Abstract
Purpose
To evaluate the efficacy of 177Lu-DOTA0-Tyr3-octreotate (177Lu-DOTATATE) radionuclide therapy in patients with inoperable or metastatic neuroendocrine tumours (NETs), (PROSPERO ID CRD42019130755).
Methods
All published clinical studies of NETs treated with 177Lu-DOTATATE were identified based on systematic searches in the PubMed, EMBASE, Cochrane Library, Web of Science and ClinicalTrials.gov databases up to January 2019. Among these studies, only the reports evaluated with the “Response Evaluation Criteria in Solid Tumours (RECIST)” or “Southwest Oncology Group (SWOG)” criteria or both were included. We analysed the disease response rate (DRR) and disease control rate (DCR) of each group to evaluate the efficacy of 177Lu-DOTATATE.
Results
Fifteen studies were selected from 715 references. The pooled effect in the RECIST group (13 studies) was 27.58% (95% confidence interval (CI) 21.03–35.27%) for the DRR and 79.14% (95% CI 75.83–82.1%) for the DCR. In the SWOG criteria group (7 studies), the pooled effect was 20.59% (95% CI 10.89–35.51%) for the DRR and 78.28% (95% CI 74.39–81.72%) for the DCR. Therefore, the RECIST and SWOG groups showed similar DRRs and DCRs after177Lu-DOTATATE treatment, indicating that 177Lu-DOTATATE treatment has excellent efficacy with a control rate of approximately 78–79%. Moreover, adverse effects of 177Lu-DOTATATE were minimal, including fatigue, nausea, vomiting and hormonal disorders.
Conclusions
For patients with inoperable or metastatic NETs, 177Lu-DOTATATE is an effective treatment with minimal side effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine tumours (NETs) originate from neuroendocrine cells, but NETs can occur in most organs of the body because neuroendocrine cells are distributed throughout the body. The incidence of NETs has increased more rapidly than that of other tumours, especially in the past 30 years. According to registries of the Surveillance, Epidemiology, and End Results (SEER) Program, the annual age-adjusted incidence of NETs has increased 6.4-fold from 1973 (1.09 per 100,000 people) to 2012 (6.98 per 100,000 people) in Europe and the USA (Dasari et al. 2017). Moreover, with various clinical manifestations, these tumours are easily misdiagnosed. Approximately, 50% of patients are found to be in metastatic stages at the time of initial diagnosis (Hallet et al. 2015). The most common NETs develop from organs in the digestive system, such as the stomach, intestines and pancreas, which account for approximately two-thirds of all NETs.
Surgery is generally the preferred treatment for NETs, but it is not suitable for metastatic disease. For advanced stages of NETs, somatostatin analogue (SSTA) therapy, chemotherapy and peptide receptor radionuclide therapy (PRRT) are commonly used, with response rates ranging from 6 to 70% (Oberg and Jelic 2009). Among these treatments, PRRT has been used in the treatment of NETs for more than 30 years and has yielded promising results. Currently, 177Lu is a popular diagnostic and therapeutic radionuclide, which is used in a number of clinical trials. Unlike 111indium (van Binnebeek et al. 2016), 90Y (Valkema et al. 2006) and other radionuclides, 177lutetium is a medium-energy β-emitter with a maximum energy of 0.5 MeV and a maximal tissue penetration of 2 mm, which provides suitable irradiation of small tumours (van Essen et al. 2010; Kam et al. 2012). Two types of 177Lu-PRRT are available: Tyr3-octreotide (TOC) and Tyr3-octreotate (TATE). They can bind to malignant cells overexpressing somatostatin receptor type 2 (SSTR2). However, TATE has a ninefold higher affinity for SSTR2 than TOC (Velikyan et al. 2012). Once bound, 177Lu-DOTATATE accumulates within tumour cells and delivers cytotoxic radiation to kill these cells. Recently, a randomized controlled clinical trial evaluated the efficacy and safety of 177Lu-DOTATATE through a comparison with octreotide (LAR) alone (Strosberg et al. 2017). They found that 177Lu-DOTATATE not only reduced the symptoms caused by hormone oversecretion, but also prolonged disease-free survival with a significantly higher response rate. Therefore, 177Lu-DOTATATE may be a valuable and effective therapy for NETs; however, only a few systematic reviews and meta-analyses (Kim et al. 2015; Severi et al. 2017; Mujica-Mota et al. 2018) have been performed to evaluate its efficacy. The increase in the use of 177Lu-DOTATATE in clinical trials (Strosberg et al. 2017; del Prete et al. 2018; Kalshetty et al. 2018) motivated us to carry out a systematic review and meta-analysis of 177Lu-DOTATATE for the treatment of inoperable or metastatic NETs. We hope to provide guidance for clinical practice, health-care decision making and future research through this meta-analysis. The PROSPERO database registered our systematic review as CRD42019130755.
