveratrol-mediated suppression of NF-κB is insignificant for melanoma cell death. Thus, resveratrol might be an efficient chemotherapeutic agent against melanoma via suppression of MITF specifically.
Experimental procedures
Materials
Most of the chemicals, unless specified and antibodies against MITF, BrdU, and tubulin were obtained from Sigma Aldrich Chemicals (St Louis, MO, USA). Penicillin, streptomycin, neomycin, RPMI 1640, DMEM medium, fetal bovine serum (FBS), were obtained from Life Technologies (Grand Island, NY). DAPI was obtained from Molecular Probes (Eugene, OR). Antibody against PARP was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody against cleaved caspase 3 and MITF was purchased from Cell Signaling Technologies (Boston, MA, USA). The gel shift NF-κB and MITF oligonucleotides and all the primers (forward and reverse) for PCR were synthesized.
Cell lines
The human cell lines used in this study were as follows: A375 (melanoma), PC3 (prostate carcinoma), HT 29 (colon cancer), and MDA MB-231 (breast carcinoma) were obtained from American Type culture collection (Manassas, VA, USA).
Cytotoxicity assay
Cytotoxicity was assayed by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay as described previously [29]. Briefly, 5 × 103 cells/well of a 96 well plate were treated with different agents and the cell viability was determined by incubating the cells with the 20 μl MTT dye (5 mg/ml in PBS) for 3 h at 37 °C. The cells were incubated with 100 μl lysis buffer (20 % SDS in 50 % dimethylformamide) for 1 h at 37 °C, and the absorbance was read at 570 nm.
Determination of cell viability using propidium iodide (PI) exclusion flow cytometry
1 x 106 cells were given desired treatment, harvested after trypsinization, washed once with PBS and suspended in 1 ml PBS. 2 μl of 2 mg/ml PI (4 μg/ml final) was added and incubated for 5 min. Immediately, cells were analyzed for PI fluorescence on BD Accuri™ C6—Flow Cytometer.
DNA fragmentation assay
Cells were harvested after treatment by scraping, followed by centrifugation at 5000 rpm for 5 min and washed with PBS. The pellet was resuspended in 500 μl of lysis buffer (10 mM Tris-HCI, pH 8.0, 10 mM NaCl, I0 mM EDTA, 1 % SDS) and kept for 2 min at room temperature. 20 μl of RNase (20 mg/ml) was added and kept at 37 °C for 2 h. 20 μl of Proteinase K (20 mg/ml) was added and kept for at 37 °C till the mixture became clear. Each 250 μl of phenol/chloroform was added and centrifuged at 13000 rpm for 10 min. The supernatant was taken out. 100 μl of 3 M sodium acetate was added. 400 μl of isopropanol was added and kept at -20 °C for 15 min. Centrifuged at 13000 rpm for 15 min. Supernatant was discarded and 70 % ethanol was added and centrifuged at 13000 rpm for 15 min. Supernatant was discarded and plate was kept for drying at room temperature. 50 μl of TE was added and run on 1.5 % agarose gel [30].
Live and dead assay
The cytotoxicity was determined by the Live/Dead assay (Molecular Probes, Eugene, OR) as described previously [31]. Briefly, cells, after different treatments were stained with ‘Live & Dead’ cell assay reagent (5 μM ethidium homodimer, 5 μM calcein-AM). Red (as dead) and green (as live) cells were analyzed under a fluorescence microscope.
Cell cycle analysis
Cells (log phase culture) were treated with various concentrations of the resveratrol or vemurafenib. After treatment, the cells were washed with PBS, trypsinized and pelleted down. One more PBS wash was given and the pellet was resuspended in PBS (100 μl). 70 % chilled ethanol (900 μl) was added while vortexing and incubated in −20 °C for 1–2 days. Spun the cells at 2500 rpm for 3 min at room temperature, ethanol was removed carefully and gave a PBS wash. Resuspended the pellet in PBS (50 μl). A solution containing propidium iodide (20 μg/ml), Triton X-100 (0.1 %) and RNase A (0.2 mg/ml) was added to the samples and inverted immediately. Samples were incubated for 30 min at 37 °C in dark. Samples were then analyzed in flow cytometer (FACS Calibur; Becton–Dickinson, San Jose, CA) after gating (FL2-A/FL2-W) the single nuclei population and data were analyzed using FACS Diva software. For assaying BrdU positive S-phase cells, cells were treated for 24 h, as indicated. BrdU (10 μM) was added for 30 min. Cells were trypsinized, harvested, fixed in 70 % ethanol for overnight, and washed twice with PBS containing 1 % BSA. DNA was denatured with 2 N HCl/0.5 % Triton X-100 for 30 min and acid was neutralized with 0.1 M sodium tetraborate. Cells were washed once with PBST [0.5 % Tween 20 (v/v) in phosphate buffered saline] with 1 % BSA and incubated in the same with mouse anti-BrdU Ab for 30 min. Cells were spun down and again incubated in PBST with goat anti-mouse IgG tagged with Alexa Fluor-488. Cells were washed in PBS and stained with 2 µM of Hoechst 33342 and samples were analyzed in flow cytometer (FACS Calibur; Becton–Dickinson, San Jose, CA).
