Abstract
Background
Peptide receptor radiation therapy (PRRT) using [177Lu-DOTA0-Tyr3]-octreotate is a new, promising option for treatment of disseminated gastroenteropancreatic neuroendocrine tumors (GEPNETs).
Methods
During 2006-2008, 26 patients with disseminated GEPNETs were treated with 177Lu-octreotate. Radiologic response (RECIST), biochemical response [plasma chromogranin-A (P-CgA)], hematologic toxicity [Common Toxicity Criteria (CTC)], absorbed dose to the kidneys (conjugate view method), and glomerular filtration rate (GFR) were analyzed.
Results
177Lu-octreotate (8 GBq) was given one to five times (median = 3) with a 6-week interval between each. Sixteen of the 26 patients were evaluated radiologically; 6 (38%) had partial response (PR), 8 (50%) had stable disease (SD), and 2 (13%) had progressive disease (PD). Seventeen of the 26 patients were evaluated biochemically; 6 (35%) showed a ≥30% decrease, 8 (47%) showed a ≥20% increase, and 3 (18%) showed neither a ≥30% decrease nor a ≥20% increase. The mean absorbed dose to the kidneys was 24 Gy. With a dose limit of 27 Gy to the kidneys, 10 patients did not receive the planned four treatments, while four patients had the potential to receive additional treatment. A significant reduction (p = 0.0013) of GFR was observed at follow-up. Three patients experienced CTC grade 3 hematologic toxicity.
Conclusions
By using the absorbed dose to the kidneys as a limiting factor, treatment with 177Lu-octreotate can be individualized, e.g., overtreatment can be avoided and patients with the potential to receive additional treatment can be identified. Further studies are needed to define tolerance doses to the kidneys so that treatment can be optimized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The survival of patients with disseminated gastroenteropancreatic neuroendocrine tumors (GEPNETs) has improved during the last few decades. Surgery with excision of the primary tumor, lymph node metastases, and liver metastases is still the only curative treatment available. In selected cases palliative liver resection is indicated [1]. Bilobar hepatic metastases can be treated with hepatic artery embolization to reduce hormonal symptoms and prolong survival in patients with midgut carcinoid [2]. Palliative treatment includes medical therapy for hormonal symptoms, e.g., somatostatin analogs and interferon-α can each be effective agents [3]. Biotherapy with interferon-α has resulted in tumor regression in 15-20% of the patients who received it, but it has not been proven to prolong survival [3–5]. Chemotherapy has resulted in only limited response rates with considerable side effects [6, 7].
Peptide receptor radiation therapy (PRRT) has recently been developed to treat somatostatin receptor-expressing GEPNET tumors. Promising results have been achieved by using the radiopharmaceutical [177Lu-DOTA0-Tyr3]-octreotate. In a previously published series, [177Lu-DOTA0-Tyr3]-octreotate treatment was found to induce complete response (CR), partial response (PR), or minor response (MR) in 2, 26, and 19%, respectively [8]. The absorbed dose to the kidneys and the bone marrow is the limiting factor for use of PRRT.
To evaluate the radiologic response, examination with computed tomography (CT) using the RECIST criteria offers one established method for evaluation of therapeutic effects [9]. The general neuroendocrine (NE) tumor marker plasma chromogranin A (P-CgA) correlates well with tumor burden [10–12]. Therefore, the biochemical response can be used as a simple but reliable method to evaluate tumor response. Development of a radiopharmaceutical for PRRT has included both new somatostatin analogs with high affinity for sstr and selection of isotopes with a suitable range, e.g., octreotate has a twofold higher affinity for sstr 2 than octreotide, and 177Lu has a suitable range for tumors up to 1 mm in diameter, whereas 90Y is more suitable for larger tumors [13, 14]. One way to optimize PRRT is to measure the absorbed dose to the kidneys. This could ensure that the dose limit is approached but not exceeded.
The aims of the present study were to evaluate the radiologic and biochemical responses to PRRT using [177Lu-DOTA0-Tyr3]-octreotate of 26 consecutive patients with disseminated GEPNETs and to evaluate if measurement of absorbed dose to the kidneys could serve as a tool for optimization of the treatment.
Materials and methods
Patients
Twenty-six patients were treated with [177Lu-DOTA0-Tyr3]-octreotate at our unit during 2006-2008. They all had disseminated GEPNET tumors (10 endocrine pancreatic tumors, 10 midgut carcinoids, 3 rectal carcinoids, 1 pulmonary carcinoid, 1 duodenal gastrinoma, 1 neuroendocrine presacral tumor). Multimodal treatment with combinations of surgery (primary and repeated), chemotherapy, somatostatin analogs, interferon-α, hepatic artery embolization, radiofrequency ablation, external radiation, and, in four patients, liver transplantation was used prior to [177Lu-DOTA0-Tyr3]-octreotate treatment (Table 1).