Materials and methods
Literature search strategy
This systematic review and meta-analysis followed the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Liberati et al. 2009). The reviewers performed searches in PubMed, Embase, Cochrane Library and Web of Science to obtain articles published on or before Jan. 18, 2019. Meanwhile, a manual search was performed for ongoing studies registered on ClinicalTrials.gov. We selected articles that reported the outcomes of patients with inoperable or metastatic NETs who were treated with 177Lu-DOTATATE and evaluated the response according to either the Response Evaluation Criteria in Solid Tumours (RECIST) (Albain et al. 2002; Hodi et al. 2016) or the Southwest Oncology Group (SWOG) (Albain et al. 2002) criteria or both. The search terms were MESH terms and free text words (Stroup et al. 2000) as follows: {(“neuroendocrine tumor*” [Mesh] OR “neuroendocrine tumour*” OR “neuroendocrine tumor*” OR “neuroendocrine neoplasm*” OR “neuroendocrine cancer*” OR “neuroendocrine carcinoma*”) AND (Lutetium [Mesh] OR *lutetium OR *Lu OR PRRT)}. The reviewers independently searched for articles that reported the outcomes of patients treated with 177Lu-DOTATATE.
Study selection
Regarding 177Lu-DOTATATE, we selected original research articles including ≥ 10 patients who had inoperable or metastatic NETs and received 177Lu-DOTATATE radionuclide therapy. If several articles were published by a single centre or a group of centres, then only the study with the most relevant patients for this meta-analysis was included. However, if the second of two articles from a centre evaluated > 50% of the patients who were unreported in the first article, then we included both articles. All included articles were limited to human studies and those written in English. Two reviewers independently performed the searches and reviewed the quality of each study according to the methodological index for non-randomized studies (MINORS) (Slim et al. 2003). Initial assessments were based on titles and abstracts, and studies lacking original data, in vitro experiments duplicating a study that had already been recovered from the literature search or articles reporting only biodistributions were excluded. Review articles, meta-analyses, abstracts, editorials and case studies were excluded. Early studies that did not provide renal protection with amino acid infusions were also excluded. Disagreements concerning eligibility were resolved by discussion between the authors (JZ and LC). If an agreement could not be reached, then a third arbiter (YC) was consulted.
Data extraction
The following baseline characteristics were extracted from each included study by the two reviewers, JZ and LC: first author, year of publication, centre, number of patients, study design, radiotherapy dose, response criteria and side effects. As treatment end points in the articles, we extracted articles that classified the objective treatment response by the RECIST or SWOG criteria. The studies were grouped according to the response criteria used for evaluation. Tumour response data were recorded as disease response rates (DRRs) and disease control rates (DCRs). DRRs is the ratio of patients with complete response (CR) and partial remission (PR), whereas DCRs are defined as the sum of the ratios, including CR, PR, minor response (MR) and stable disease (SD). Any information omitted from the published articles was requested from the study investigators via email.
Statistical analysis
We performed patient-based evaluations for each study, and the effect sizes were based on the proportions of DRRs and DCRs with 95% confidence intervals (CIs). The pooled proportions are presented with fixed-effects and random-effects models when applicable. Heterogeneity among the studies was assessed using Cochran’s Q and I2 statistics as described previously (Higgins and Thompson 2002). The possibility of publication bias was assessed using a funnel plot with Egger’s test and an influence analysis. A sensitivity analysis was used to analyse the stability of the test results. Differences were statistically significant at P < 0.05. All analyses were carried out using Microsoft Excel, version 365 (Microsoft, Corp. USA) and R statistical software with the meta package (Schwarzer 2012), version 3.5.2 (R Development Core Team 2011).