Annexin V-PE-7AAD assay
Cells were treated and assayed for cell death using Annexin V-PE apoptosis detection kit (BD Pharmingen™), according to manufacturer’s protocol.
Real time quantitative reverse transcriptase (RT)-PCR
5 μg of total RNA, isolated by TRIzol method (Gibco BRL, Grand Island, NY) was reverse transcribed into cDNA using Superscript-III reverse transcriptase (Lifetechnologies, Grand Island, NY), followed by the amplification of the gene of interest using gene specific primers for VEGF-A, cyclin D1, CDK2, MITF and GAPDH. PCR was performed and amplified products were separated by agarose gel electrophoresis (2 %) and visualized by ethidium bromide staining for semi-quantitative PCR [29] and qRT-PCR was done using SYBR green based mastermix (Thermo Fisher Scientific, MA, USA). The primer sequence and product sizes are as follows: MITF: 103 bp {forward} 5′-CCGTCTCTCACTGGATTGGT-3′,{reverse} 5′-TACTTGGTGGGGTTTTCGAG-3′, Cyclin D1: 146 bp {forward}5′-GGATGCTGGAGGTCTGCGA-3′,{reverse}5′-AGAGGCCACGAACATGCAAG-3′; CDK2: 239 bp {forward}5′-CGGATCTTTCGGACTCTGGG-3′,{reverse} 5′-ACTGGCTTGGTCACATCCTG-3′;VEGF A: (344 bp) {forward}5′-ATGAACTTTCTGCTGTCTTGGGT-3′,{reverse}5′-TGGCCTTGGTGAGGTTTGATCC-3′; Angiopoietin 1: (500 bp) {forward}5′-GCCTACACTTTCATTCTTCCAGA-3′, {reverese} {forward}5′-TCTTCCTTGTGTTTTCCTTCCAT-3′; GAPDH: (192 bp) {forward}5′-ACCTGCCAAATATGATGAC-3′, {reverse}5′-TCATACCAGGAAATGAGCTT-3′.
Lactate dehydrogenase (LDH) release assay
Necrosis of cells was assayed by measuring LDH, the cytosolic marker, from treated cells’ supernatant. Culture supernatants were incubated with the substrate solution (230 mM sodium pyruvate and 5 mM NADH in 0.1 M phosphate buffer, pH 7.5) and rate of decrease in absorbance at 340 nm was measured.
PARP and caspase 3 cleavages
The PARP and caspase 3 cleavage was determined using anti-PARP and caspase 3 (cleaved fragment) antibodies by Western blot.
Transcription factors assay by electrophoretic mobility shift assay (EMSA)
To determine NF-κB and MITF DNA binding activity, EMSA were conducted essentially as described [32]. Briefly, 12 µg nuclear extract proteins were incubated with 32P end-labeled double-stranded NF-κB or MITF oligonucleotides for 30 min at 37 °C, and the DNA–protein complex was separated from free oligonucleotide on 6.6 % native polyacrylamide gels. The oligos used for EMSA were as follows: NF-κB: {forward} 5′-TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGG–3′, {reverse} 5′–CCACGCCTCCCTGGAAAGTCCCCAGCGGAAAGTCCCTTGTAACAA–3′, MITF: {forward} 5′-CGCTGCACAGAGCATGTGACCCCAGAGGCC–3′, {reverse} 5′–GGCCTCTGGGGTCACATGCTCTGTGCAGCG–3′.
MITF dependent reporter gene [secretory alkaline phosphatase activity (SEAP)] assay
The promoter of tyrosinase (-200 to +80), which is an MITF dependent gene was cloned into pSEAP2-Basic vector. The primers used were as follows: Tyr promoter {forward} 5′- GTAGGTACCACCATAAGAATTAAACTATT-3′,{reverse}5′- GCTCTCGAGTTCCTCTAGTCCTCACAAGG-3′. A375 cells were transfected with Tyr promoter-SEAP plasmid (1 μg/well in a 24-well plate) or vector control using lipofectamine 2000. Desired treatments were given after 12 h of transfection for another 24 h. Culture-condition medium was collected and 25 μl was analyzed for alkaline phosphatase activity using 4-methyl umbelliferyl phosphate as substrate, according to CLONTECH protocol (Palo Alto, CA, USA).
NF-κB and MITF knock down experiments
A375 cells, stably knocking down Rel A, a component of NF-κB were generated using both scrambled and Rel A specific shRNAs and lentiviral packaging system. Stable transfectants were selected using 4 μg/ml puromycin. MITF shRNA was generated by cloning double stranded oligonucleotides in pGFP-V-RS plasmid, using BamHI and HindIII [33]. The following oligonucleotides were ordered with overhangs: First strand–5′-GATCCGATCCAAACTGGAAGACATACGTGTGCTGTCCGTATGTCTTCCAGTTTGGATCTTTTTA-3′, complimentary strand–5′-AGCTTAAAAAGATCCAAACTGGAAGACATACGGACAGCACACGTATGTCTTCCAGTTTGGATCG-3′. A375 cells were transfected with both vector control and MITF shRNA vector using Lipofectamine 2000 and media change was done after 12 h. Cells were incubated for 48 h, before treating with resveratrol for another 24 h.