Indications for treatment
The indications for PRRT were progressive disease despite conventional multimodal therapy and a tumor uptake of the radiopharmaceutical of at least twice the physiologic liver uptake.
Treatment protocol and follow-up
The patients were scheduled for four treatment sessions, each with 8 GBq of [177Lu-DOTA0-Tyr3]-octreotate, 6 weeks apart. The absorbed dose to each kidney was calculated after each session with the conjugate view method. The total absorbed dose limit to each kidney was 27 Gy. The radiologic response was evaluated by measuring tumor size using RECIST criteria [complete response (CR), disappearance; partial response (PR), ≥30% decrease; stable disease (SD), neither PR nor PD; progressive disease (PD), ≥20% increase together with no CR, PR, or SD documented before PD] on CT scans taken before and after treatment. Biochemical response was evaluated by comparing P-CgA before and after treatment. Hematological toxicity was evaluated according to the Common Toxicity Criteria (CTC) scale. Renal function was assessed by measuring glomerular filtration rate (GFR) by either by Cr-EDTA or iohexol clearance.
Statistical analysis
The change in tumor size according to RECIST criteria and the change in GFR were analyzed using Wilcoxon matched-pairs signed rank sum test; p < 0.05 was considered significant.
Results
Absorbed doses to the kidneys
The mean absorbed dose to the kidneys was 24 Gy ± 5.9 (SEM). Four treatment sessions were given as planned to 11 of the 26 patients. One patient had five sessions, while 10 had fewer than four sessions since they had reached the kidney dose limit after two or three sessions. Four patients did not reach the dose limit and thus could have had additional treatment.
Radiological response
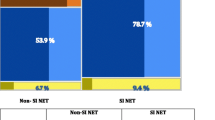
Sixteen of the 26 patients could be evaluated radiologically. Ten either lacked suitable lesions (not measurable on CT, n = 3), died of disease before follow-up (n = 3), or were due to be followed up (n = 4). The radiologic tumor response varied from a 54% increase of tumor size (progression) to a 79% decrease (regression). Six of the 16 (38%) showed partial response (PR) according to RECIST criteria. Eight (50%) showed stable disease (SD) and two (13%) showed progressive disease (PD). No complete remissions (CR) were observed. The radiologic response was not significant, p = 0.15 (Fig. 1).
Sixteen of 26 patients could be evaluated radiologically. The radiologic tumor response varied from a 54% increase of tumor size (progression) to a 79% decrease (regression). Using the RECIST criteria, 6 of 16 patients (38%) showed partial response (PR), 8 of 16 (50%) showed stable disease (SD), and 2 of 16 (13%) showed progressive disease (PD)
Biochemical response
Seventeen of 26 patients were evaluated biochemically by measuring P-CgA. Nine patients either did not have elevated P-CgA before treatment (n = 4), died of disease before follow-up (n = 3), or were due to be followed up (n = 2). Six (35%) showed a ≥30% decrease, eight (47%) showed a ≥20% increase, and three (18%) showed neither a ≥30% decrease nor a ≥20% increase (Fig. 2).
Seventeen of 26 patients could be evaluated biochemically. The biochemical response varied from a 372% increase to a 72% decrease of P-CgA values. When applying the same response limits as in the RECIST criteria to the biochemical response, 6 of 17 patients (35%) showed partial response (PR), 3 of 17 (18%) showed stable disease (SD), and 8 of 17 (47%) showed progressive disease (PD)
Adverse effects
Three patients experienced CTC grade 3 hematologic toxicity with low platelet counts. Nadir values were 12, 19, and 38 × 109/l and appeared at 40–45 days after treatment. The mean GFR before treatment was 80 ml/1.73 m2/min ± 4 (SEM). At follow-up the mean GFR was 70 ml/1.73 m2/min ± 4 (SEM). The decrease in GFR was significant, p = 0.0013.
Discussion
PRRT with [177Lu-DOTA0,Tyr3]-octreotate has recently been introduced as a palliative treatment for patients with GEPNETs [8]. This radiopharmaceutical binds specifically to sstr 2 and 5 resulting in uptake and internalization of the radionuclide. The first attempts of systemic treatment with [111In-DTPA0]-octreotide resulted in symptomatic relief and reduction in biochemical markers but rarely a radiologic response [15–17]. [90Y-DOTA,Tyr3]-octreotate improved the results and led to partial regression in 7–24% of treated patients [18–20]. Treatment using [177Lu-DOTA0,Tyr3]-octreotate has, so far, shown the most promising results, with tumor response in up to 47% of treated patients [8].
