Abstract
Purpose
The incidence of Urothelial carcinoma of bladder (UBC) is gradually increasing by changing lifestyle and environment. The development of a tumor has been noted to be accompanied by modifications in the extracellular matrix (ECM) consisting of CD44, hyaluronic acid (HA) and its family members. The importance of CD44 splice variants and HA family members has been studied in UBC.
Methods
The cohort of study included 50 UBC patients undergoing radical cystectomy and 50 healthy subjects. The molecular expression of CD44 and HA family members was determined. Effect of CD44 variant-specific silencing on downstream signaling in HT1376 cells was investigated. Combinatorial treatment of 4-MU (4-methylumbelliferone) with cisplatin or doxorubicin on chemosensitivity was also explored.
Results
Higher expression of HA, HAS2, and CD44 was observed in Indian UBC patients which also showed the trend with severity of disease. Splice variant assessment of CD44 demonstrated the distinct role of CD44v3 and CD44v6 in bladder cancer progression. shRNA-mediated downregulation of CD44v3 showed an increase effect on cell cycle, apoptosis and multiple downstream signaling cascade including pAkt, pERK and pSTAT3. Furthermore, 4-MU, an HA synthesis inhibitor, observed to complement the effect of Cisplatin or Doxorubicin by enhancing the chemosensitivity of bladder cancer cells.
Conclusions
Our findings exhibit involvement of CD44 splice variants and HA family members in UBC and significance of 4-MU in enhancing chemosensitivity suggesting their novel therapeutic importance in disease therapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urothelial Carcinoma of Bladder (UBC) is the ninth most prevalent cancer and thirteenth most common cause of cancer death worldwide (Ploeg et al. 2009). Incidence in developing countries is higher compared to developed countries (Jemal et al. 2005; Parkin 2008). As per National Cancer Registry Programme, in India, the incidence rate of the urinary bladder cancer is 3.67% in males and 0.83% in females (per 100,000 annually) (Parkin 2008).
Tumor microenvironment (TME) consists of various components that are classified into three main groups: cells of haematopoietic origin, cells of mesenchymal origin and non-cellular components (Extracellular matrices: ECM). ECM comprises of collagens, glycoproteins, proteoglycans, and hyaluronan, which acts as a physical scaffold to which tumor cells attach and migrate. Hyaluronic acid (HA), a non-sulphated glycosaminoglycan, is a ubiquitous component of the ECM that provides tissue homeostasis and maintains ECM architecture. In TME, HA is able to transmit signals originating from ECM into the cell, changes its metabolism, and linked to the promotion of cell migration and metastasis (Stern et al. 2006; Toole and Hascall 2002). HA is synthesized by transmembrane protein Hyaluronic acid synthase (HAS), having three isoforms: HAS1, HAS2, and HAS3, that synthesizes HA at different rates. HAS1 and HAS2 have been reported to be involved in the progression of bladder cancer (Jamshidian et al. 2014; Kramer et al. 2011).
HA attains its effect by interacting with its primary receptor CD44 and this binding is not hindered by variations in membrane proximal domain (He et al. 1992; Knudsen et al. 1999; Toole, 2004; Turley et al. 2002). Pericellular HA from tumor cells has been shown to bind CD44 and induces a lipid raft-associated, signaling complex-containing Src, phosphorylated ErbB2 (p-ErbB2), CD44, ezrin, phosphoinositide 3-kinase, and chaperone molecules Hsp90 and cdc37. This complex is apparently necessary for promoting survival activities of tumor cells (Ghatak et al. 2005; Misra et al. 2006). HA-activated CD44 can also associate and activate the member of the Src family of proteins, Lyn, which in turn activates PI3-K and AKT to promote resistance to apoptosis (Ilangumaran et al. 1998). CD44 also activates MAPK/ERK pathway to protect tumor cells from spontaneous and drug-induced apoptosis (Herishanu et al. 2011; Kouvidi et al. 2011; Meran et al. 2011). Interaction of HA with CD44 induces EMT transition via activation of numerous downstream pathways (Lamouille et al. 2014). HA synthesis is inhibited by 4-Methylumbelliferone (4-MU). In tumor cells, 4-MU has been reported to hamper proliferation, motility and invasion by inhibiting variety of HA signaling events, including downregulation of p-ErbB2, pAkt and MMPs (Saito et al. 2013; Urakawa et al. 2012).
CD44 gene comprised of 19 exons of which the first 5 and last 5 exons are constant encoding the shortest isoform of CD44, i.e., the CD44 standard (CD44 s). The middle nine exons are assembled with the standard CD44 isoform and undergo alternative splicing to generate multiple CD44 variants (CD44v) (Chen et al. 2018). This differential splicing process resulted in more than twenty CD44 isoforms that regulate and participate in cellular differentiation. Specific CD44 variants have been found to be expressed in certain metastatic cancers (Gee et al. 2004; Hamilton et al. 2007). CD44v6 is suggested to be involved in breast and colorectal cancers, whereas CD44v3 is implicated in chronic myeloid leukemia (Akisik et al. 2002; Basakran 2015; Olsson et al. 2011). These studies indicate a cancer-specific expression of CD44 variants that has not been reported in UBC so far. Taken together, role of these HA-family-member molecules including specific variant of CD44 along with HA synthesis inhibitor has not been established in UBC.