Statistical analysis
Results were expressed as mean ± SEM of at least three independent experiments. Statistical analyses of the samples were done by unpaired student’s t test or one-way ANOVA with Tukey’s multiple comparisons test, wherever applicable. The p < 0.05 was considered to be significant. All statistical analyses were done using Graphpad Prism 6.0.
Results
Several inhibitors, especially resveratrol were used in this study do not show any cytolysis as determined by the lactate dehydrogenase (LDH) assay.
Resveratrol potently induces melanoma cell death
In order to detect the role of several inhibitors in the aggressive melanoma cell growth, A375 cells were incubated with different concentrations of resveratrol, azadirachtin or thiadiazolidine derivative (P3-25) or 10 μM vemurafenib for 72 h in triplicate. MTT assay was done to determine the cell death. Resveratrol increased the cell death than azadirachtin and P3-25 (Fig. 1a). The experiment was repeated thrice, the data was plotted as mean percentage of cell death ± SEMVemurafenib, a mutant B-Raf inhibitor is considered for positive control to block melanoma cell growth. Resveratrol increased cell death in a concentration-dependent manner than azadirahtin or P3-25 as shown by the increased number of dead cells as determined by Live and Dead assay (Fig. 1b) or PI exclusion flow cytometry (Fig. 1c). All these data suggest that resveratrol increased melanoma cell death than azadirachtin or P3-25.
Effect of resveratrol on cell death a A375 cells (5000/well of 96-well plate in triplicate) were cultured overnight and incubated with different concentrations of resveratrol, azadirachtin or P3-25 along with 10 µM of vemurafenib for 72 h. MTT assay was done and indicated in percentage of cell death, considering the untreated cells’ value as 0 % cell death. The experiment was repeated at least thrice and the data were plotted as mean ± SEM.**P < 0.01; ***P < 0.001; ****P < 0.0001 (one-way ANOVA and Tukey’s multiple comparisons test). b Cells were treated with different concentrations of resveratrol, azadirachtin or P3-25 along with 10 µM of vemurafenib or sorafenib for 24 h and live and dead assay was done. c Cells were treated similarly, along with 5 µM of paclitaxel for cell death and flow cytometry was done for PI exclusion
Resveratrol-mediated cell death is more rigorous in melanoma cells
As cell signaling varies from cell type to cell type, we incubated PC3, HT29, and MDA MB-231 cells along with A375 melanoma cells with different concentrations of resveratrol for 72 h. A375 cells showed pronounced cell death than PC3, HT29, or MDA MB-231 cells at any concentrations of resveratrol as determined by MTT assay (Fig. 2a). To determine the role of NF-κB, these cells were treated with different concentrations of resveratrol, nuclear extracts (NE) were prepared and gel shift assay was done. Basal NF-κB activity is more in A375 cells and resveratrol efficiently suppressed this activity (Fig. 2b). These data further suggest that resveratrol induces prominent cell death in melanoma cells.
Effect of resveratrol on cell death in different cell types a A375, PC3, HT29 and MDA MB-231 cells were treated with different concentrations of resveratrol ranging from 0 to 200 µM for 72 h and MTT assay was done. The percentage of cell death ± SD was indicated considering untreated cells as 100 % viable. b All four cell lines were treated with 0, 25, 50 and 100 μM of resveratrol, nuclear extracts were isolated and Gel shift assay was done for NF-κB
Resveratrol increases melanoma cell apoptosis, but not necrosis
To further confirm the mechanism of cell death, A375 cells were incubated with different concentrations of resveratrol, sorafenib (Raf inhibitor), vemurafenib (specific mutant B-Raf inhibitor), doxorubicin and oleandrin (positive controls for apoptosis) for 24 h, genomic DNA was isolated, ran on 2 % agarose gel, and inter-nucleosomal DNA fragmentation was observed. Resveratrol is potently inducing DNA fragmentation, which is comparable to doxorubicin (Fig. 3a). The caspase-dependent fragmentation of poly (ADP-ribose) polymerase (PARP) and the amount of cleaved caspase-3 were increased upon resveratrol treated cells for 24 h than vemurafenib or sorafenib (Fig. 3b). These data suggest that resveratrol is more potent to induce apoptosis in melanoma cells than known inhibitors of B-Raf at 24 h of treatment. Some of the small molecules used to induce cell death via necrosis of cells. However, the effect of resveratrol on melanoma cell death is either due to programmmed cell death or necrosis was determined by assaying the release of the LDH in the culture supernatant in the treated cells. Even 200 µM of resveratrol or 10 µM of vemurafenib or sorafenib did not increase LDH release than 5 µM doxorubicin treated cells (Fig. 3c). This result suggests that resveratrol does not induce cell death via necrosis.