The present study describes the outcome of [177Lu-DOTA0,Tyr3]-octreotate treatment in 26 consecutive patients evaluated radiologically (RECIST criteria) and biochemically (P-CgA). The follow-up time was relatively short (mean = 5 months). The rate of partial response (38%) is in line with that of previous reports. We observed a weak correlation between radiologic and biochemical responses. A possible explanation for this could be the relatively short follow-up time, i.e., because the P-CgA value reflects both the functional status of the tumor cells and tumor volume, the biochemical responses and radiologic responses can be anticipated not to be synchronous [11]. P-CgA levels are also affected by renal function, which, as shown in the present study, is affected by PRRT [21]. The adverse effects of PRRT reported by other centers have generally been mild. However, the significant reduction in the GFR observed in the present study indicates the importance of the kidney as a dose-limiting organ.
A prerequisite for successful PRRT is high expression of sstr in the tumors. Usually, the selection of suitable patients is based on tumor uptake on octreotide scintigraphy. The cutoff limit for treatment in the present study was a tumor-specific uptake of at least twice the physiologic uptake in the liver, a method that has been widely used. However, this type of visual or semiquantitative evaluation of the concentration of the radiopharmaceutical in the tumor should be avoided. One major reason is that the interindividual variation in the concentration of 111In-octreotide is large, up to a factor of 4 (and liver is not the most critical organ in this treatment). Another reason is that properties and limitations of the scintigraphic technique will give misleading results if these limitations are not dealt with. Instead a more careful determination of tumor uptake, an absorbed dose should be determined.
Several more specific methods for the selection of patients for this treatment have been proposed. Tumor-to-blood (T/B) ratios of the radiopharmaceutical give a relatively accurate measurement of the tumor-specific uptake [22, 23]. Quantification of sstr expression in tumor biopsies gives a precise profile of the sstr subtypes expressed [24, 25]. A suitable radiopharmaceutical with a high affinity for the particular subtypes expressed can be selected in order to obtain an optimal tumor-specific uptake. In the future, a combination of pharmacologic treatment and tumor-specific PRRT may result in better response rates in patients with NE tumors [26].
The tumor burden of our patients was relatively large and the patients had undergone several modalities of conventional treatment before PRRT. Careful selection of patients with regard to sstr expression could be one way to treat patients with smaller tumor burdens, e.g., as completion therapy after surgical resection.
In an animal model of a human carcinoid tumor (GOT 1), a very high rate of CR was accomplished [27, 28]. The GOT 1 tumors have preserved T/B ratios and sstr profiles compared to the original tumor. The results from this model suggest that a higher response rate could be achieved if the amount of activity administered, using an optimal treatment schedule, could be increased. In the present study, measurement of the absorbed dose to the kidneys identified four patients who could have received additional treatment without exceeding the dose limit of the kidney. Our results therefore indicate that such measurements could contribute to individualized treatment. Further studies to define tolerance doses to the kidneys and development of more precise methods for determination of the absorbed doses to the kidneys could contribute to optimization of treatment schedules.
References
Wängberg B, Westberg G, Tylén U et al (1996) Survival of patients with disseminated midgut carcinoid tumors after aggressive tumor reduction. World J Surg 20:892–899, discussion 899
Swärd C, Johanson V, Nieveen van Dijkum E et al (2009) Prolonged survival after hepatic artery embolization in patients with midgut carcinoid syndrome. Br J Surg 96:517–521
Öberg K (2000) Interferon in the management of neuroendocrine GEP-tumors: a review. Digestion 62(Suppl 1):92–97
Kölby L, Persson G, Franzén S et al (2003) Randomized clinical trial of the effect of interferon alpha on survival in patients with disseminated midgut carcinoid tumours. Br J Surg 90:687–693
Öberg K (2003) Diagnosis and treatment of carcinoid tumors. Expert Rev Anticancer Ther 3:863–877