Results
Literature search
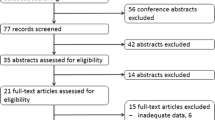
The electronic searches for studies of 177Lu-DOTATATE returned 715 hits. A total of 679 records were identified through the database searches, and 36 additional records were identified through a manual search of ClinicalTrials.gov database. In total, 453 records remained after duplicate records were removed. After screening the titles and abstracts, 262 records remained. However, 61 reviews, 50 preclinical studies, 59 conference abstracts and 14 case studies with fewer than ten patients did not meet the inclusion criteria. An additional 60 studies were excluded, as the compounds used in those studies were labelled using other precursors. After reviewing the full text articles of the remaining 16 studies, 15 articles with 872 patients were eligible for inclusion in the study. The review procedure is shown in Fig. 1.
Then, the quality assessment was conducted on 15 studies based on the MINORS, and the research generally met most quality standards. Most included studies provided tumour response data analysed with either the RECIST (Sward et al. 2010; Bodei et al. 2011, 2016; van Vliet et al. 2013; Delpassand et al. 2014; Ezziddin et al. 2014; Sabet et al. 2015; Soydal et al. 2016; del Prete et al. 2017, 2018; Hamiditabar et al. 2017; Strosberg et al. 2017; Kalshetty et al. 2018) or SWOG (Sansovini et al. 2013; van Vliet et al. 2013; Ezziddin et al. 2014; Paganelli et al. 2014; del Prete et al. 2017, 2018) criteria, while 5 studies analysed the data with both sets of criteria and were thus included in both the RECIST and SWOG groups (van Vliet et al. 2013; Ezziddin et al. 2014; Sabet et al. 2015; del Prete et al. 2017, 2018). However, the data from studies by del Prete et al. (2017, 2018) group were collected from the same institutions, and the author mentioned that the latter study was independent of their retrospective cohort of patients treated with empiric PRRT before April 2016 (del Prete et al. 2018); thus, we included both studies. Moreover, one randomized controlled trial evaluating the efficacy and safety of 177Lu-DOTATATE (Strosberg et al. 2017) with only DRR results was also included in the RECIST subgroup. Therefore, 872 patients were included in this study, and all treated patients were divided into two groups: the RECIST (777 patients) and SWOG (515 patients) groups, as shown in Table 1. These patients were treated with two to five cycles of 177Lu, with an activity level of approximately 7.4 GBq for each cycle, the mentioned time interval between each cycle was 6–14 weeks and the activity level of the last cycle ranged from 3.7 to 15.9 GBq, resulting in a cumulative activity level between 3.7 and 78.6 GBq.
Efficacy of 177Lu-DOTATATE for the treatment of NETs
To evaluate the efficacy of 177Lu-DOTATATE for the treatment of NETs, DRR and DCR data were analysed here. The pooled rates were analysed with both fixed-effects model and random-effects models, as shown in Table 2. A total of 218 patients had effective responses to 177Lu-DOTATATE in 15 eligible studies in the RECIST group with 777 patients. The test of heterogeneity showed a highly significant result for the DRR (I2 = 74.3%), which were analysed using a random-effects model. The DRR ranged from 8.69 to 57.35%, with an average effect of 27.58% (95% CI 21.03–35.27%) as shown in Fig. 2a. The DCR ranged from 71.87 to 90.16%, with slight heterogeneity (I2 = 19.1%). Fixed-effect model analysis showed an average DCR of 79.14% (95% CI 75.83–82.1%) as shown in Fig. 2b.
Seven eligible studies with 515 patients were included in the SWOG group analysis. A total of 135 patients had effective outcomes with 177Lu-DOTATATE treatment. The test of heterogeneity showed a highly significant result for the DRR (I2 = 88.1%). The SWOG criteria group had a DRR ranging from 8.69 to 60.29%, with a pooled effect of 20.59% (95% CI 10.89–35.51%) in the random-effects model. The forest plot is shown in Fig. 3a. The DCR ranged from 73.91 to 91.8%, with moderate heterogeneity (I2 = 49.8%). The fixed-effects model showed an average DCR of 78.28% (95% CI 74.39–81.72%) in Fig. 3b.