Activation of apoptosis but not necrosis by resveratrol in melanoma a A375 cells were treated with 50, 100 and 200 μM of resveratrol; 10 μM each of vemurafenib or sorafenib; 5 μM of doxorubicin; or 100 ng/ml of oleandrin. Genomic DNA was islolated and checked for fragmentation on a 2 % agarose gel. b Cells were treated with resveratrol, venurafenib or sorafenib, lysates were prepared and probed for apoptotic markers such as cleaved PARP and cleaved caspase-3. c Supernatants were collected from treated cells and LDH assay was done for observing cytolysis, considering Triton X-100 treated cells as 100 %. Data, obtained from three independent experiments were indicated in percentage of cytolysis as mean ± SEM.****P < 0.0001 (one-way ANOVA and Tukey’s multiple comparisons test)
Resveratrol arrests melanoma cells at S phase
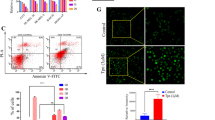
To understand the resveratrol-mediated cell death on melanoma cells either by arresting any cell cycle phases, cells were treated with different concentrations of resveratrol and 10 μM of vemurafenib for 24 h. Cell cycle phases were analyzed in Flow cytometer. Almost 40 to 60 % cells were stayed at S phase at 25 to 100 μM concentrations of resveratrol. Vemurafenib did not show any increase in the cell population at this phase as indicated in the bar diagram for S phase population (Fig. 4a). The cell population those were on sub G0/G1 phase are considered as apoptotic population and indicated in percentage (Fig. 4b). Percentages of both early (Q3) and late (Q2) phases of apoptosis was increased by resveratrol, which was comparable to paclitaxel (Fig. 4c). S-phase arrest of cell cycle was further confirmed by BrdU flow cytometry. Almost 2-fold increase in S-phase population upon treatment with 50 µM of resveratrol (Fig. 4d1, d2), further suggests that it can indeed arrests the cell cycle at S-phase.
Effect of resveratrol on cell cycle a A375 ells were treated with 25, 50 and 100 µM of resveratrol or 10 µM of vemurafenib for 24 h, stained with PI and cell cycle analysis was done using flow cytometry. Percentage of S-phase population was plotted for showing S-phase cell cycle arrest. b Percentage of Sub G0/G1 population was plotted for showing apoptotic population. Data indicated as mean cell population in percentage ± SEM.*P <0.05; ****P <0.0001 (one-way ANOVA and Tukey’s multiple comparisons test). c Cells were treated similarly, stained with annexin V-PE and 7-AAD and flow cytometry was done. 5 µM of paclitaxel was used as positive control. d1 Cells were treated with 50 µM of resveratrol for 24 h, labelled with BrdU and stained with anti-BrdU Ab tagged with Alexa Fluor 488 and Hoechst 33342 and analyzed using flow cytometry. d2 The cell population for the same experiment were indicated in percentage
Resveratrol inhibits DNA binding activity of MITF
As melanoma cells are more sensitive to resveratrol for cell death, the factor s behind the melanoma-specific cell death are determined. The transcription factor MITF is known for melanoma progression. To detect the role of resveratrol, the MITF DNA binding was determined. Resveratrol, but not the azadirachtin, P3-25 or vemurafenib inhibited MITF DNA binding activity as detected from nuclear extracts (NE) from treated cells (Fig. 5a) and MITF dependent SEAP activity (Fig. 5d). The MITF DNA binding activity was inhibited by resveratrol in a dose-dependent manner as determined from the whole cell extracts (WCE) (Fig. 5b) and MITF dependent SEAP activity (Fig. 5e). In fact vemurafenib is activating MITF dependent SEAP activity. Resveratrol did not interfere MITF DNA binding as determined from the NE, treated with different concentrations of resveratrol in vitro (Fig. 5c). This result suggests that resveratrol inhibits the MITF DNA binding activity in the cells, but does not interact with the DNA binding site. Resveratrol-mediated decrease in MITF DNA binding required 24 h pretreatment of it as determined from the DNA binding activity of resveratrol-treated cells’ NE from different times (Fig. 5f). MITF inhibition of resveratrol is prominent in A375 melanoma, compared to other cell types, as it is melanocytic transcription factor (Fig. 5g).