Öberg K (1993) The use of chemotherapy in the management of neuroendocrine tumors. Endocrinol Metab Clin North Am 22:941–952
Rougier P, Mitry E (2000) Chemotherapy in the treatment of neuroendocrine malignant tumors. Digestion 62(Suppl 1):73–78
Kwekkeboom DJ, Teunissen JJ, Bakker WH et al (2005) Radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol 23:2754–2762
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors, European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Hsiao RJ, Neumann HP, Parmer RJ et al (1990) Chromogranin A in familial pheochromocytoma: diagnostic screening value, prediction of tumor mass, and post-resection kinetics indicating two-compartment distribution. Am J Med 88:607–613
Kölby L, Bernhardt P, Swärd C et al (2004) Chromogranin A as a determinant of midgut carcinoid tumour volume. Regul Pept 120:269–273
Stridsberg M, Öberg K, Li Q et al (1995) Measurements of chromogranin A, chromogranin B (secretogranin I), chromogranin C (secretogranin II) and pancreastatin in plasma and urine from patients with carcinoid tumours and endocrine pancreatic tumours. J Endocrinol 144:49–59
Bernhardt P, Benjegard SA, Kölby L et al (2001) Dosimetric comparison of radionuclides for therapy of somatostatin receptor-expressing tumors. Int J Radiat Oncol Biol Phys 51:514–524
de Jong M, Breeman WA, Valkema R et al (2005) Combination radionuclide therapy using 177Lu- and 90Y-labeled somatostatin analogs. J Nucl Med 46(Suppl 1):13S–17S
Andersson P, Forssell-Aronsson E, Johanson V et al (1996) Internalization of indium-111 into human neuroendocrine tumor cells after incubation with indium-111-DTPA-D-Phe1-octreotide. J Nucl Med 37:2002–2006
Krenning EP, de Jong M, Kooij PP et al (1999) Radiolabelled somatostatin analogue(s) for peptide receptor scintigraphy and radionuclide therapy. Ann Oncol 10(Suppl 2):S23–S29
van Essen M, Krenning EP, Bakker WH et al (2007) Peptide receptor radionuclide therapy with 177Lu-octreotate in patients with foregut carcinoid tumours of bronchial, gastric and thymic origin. Eur J Nucl Med Mol Imaging 34:1219–1227
Paganelli G, Zoboli S, Cremonesi M et al (2001) Receptor-mediated radiotherapy with 90Y-DOTA-D-Phe1-Tyr3-octreotide. Eur J Nucl Med 28:426–434
Waldherr C, Pless M, Maecke HR et al (2001) The clinical value of [90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: a clinical phase II study. Ann Oncol 12:941–945
Valkema R, De Jong M, Bakker WH et al (2002) Phase I study of peptide receptor radionuclide therapy with [In-DTPA]octreotide: the Rotterdam experience. Semin Nucl Med 32:110–122
Hsiao RJ, Mezger MS, O’Connor DT (1990) Chromogranin A in uremia: progressive retention of immunoreactive fragments. Kidney Int 37:955–964
Forssell-Aronsson E, Bernhardt P, Nilsson O et al (2004) Biodistribution data from 100 patients i.v. injected with 111In-DTPA-D-Phe1-octreotide. Acta Oncol 43:436–442
Forssell-Aronsson E, Fjälling M, Nilsson O et al (1995) Indium-111 activity concentration in tissue samples after intravenous injection of indium-111-DTPA-D-Phe-1-octreotide. J Nucl Med 36:7–12
Kölby L, Wängberg B, Ahlman H et al (1998) Somatostatin receptor subtypes, octreotide scintigraphy, and clinical response to octreotide treatment in patients with neuroendocrine tumors. World J Surg 22:679–683
Kubota A, Yamada Y, Kagimoto S et al (1994) Identification of somatostatin receptor subtypes and an implication for the efficacy of somatostatin analogue SMS 201-995 in treatment of human endocrine tumors. J Clin Invest 93:1321–1325
de Visser M, Verwijnen SM, de Jong M (2008) Update: improvement strategies for peptide receptor scintigraphy and radionuclide therapy. Cancer Biother Radiopharm 23:137–157
Kölby L, Bernhardt P, Ahlman H et al (2001) A transplantable human carcinoid as model for somatostatin receptor-mediated and amine transporter-mediated radionuclide uptake. Am J Pathol 158:745–755
Kölby L, Bernhardt P, Johanson V et al (2005) Successful receptor-mediated radiation therapy of xenografted human midgut carcinoid tumour. Br J Cancer 93:1144–1151
Acknowledgments
This work was supported by grants from the Swedish Research Council (5220), the Swedish Cancer Society (654, 4956), and the King Gustav V Jubilee Clinic Cancer Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Swärd, C., Bernhardt, P., Ahlman, H. et al. [177Lu-DOTA0-Tyr3]-Octreotate Treatment in Patients with Disseminated Gastroenteropancreatic Neuroendocrine Tumors: The Value of Measuring Absorbed Dose to the Kidney. World J Surg 34, 1368–1372 (2010). https://doi.org/10.1007/s00268-009-0387-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-009-0387-6