Publication bias and sensitivity analysis
The funnel plot was drawn along the horizontal axis, and standard error was plotted along the vertical axis with each study for the DRRs (Fig. 4a) and DCRs (Fig. 4b) in the RECIST group. Visual inspection revealed asymmetry for both the DRR and DCR in the RECIST group, reflecting a possible publication bias, although Egger’s test was not statistically significant for the DRR (t = − 0.6699, P = 0.52, Fig. 5a) or DCR (t = − 0.8476, P = 0.42, Fig. 5b). Next, we performed sensitivity analyses by omitting one of the studies each time and observing the influence on the total combined effect. The results showed no significant changes in the combined results for the DRR (Fig. 6a) or DCR (Fig. 6b) after excluding any one of the studies, suggesting that the combined effect results have good stability and reliability. After eliminating each study in turn, the merger rate of the remaining studies was approximately 0.29 or 0.79, and no significant change was observed.
Adverse effects
In all studies included here, 177Lu-DOTATATE was tolerable and safe, with few serious adverse reactions such as fatigue, nausea, vomiting, hormonal disorders and nephrotoxicity. In fact, some of adverse reactions were related to pre-medication with agents such as everolimus, capecitabine and temozolomide. Some of them were transient, the reactions often disappeared very quickly. However, the renal metabolic pathway of this medicine may cause renal toxicity. It can be reduced or avoided by using renal protective medicines. Most clinical trials are generally routine in renal protection. If there is no indication of renal protection in early clinical trials, renal toxicity and side effects are too large. Thus, we excluded some early studies that did not provide renal protection with amino acid infusions as an exclusion criterion. The two forms of 177Lu-DOTATATE differed in terms of adverse effects. Toxicity was evaluated according to National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (CTCAE) (Trotti et al. 2003). van Essen et al. (2010) reported grade 4 haematologic toxicity in one patient. Sansovini et al. (2013) found that one patient developed grade 3 renal toxicity.
Discussion
With the increasing rate of NETs over the last 30 years, effective therapeutic methods are needed. In recent decades, new small-molecule peptides with high-affinity binding to tumour cell receptors have been rapidly developed. Among all types of therapeutic methods, PRRT has been widely explored for its role in patients with NETs (Werner et al. 2015). Currently, 177Lu has attracted much attention, as the combined use of 177Lu and 68 Ga can play the role of integrated diagnosis and treatment in PRRT.
In this meta-analysis, the tumour response results of the studies were separated according to either the RECIST or SWOG criteria. Although the analysed results in the RECIST group seem similar to those in the SWOG group, several differences exist between these sets of criteria in terms of the definitions used. For the RECIST, the longest diameter of up to five lesions per organ and up to ten lesions in total is measured. For the SWOG, the sum of the products of the perpendicular diameters of up to three lesions per organ is calculated. They also have different definitions of the response criteria for CR, PR, SD and progressive disease. Because these two sets of response criteria are very different, the outcomes of the studies should be analysed separately. 177Lu-DOTATATE treatment resulted in a DRR of 27.58% (95% CI 21.03–35.27%) in the RECIST group and 20.59% (95% CI 10.89–35.51%) in the SWOG criteria group, but the results were highly inconsistent (I2 = 74.3% and I2 = 88.1%, respectively), indicating that the DRRs varied widely among the included studies. This might be related to the heterogeneous PRRT dose regimen used, such as two to five cycles, 6–14 weeks interval and different dose for each cycle of treatment used. Efficacy determination was in a variable range, since therapy regimens were different.