Effect of resveratrol on the activity of MITF transcription factor a A375 ells were treated with 50 and 100 µM of resveratrol or azadirachtin, 50 and 100 nM of P3-25, or 10 µM of vemurafenib for 24 h, nuclear extracts (NE) were isolated and EMSA was done for MITF DNA binding. b Cells were treated with different concentrations of resveratrol or 10 µM of vemurafenib for 24 h, whole cell extracts (WCE) were prepared and EMSA was done for MITF. c NE were isolated from untreated cells, incubated with different concentrations of resveratrol in an in vitro binding reaction and EMSA was done for MITF. d A375 cells were transfected with Tyr promoter—SEAP vector, treated with resveratrol, azadirachtin, P3-25 or vemurafenib for 24 h, SEAP assay was done for culture-conditioned medium and plotted as fold change over control. e The transfected cells were incubated with different concentrations of resveratrol for 24 h and SEAP assayed. Data were obtained from at least three independent experiments and plotted as mean ± SEM.*P < 0.05; **P < 0.01; ****P < 0.0001 (one-way ANOVA and Tukey’s multiple comparisons test). f A375 cells were treated with 100 μM of resveratrol for different time intervals, NE and cytoplasmic extracts (CE) were prepared and MITF DNA binding was assayed by EMSA. g MDA MB-231, A375, HT29 and PC3 cells were treated with different concentrations of resveratrol for 24 h. MITF DNA binding was assayed from NE
Resveratrol inhibits amount of MITF and its dependent genes
Resveratrol-mediated downregulation of MITF was further confirmed by determining the amount of it by Western blot. Resveratrol, but not azadirachtin or P3-25 decreased the amount of MITF (Fig. 6a). Resveratrol treatment led to decrease in the amount of MITF at 24 h as determined by Western blot (Fig. 6b) and 50 μM concentration of it showed almost complete inhibition (Fig. 6c). Resveratrol inhibited the expression of cyclin D1, CDK2 and MITF in a dose-dependent manner as determined by the qRT-PCR (Fig. 6d1–d3). All these data suggest that resveratrol inhibits MITF DNA binding and its dependent gene expression.
Effect of resveratrol on expression of MITF and its dependent genes a A375 cells were treated with 50 and 100 µM of resveratrol or azadirachtin, 50 and 100 nM of P3-25, or 10 µM of vemurafenib for 24 h. Whole cell extracts (WCE) were prepared and amount of MITF was detected by Western blot. Same blot was reprobed for tubulin. b Cells were treated with 100 μM resveratrol for different times and MITF expression was determined by Western blot from WCE. c Cells were treated with different concentrations of resveratrol for 24 h and MITF expression was measured by Western blot. d1, d2 and d3 A375 cells were treated with 100 µM of resveratrol for 24 h, total RNA was isolated and qRT-PCR was done for cyclin D1 (d1), CDK2 (d2), and MITF (d3). Data were plotted in fold ± SEM.**P < 0.01; ***P < 0.001; ****P < 0.0001 (unpaired Student’s t-test)
Inhibition of MITF, but not NF-κB is the reason for resveratrol-mediated cell death
As resveratrol has shown to inhibit NF-κB, the important transcription factor for cell proliferation it is important to understand its role in melanoma cell death mediated by resveratrol. The basal activity of NF-κB and MITF was inhibited by resveratrol in A375 melanoma cells. These amounts were partially reduced upon treatment of suboptimal concentration of IKK complex inhibitor, BAY (Fig. 7a). MDA MB-231, a non-melanoma cell line did not show high basal amount of NF-κB or MITF. BAY treatment did not show sufficient cell death in A375 cells, but resveratrol showed almost 60 % cell death alone or in combination of BAY as determined by MTT assay (Fig. 7c) or the cleavages of caspase 3 or PARP (Fig. 7b). In non-melanoma cell line MDA MB-231, resveratrol enhances partial cell death only. P3-25, which was shown to inhibit NF-κB [34] is showing similar cell death in both melanoma and non-melanoma, where as resveratrol is showing significantly more cell death in melanoma only (Fig. 7d). Knocking down of Rel A, a component of NF-κB did not show any increase in cell death upon resveratrol treatment (Fig. 7e). The knock down of Rel A was determined from stable knock down cells using shRNA of Rel A by Western blot (Fig. 7f). These data suggest that inhibition of NF-κB does not have prominent role on resveratrol mediated melanoma cell death.
Effect of NF-κB inhibitors on resveratrol-mediated downregulation of NF-κB, MITF, and cell death a A375 and MDA-MB-231 cells were treated with 100 µM of resveratrol, 5 µM BAY, or in combination for 24 h, nuclear extracts were prepared and EMSA was done for NF-κB and MITF. b Cells were treated in the same way, whole cell lysates were prepared and western blotting was done for both cleaved caspase-3 and PARP, which were then reprobed for tubulin. c Both cell types were treated as mentioned above and MTT was done and cell death was indicated in mean percentage ± SEMd Both cell types were treated with 100 μM of resveratrol or 100.nM of P3-25, MTT assay was done and cell death was plotted in mean percentage ± SEM.****P < 0.0001 (one-way ANOVA and Tukey’s multiple comparisons test). e A375 cells stably over expressing scrambled and Rel A shRNA were treated with 100 µM of resveratrol for 24 h, lysates were prepared and probed for PARP, Rel A and GAPDH. f Successful knock down of Rel A was shown by Western blot
MITF overexpression inhibits and knock down aggravates resveratrol-induced cell death
To determine the role of MITF on resveratrol-mediated melanoma cell death, cells were transfected with Vector-GFP and MITF-GFP constructs. Cells were treated with resveratrol followed by incubation with PI. The cells either GFP positive or non-GFP showed fragmented nuclei in Vector-GFP transfected cells. GFP positive cells showed intact nuclei upon treatment with resveratrol in MITF-GFP transfected cells (Fig. 8a). MITF overexpression which was determined Western blot (Fig. 8c) decreased the cleavage of PARP upon resveratrol treatment (Fig. 8b). MITF knock down which was determined by Western blot in MITF shRNA transfected cells (Fig. 8e) increased cleavage of PARP and resveratrol treatment further enhanced this cleavage (Fig. 8d). These data suggest that MITF transfected cells were protected from resveratrol mediated cell death and knocked down cells were more sensitive.
Effect of MITF on resveratrol-induced cell death a A375 cells were transfected with both EGFP vector control and EGFP-MITF for 6 h followed by culture for 12 h. Cells were then treated with 50 µM of resveratrol for 24 h and stained with PI. Images were taken using fluorescent microscope. b Both vector control and MITF over expressed cells were treated with 100 μM of resveratrol for 24 h and PARP cleavage was analysed using Western blot. c Over expression of EGFP-MITF was shown using anti-MITF antibody. d A375 cells were transiently transfected with shRNA of control and MITF, treated with 100 µM of resveratrol, lysates were prepared and probed for PARP, MITF and GAPDH. e Knock down of MITF was shown by Western blot from the lysates
Discussion
Out of all dermatological cancers, melanoma accounts for 4 % but 80 % death is due to this [35]. It poses an aggressiveness i.e. the 86 % patents survive less than 5 years. Thus, coining novel therapeutics to cut the life line of melanoma is always valuable. We have tested few inhibitors that basically induce cell death in this study to regulate the aggressive melanoma cell progression. Azadirachtin and thiadiazolidine derivative, P3-25 are known to induce cell death in several tumor cells [32, 34–39]. Resveratrol, a polyphenol is known to induce cell death in many tumors and currently used as ant-arthritic and cardioprotective agent [40–43]. Upon treatment of these inhibitors, resveratrol is the most potent inducer of melanoma cell death. Though all these inhibitors inhibited NF-κB, but the melanoma cell death is independent of this NF-κB inhibition. Resveratrol-mediated cell death is more in melanoma cells than other cell types, like breast, prostate, or colon cancer cells. This prompts us to look for the molecular basis of resveratrol action in melanoma.
As B-Raf is important upstream kinase which regulates melanoma proliferation, we have checked the effect of resveratrol on B-Raf action. Though, B-Raf is mutated in most of the melanoma and mutant form leads to gain of function in terms of cell proliferation [6, 7], we have used inhibitors of both wild type and mutant form of B-Raf to look the melanoma cell proliferation. Vemurafenib, a mutant B-Raf inhibitor had some role in cell death at 72 h of treatment, but 24 h treatment did not show prominent cell death as determined by multiple assays. Sorafenib, a general Raf kinase inhibitor did not show much cell death even at 72 h of treatment. Inhibition of NF-κB either by P3-25, having double-edged sword effect to inhibit it [32] or azadirachtin, inhibits cytokine and growth factor receptors to block NF-κB has not much effect to block melanoma cell growth. Though, resveratrol inhibited basal activity of NF-κB, but the inhibition of this factor is not mattered much for melanoma cell death.
Melanoma cell proliferation depends upon the activation of transcription factor MITF and its dependent genes like cyclin D1 and CDK2. We have found that resveratrol specifically inhibited MITF DNA binding activity, but not by azadirachtin, P3-25 or vemurafenib. As the transcription factor protein remains in the nucleus, the nuclear protein when incubated with resveratrol the MITF DNA binding activity was not altered. These data suggest that being a small molecule resveratrol does not interfere in the DNA binding activity. Resveratrol not only decreases the DNA binding activity of MITF in the nuclear extracts, but also in the whole cell extract protein. As this is not interacted with the DNA binding site, the decrease in the amount of MITF was observed as determined by Western blot and qRT-PCR. These data suggest that resveratrol is exerting its effect by decreasing the expression of MITF.
Chemoprevention is considered a promising strategy in the field of cancer therapy and suppressing or reversing the process of tumor formation has gained much attention [44, 45]. Classic cytotoxic agents, known to act on the cell cycle, continue to underline first line treatments in oncology. Therefore, novel targeted therapeutics, especially, these modulating the cell cycle checkpoint have emerged as an attractive candidate for new cancer therapies [46]. As cyclins and CDKs are involved in cell cycle regulation and considering the progressive melanoma cells behavior we have looked into the effect of resveratrol on cell cycle regulation. Flow cytometric analysis of DNA content showed a dose-dependent block in the S phase and an increase in the sub G0/G1 (apoptotic) cell population. At 50 µM concentration almost 66 % of the live population was arrested in the S phase, though there are several reports suggesting the resveratrol-mediated arrest of cell cycle at G1- and S-phases in various tumor cell types [47, 48]. Several NF-κB-dependent genes, expressed in cells regulate the cell cycle and cell death. Inhibition of NF-κB did not show much cell death in melanoma cells. NF-κB inhibition did not show any additive cell death in melanoma cells upon resveratrol treatment. Partial cell death was observed in non-melanoma cells upon NF-κB inhibition. These data suggest that resveratrol-mediated inhibition of NF-κB does not interfere in melanoma cell death significantly. Resveratrol increased cell death in EGFP positive vector transfected cells, but not in MITF-EGFP transfected cells further suggested that MITF is the major determinant for resveratrol-mediated melanoma cell death. Overall, our data suggest that resveratrol-mediated downregulation of MITF, but not NF-κB is the main reason of melanoma cell death.