177Lu-DOTATATE treatment resulted in a DCR of 79.14% (95% CI 75.83–82.1%) in the RECIST group and 78.28% (95% CI 74.39–81.72%) in the SWOG group. These data reflected homogenous results (I2 = 19.1% and I2 = 49.8%, respectively), signifying consistency among the included studies. The results showed that the therapeutic effects of 177Lu-DOTATATE were practical. Furthermore, a multicentre randomized clinical trial (NETTER-1, NCT01578239) compared 177Lu-labelled PRRT with supportive care using octreotide achieved good results, with an estimated rate of progression-free survival of 65.2% (95% CI, 50.0 to 76.8%) in the 177Lu-DOTATATE group compared to only 10.8% (95% CI, 3.5 to 23.0%) in the control group after 20 months of treatment. Here, our results showed that the DCR was 79.14% or 78.28% which is consistent with the reported data, suggesting that 177Lu-DOTATATE is an effective therapy for NETs.
Previous studies had reported that 177Lu-DOTATOC, 90Y-DOTATATE and 90Y-DOTATOC all have beneficial therapeutic effects. However, we pay attention to TATA, because the binding force between TATE and SSTR2 is much higher than that between TOC and SSTR2. The clinical literature on radionuclide-labelled TATE was significantly higher than that of TOC. A previous meta-analysis reported that 177Lu-labelled PRRT (Kim et al. 2015) yielded a DCR of 81% (95% CI 71–91%) in the RECIST group and 82% (95% CI 71–91%) in the SWOG criteria group. Seven studies were included, only one labelled with TOC. Other studies compared 177Lu-PRRT with 90Y-PRRT (Severi et al. 2017) or chemotherapy (Mujica-Mota et al. 2018). The risk of side effects of radionuclide therapy with 90Y was higher than with 177Lu (Valkema et al. 2005). Our results incorporate recently reported clinical trial data, which confirm the efficacy of 177Lu-DOTATATE in the treatment of metastatic NETs.
This study combined 15 original studies and demonstrated the therapeutic effect of 177Lu-DOTATATE on NETs. However, our study also has certain limitations. On one hand, although we tried our best to search for relevant research, we may have ignored some studies that were not published online. On the other hand, the study is limited by the characteristics of a single-rate meta-analysis, and the heterogeneity was high. Even in the classification analysis, partial heterogeneity cannot be ruled out.
Conclusion
In conclusion, the results of this meta-analysis indicate that 177Lu-DOTATATE is effective and safe for the treatment of NETs. However, although 177Lu-DOTATATE has entered phase III clinical trials in some countries, no unified standard for the dose or frequency of delivery and no data on dosage standards or long-term adverse reactions are available. Moreover, additional 177Lu-DOTATATE clinical data from Asian samples are needed to verify our conclusion. High-quality original research, especially randomized controlled clinical studies, are needed to provide more evidence for the clinical application of 177Lu-DOTATATE.
References
Albain KS, Crowley JJ, Turrisi AT, Gandara DR, Farrar WB, Clark JI, Beasley KR, Livingston RB (2002) Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest oncology group phase II study, SWOG 9019. J Clin Oncol 20:3454–3460. https://doi.org/10.1200/jco.2002.03.055
Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, Bartolomei M, Lombardo D, Ferrari ME, Sansovini M, Chinol M, Paganelli G (2011) Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging 38:2125–2135. https://doi.org/10.1007/s00259-011-1902-1
Bodei L, Kidd M, Modlin IM, Severi S, Drozdov I, Nicolini S, Kwekkeboom DJ, Krenning EP, Baum RP, Paganelli G (2016) Measurement of circulating transcripts and gene cluster analysis predicts and defines therapeutic efficacy of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors. Eur J Nucl Med Mol Imaging 43:839–851. https://doi.org/10.1007/s00259-015-3250-z
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC (2017) Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 3:1335–1342. https://doi.org/10.1001/jamaoncol.2017.0589
Delpassand ES, Samarghandi A, Zamanian S et al (2014) Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas 43:518–525. https://doi.org/10.1097/mpa.0000000000000113
del Prete M, Buteau FA, Beauregard JM (2017) Personalized 177Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: a simulation study. Eur J Nucl Med Mol Imaging 44:1490–1500. https://doi.org/10.1007/s00259-017-3688-2
del Prete M, Buteau FA, Arsenault F, Saighi N, Bouchard LO, Beaulieu A, Beauregard JM (2018) Personalized 177Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: initial results from the P-PRRT trial. Eur J Nucl Med Mol Imaging 46:728–742. https://doi.org/10.1007/s00259-018-4209-7
Development Core Team R (2011) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria
Ezziddin S, Khalaf F, Vanezi M, Haslerud T, Mayer K, Zreiqat A, Willinek W, Biersack HJ, Sabet A (2014) Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 41:925–933. https://doi.org/10.1007/s00259-013-2677-3
Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S (2015) Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 121:589–597. https://doi.org/10.1002/cncr.29099
Hamiditabar M, Ali M, Roys J, Wolin EM, O'Dorisio TM, Ranganathan D, Tworowska I, Strosberg JR, Delpassand ES (2017) Peptide receptor radionuclide therapy with 177Lu-octreotate in patients with somatostatin receptor expressing neuroendocrine tumors: six years' assessment. Clin Nucl Med 42:436–443. https://doi.org/10.1097/rlu.0000000000001629
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. https://doi.org/10.1002/sim.1186
Hodi FS, Hwu WJ, Kefford R et al (2016) Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 34:1510–1517. https://doi.org/10.1200/jco.2015.64.0391
Kalshetty A, Ramaswamy A, Ostwal V, Basu S (2018) Resistant functioning and/or progressive symptomatic metastatic gastroenteropancreatic neuroendocrine tumors: efficacy of 177Lu-DOTATATE peptide receptor radionuclide therapy in this setting. Nucl Med Commun 39:1143–1149. https://doi.org/10.1097/mnm.0000000000000926
Kam BL, Teunissen JJ, Krenning EP, de Herder WW, Khan S, van Vliet EI, Kwekkeboom DJ (2012) Lutetium-labelled peptides for therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging 39:S103–S112. https://doi.org/10.1007/s00259-011-2039-y
Kim SJ, Pak K, Koo PJ, Kwak JJ, Chang S (2015) The efficacy of 177Lu-labelled peptide receptor radionuclide therapy in patients with neuroendocrine tumours: a meta-analysis. Eur J Nucl Med Mol Imaging 42:1964–1970. https://doi.org/10.1007/s00259-015-3155-x
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1–e34. https://doi.org/10.1016/j.jclinepi.2009.06.006
Mujica-Mota R, Varley-Campbell J, Tikhonova I et al (2018) Everolimus, lutetium-177 DOTATATE and sunitinib for advanced, unresectable or metastatic neuroendocrine tumours with disease progression: a systematic review and cost-effectiveness analysis. Health Technol Assess 22:1–326. https://doi.org/10.3310/hta22490
Oberg K, Jelic S, Esmo Guidelines Working Group (2009) Neuroendocrine gastroenteropancreatic tumors: ESMO clinical recommendation for diagnosis, treatment and follow-up. Ann Oncol 20(150):153. https://doi.org/10.1093/annonc/mdp158
Paganelli G, Sansovini M, Ambrosetti A, Severi S, Monti M, Scarpi E, Donati C, Ianniello A, Matteucci F, Amadori D (2014) 177 Lu-Dota-octreotate radionuclide therapy of advanced gastrointestinal neuroendocrine tumors: results from a phase II study. Eur J Nucl Med Mol Imaging 41:1845–1851. https://doi.org/10.1007/s00259-014-2735-5
Sabet A, Dautzenberg K, Haslerud T, Aouf A, Sabet A, Simon B, Mayer K, Biersack HJ, Ezziddin S (2015) Specific efficacy of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced neuroendocrine tumours of the small intestine. Eur J Nucl Med Mol Imaging 42:1238–1246. https://doi.org/10.1007/s00259-015-3041-6
Sansovini M, Severi S, Ambrosetti A, Monti M, Nanni O, Sarnelli A, Bodei L, Garaboldi L, Bartolomei M, Paganelli G (2013) treatment with the radiolabelled somatostatin analog Lu-DOTATATE for advanced pancreatic neuroendocrine tumors. Neuroendocrinology 97:347–354. https://doi.org/10.1159/000348394