Abbreviations
- BAY:
-
BAY 11-7082
- BrdU:
-
5-Bromo-2′-deoxyuridine
- CE:
-
Cytoplasmic extract
- GFP:
-
Green fluorescent protein
- MITF:
-
Microphthalmia-associated transcription factor
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NE:
-
Nuclear extract
- NF-κB:
-
Nuclear transcription factor kappa B
- PARP:
-
Poly (ADP-ribose) polymerase
- P3-25:
-
5-(4-Methoxyarylimino)-2-N-(3,4-dichlorophenyl)-3-oxo-1,2,4-thiadiazolidine
- PI:
-
Propidium iodide
- SEAP:
-
Secretory alkaline phosphatase
- VEGF:
-
Vascular endothelial growth factor
References
Ricci MS, Zong WX (2006) Chemotherapeutic approaches for targeting cell death pathways. Oncologist 11:342–357
American Cancer Society (2014) Cancer Facts & Figures 2014. Atlanta: American Cancer Society
Poynter JN, Elder JT, Fullen DR et al (2006) BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma Res 16:267–273
Davies H, Bignell GR, Cox C et al (2002) Mutations of the BRAF gene in human cancer. Nature 417:949–954
Pollock PM, Harper UL, Hansen KS et al (2003) High frequency of BRAF mutations in nevi. Nat Genet 33:19–20
Dhomen N, Reis-Filho JS, da Rocha Dias S et al (2009) Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell 15:294–303
Halilovic E, Solit DB (2008) Therapeutic strategies for inhibiting oncogenic BRAF signaling. Curr Opin Pharmacol 8:419–426
Roos WP, Quiros S, Krumm A et al (2014) B-Raf inhibitor vemurafenib in combination with temozolomide and fotemustine in the killing response of malignant melanoma cells. Oncotarget 5:12607–12620
Nazarian R, Shi H, Wang Q et al (2010) Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 468:973–977
Spagnolo F, Ghiorzo P, Orgiano L et al (2015) BRAF-mutant melanoma: treatment approaches, resistance mechanisms, and diagnostic strategies. OncoTargets Therapy 8:157–168
Levy C, Khaled M, Fisher DE (2006) MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med 12:406–414
Cheli Y, Ohanna M, Ballotti R, Bertolotto C (2010) Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment Cell Melanoma Res 23:27–40
Dynek JN, Chan SM, Liu J, Zha J, Fairbrother WJ, Vucic D (2008) Microphthalmia-associated transcription factor is a critical transcriptional regulator of melanoma inhibitor of apoptosis in melanomas. Cancer Res 68:3124–3132
Robson EJ, He SJ, Eccles MR (2006) A PANorama of PAX genes in cancer and development. Nat Rev Cancer 6:52–62
Haq R, Yokoyama S, Hawryluk EB et al (2013) BCL2A1 is a lineage-specific antiapoptotic melanoma oncogene that confers resistance to BRAF inhibition. Proc Natl Acad Sci USA 110:4321–4326
Hartman ML, Talar B, Noman MZ, Gajos-Michniewicz A, Chouaib S, Czyz M (2014) Gene expression profiling identifies microphthalmia-associated transcription factor (MITF) and Dickkopf-1 (DKK1) as regulators of microenvironment-driven alterations in melanoma phenotype. PLoS One 9:e95157
Carreira S, Goodall J, Aksan I et al (2005) Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature 433:764–769
Loercher AE, Tank EM, Delston RB, Harbour JW (2005) MITF links differentiation with cell cycle arrest in melanocytes by transcriptional activation of INK4A. J Cell Biol 168:35–40
Yavuzer U, Keenan E, Lowings P, Vachtenheim J, Currie G, Goding CR (1995) The Microphthalmia gene product interacts with the retinoblastoma protein in vitro and is a target for deregulation of melanocyte-specific transcription. Oncogene 10:123–134
Wu M, Hemesath TJ, Takemoto CM et al (2000) c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev 14:301–312
Xu W, Gong L, Haddad MM et al (2000) Regulation of microphthalmia-associated transcription factor MITF protein levels by association with the ubiquitin-conjugating enzyme hUBC9. Exp Cell Res 255:135–143
Wellbrock C, Rana S, Paterson H, Pickersgill H, Brummelkamp T, Marais R (2008) Oncogenic BRAF regulates melanoma proliferation through the lineage specific factor MITF. PLoS One 3:e2734
Jang M, Cai L, Udeani GO et al (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Mgbonyebi OP, Russo J, Russo IH (1998) Antiproliferative effect of synthetic resveratrol on human breast epithelial cells. Int J Oncol 12:865–869
She QB, Bode AM, Ma WY, Chen NY, Dong Z (2001) Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res 61:1604–1610
Manna SK, Mukhopadhyay A, Aggarwal BB (2000) Leflunomide suppresses TNF-induced cellular responses: effects on NF-kappa B, activator protein-1, c-Jun N-terminal protein kinase, and apoptosis. J Immunol 165:5962–5969