Schwarzer G (2012) Meta: meta‐analysis with R. R package version 2.0‐0. https://CRAN.R-project.org/package=meta. Accessed 23 June 2017
Severi S, Grassi I, Nicolini S, Sansovini M, Bongiovanni A, Paganelli G (2017) Peptide receptor radionuclide therapy in the management of gastrointestinal neuroendocrine tumors: efficacy profile, safety, and quality of life. Onco Targets Ther 10:551–557. https://doi.org/10.2147/OTT.S97584
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J (2003) Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 73:712–716. https://doi.org/10.1046/j.1445-2197.2003.02748.x
Soydal C, Peker A, Ozkan E, Kucuk ON, Kir MK (2016) The role of baseline Ga-68 DOTATATE positron emission tomography/computed tomography in the prediction of response to fixed-dose peptide receptor radionuclide therapy with Lu-177 DOTATATE. Turk J Med Sci 46:409–413. https://doi.org/10.3906/sag-1412-11
Strosberg J, El-Haddad G, Wolin E et al (2017) Phase 3 trial of 177Lu-DOTATATE for midgut neuroendocrine tumors. New Engl J Med 376:125–135. https://doi.org/10.1056/NEJMoa1607427
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283:2008–2012. https://doi.org/10.1001/jama.283.15.2008
Sward C, Bernhardt P, Ahlman H, Wangberg B, Forssell-Aronsson E, Larsson M, Svensson J, Rossi-Norrlund R, Kolby L (2010) [177Lu-DOTA 0-Tyr 3]-octreotate treatment in patients with disseminated gastroenteropancreatic neuroendocrine tumors: the value of measuring absorbed dose to the kidney. World J Surg 34:1368–1372. https://doi.org/10.1007/s00268-009-0387-6
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176–181. https://doi.org/10.1016/s1053-4296(03)00031-6
Valkema R, Pauwels SA, Kvols LK, Kwekkeboom DJ, Jamar F, de Jong M, Barone R et al (2005) Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-Dota(0),Tyr(3)-octreotide and (177)Lu-Dota(0), Tyr(3)-octreotate. J Nucl Med 46:83s–91s. https://jnm.snmjournals.org/content/46/1_suppl/83S.
Valkema R, Pauwels S, Kvols LK, Barone R, Jamar F, Bakker WH, Kwekkeboom DJ, Bouterfa H, Krenning EP (2006) Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0, Tyr3]octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med 36:147–156. https://doi.org/10.1053/j.semnuclmed.2006.01.001
van Binnebeek S, Vanbilloen B, Baete K et al (2016) Comparison of diagnostic accuracy of (111)in-pentetreotide SPECT and (68)Ga-DOTATOC PET/CT: a lesion-by-lesion analysis in patients with metastatic neuroendocrine tumours. Eur Radiol 26:900–909. https://doi.org/10.1007/s00330-015-3882-1
van Essen M, Krenning EP, Kam BL, de Herder WW, Feelders RA, Kwekkeboom DJ (2010) Salvage therapy with 177Lu-octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumors. J Nucl Med 51:383–390. https://doi.org/10.2967/jnumed.109.068957
van Vliet EI, Krenning EP, Teunissen JJ, Bergsma H, Kam BL, Kwekkeboom DJ (2013) Comparison of response evaluation in patients with gastroenteropancreatic and thoracic neuroendocrine tumors after treatment with [177Lu-DOTA0, Tyr3]octreotate. J Nucl Med 54:1689–1696. https://doi.org/10.2967/jnumed.112.117408
Velikyan I, Xu H, Nair M, Hall H (2012) Robust labeling and comparative preclinical characterization of DOTA-TOC and DOTA-TATE. Nucl Med Biol 39:628–639. https://doi.org/10.1016/j.nucmedbio.2011.12.010
Werner RA, Bluemel C, Allen-Auerbach MS, Higuchi T, Herrmann K (2015) 68Gallium- and 90Yttrium-/ 177Lutetium: "theranostic twins" for diagnosis and treatment of NETs. Ann Nucl Med 29:1–7. https://doi.org/10.1007/s12149-014-0898-6
Funding
This study was funded by the Science and Technology Department of Sichuan Province (Grant Number 2018JPT0023).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, J., Song, Q., Cai, L. et al. The efficacy of 177Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) in patients with metastatic neuroendocrine tumours: a systematic review and meta-analysis. J Cancer Res Clin Oncol 146, 1533–1543 (2020). https://doi.org/10.1007/s00432-020-03181-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03181-2