Adhami VM, Afaq F, Ahmad N (2003) Suppression of ultraviolet B exposure-mediated activation of NF-kappaB in normal human keratinocytes by resveratrol. Neoplasia 5:74–82
Yamamoto Y, Gaynor RB (2001) Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Investig 107:135–142
Mahali S, Raviprakash N, Raghavendra PB, Manna SK (2011) Advanced glycation end products (AGEs) induce apoptosis via a novel pathway: involvement of Ca2+mediated by interleukin-8 protein. J Biol Chem 286:34903–34913
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501
Sarkar A, Sreenivasan Y, Ramesh GT, Manna SK (2004) beta-D-Glucoside suppresses tumor necrosis factor-induced activation of nuclear transcription factor kappaB but potentiates apoptosis. J Biol Chem 279:33768–33781
Manna SK, Babajan B, Raghavendra PB, Raviprakash N, Sureshkumar C (2010) Inhibiting TRAF2-mediated activation of NF-kappaB facilitates induction of AP-1. J Biol Chem 285:11617–11627
Kido K, Sumimoto H, Asada S et al (2009) Simultaneous suppression of MITF and BRAF V600E enhanced inhibition of melanoma cell proliferation. Cancer Sci 100:1863–1869
Manna P, Narang KK, Manna SK (2005) 1,2,4-Thiadiazolidine derivative inhibits nuclear transcription factor-kappaB and its dependent genes activation but induces apoptosis. Int J Cancer J Int Du Cancer 113:549–560
Miller AJ, Mihm MC Jr (2006) Melanoma. New Engl J Med 355:51–65
Li W, Wang Z, Gududuru V et al (2007) Structure-activity relationship studies of arylthiazolidine amides as selective cytotoxic agents for melanoma. Anticancer Res 27:883–888
Sorriento D, Del Giudice C, Bertamino A et al (2015) New small molecules, ISA27 and SM13, inhibit tumour growth inducing mitochondrial effects of p53. Br J Cancer 112:77–85
Priyadarsini RV, Murugan RS, Sripriya P, Karunagaran D, Nagini S (2010) The neem limonoids azadirachtin and nimbolide induce cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells. Free Radical Res 44:624–634
Srivastava P, Yadav N, Lella R et al (2012) Neem oil limonoids induces p53-independent apoptosis and autophagy. Carcinogenesis 33:2199–2207
Elmali N, Baysal O, Harma A, Esenkaya I, Mizrak B (2007) Effects of resveratrol in inflammatory arthritis. Inflammation 30:1–6
Xuzhu G, Komai-Koma M, Leung BP et al (2012) Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann Rheum Dis 71:129–135
Penumathsa SV, Maulik N (2009) Resveratrol: a promising agent in promoting cardioprotection against coronary heart disease. Can J Physiol Pharmacol 87:275–286
Wu JM, Wang ZR, Hsieh TC, Bruder JL, Zou JG, Huang YZ (2001) Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review). Int J Mol Med 8:3–17
Aggarwal BB, Takada Y, Oommen OV (2004) From chemoprevention to chemotherapy: common targets and common goals. Expert Opin Investig Drugs 13:1327–1338
Levi MS, Borne RF, Williamson JS (2001) A review of cancer chemopreventive agents. Curr Med Chem 8:1349–1362
Potter AJ, Gollahon KA, Palanca BJ et al (2002) Flow cytometric analysis of the cell cycle phase specificity of DNA damage induced by radiation, hydrogen peroxide and doxorubicin. Carcinogenesis 23:389–401
Joe AK, Liu H, Suzui M, Vural ME, Xiao D, Weinstein IB (2002) Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin Cancer Res Off J Am Assoc Cancer Res 8:893–903
Yang Q, Wang B, Zang W et al (2013) Resveratrol inhibits the growth of gastric cancer by inducing G1 phase arrest and senescence in a Sirt1-dependent manner. PLoS One 8:e70627
Acknowledgments
The Department of Biotechnology (DBT), Govt. of India supported this work. We duly thank DBT for providing fellowship to Raveendra Babu. We acknowledge Mr. Aushaq Bashir Malla, Laboratory of Cell Signaling, CDFD for helping in the Flow Cytometer work. We are very thankful to Dr.Soumen Basak, National Institute of Immunology, New Delhi for kindly providing us GE Dharmacon (New Delhi, India) lentiviral shRNA vectors for knocking down Rel A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mokhamatam, R.B., Sahoo, B.K. & Manna, S.K. Suppression of microphthalmia-associated transcription factor, but not NF-kappa B sensitizes melanoma specific cell death. Apoptosis 21, 928–940 (2016). https://doi.org/10.1007/s10495-016-1260-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-016-1260-3