In this study, we investigated expression of HA, HAS (1&2), and CD44 in patients with bladder cancer and further assessed their therapeutic potential. Variant-specific CD44 expressions were evaluated and downstream effects have been observed after their silencing. Effects of 4-MU treatment alone or in combination with chemotherapeutic drugs (cisplatin and doxorubicin) were ascertained as a plausible therapeutic approach in UBC.
Materials and methods
Study subjects and cell lines
Our study was approved by institute’s ethical committee (IEC No.: IESC/T-411/02.11.2012) and written consent from patients was obtained. All the patients were histopathologically proven cases of urothelial carcinoma of bladder (Supplementary Table 1). Patients having malignancy at any other part of the body and any urinary tract infection (UTI) were excluded. 50 clinically diagnosed cases of UBC patients with high- and low-grade muscle invasive tumors subjected to undergo radical cystectomy were enrolled. Blood samples (P), tumor tissue (T), and adjacent normal tissue (N) were obtained from them. 50 healthy control subjects were also enrolled for blood samples (C) only. Total 5 ml of venous blood was drawn from all the subjects (P) and (C) in sterile endotoxin-free vacutainers with EDTA as an anticoagulant for isolation of Peripheral Blood Mononuclear Cells (PBMCs). Tumor specimen (T) and adjacent non-tumor specimens (N) were collected from the same patient who underwent radical cystectomy in the Department of Urology, AIIMS, New Delhi. HT1376 (high-grade bladder cancer cell line) was purchased from ATCC and maintained in Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% (v/v) FBS (Fetal bovine serum) under strict aseptic condition in culture hood.
RNA extraction and reverse transcription
RNA was extracted using Trizol® (Sigma, USA) and quantified using Nanodrop™ spectrophotometer (ThermoFisher, USA). For reverse transcription of 1 μg of purified total RNA, 1 μl of random hexamer for a final concentration of 2.5 μM was added. After 10 min of incubation at 70 °C, 10× M-MuLV Reverse Transcriptase Buffer (Fermentas), M-MuLV Reverse Transcriptase (200 units/μl, Fermentas), 2 μl of 10 mM dNTP mix (Fermentas, ThermoFisher, USA), RNase Inhibitor (40 units/μl, Promega) and RNase free water were added for final volume of 20 μl, then incubated at 42 °C for 60 min and 70 °C for 10 min.
PCR analysis
Serial PCRs with five distinct human-specific primer pairs for CD44 were performed for the examination of CD44 variants (Supplementary Table 2). The PCR mixture with different primer combinations was prepared. Cycling conditions were: 94 °C for 12 min once, then 94 °C for 1 min, 65 °C for 1 min, 72 °C for 1 min for 40 cycles and extension step was 72 °C for 10 min. For quantitative PCR, the cDNA synthesized was used as a template to evaluate gene amplification (BIO-RAD CFX96, USA) with primers for HAS1, HAS2, CD44, CD44v3, and CD44v6 and SYBR green master mix (Fermentas). Cycling conditions were: denaturation at 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, 72 °C for 30 s. All primers had an optimal annealing temperature of 60 °C (Supplementary Table 2).
Immunohistochemistry and western blotting (immunoblotting)
Tissue specimens (tumor and non-tumor) were fixed in 10% formalin following which paraffin-fixed tissue embedded blocks were prepared. APES-coated slides were used for mounting tissue specimen. Antigen retrieval was performed using citrate buffer (pH 6.0) followed by washing in TBS (Tris Buffered Saline). Primary antibodies specific for HAS1, HAS2 and CD44 were then added and incubated overnight. Anti-mouse and/or anti-rabbit secondary antibodies were added followed by treatment with avidin-conjugated HRP, chromogen and counterstained with hematoxylin. Slides were then mounted and quantified.
For Immunoblotting, samples were lysed in RIPA lysis buffer and proteins were then quantified using Bradford method. Then, 30 µg protein was resolved by SDS-PAGE and transferred onto a nitrocellulose membrane. Membrane was then blocked by BSA and incubated overnight with respective antibodies (HAS1, HAS2, CD44 and various signaling proteins) (Supplementary Table 3). Then, HRP-conjugated secondary antibody was added. Chemiluminescence detection (Thermo Scientific, USA) was used to develop blots, and images were acquired by FluorChem M (ProteinSimple, California, USA) followed by quantification using ImageJ analyzer software.
Cell viability assay
10 × 103 cells were plated into each well of a 96-well plate and kept at 37 °C in a humidified atmosphere containing 5% CO2. They were treated with different concentrations of 4-methylumbelliferone (4-MU), cisplatin and doxorubicin (Sigma-Aldrich, USA) for 48 h or 72 h. 20 µl MTT reagent (5 mg/ml) was added in each well and incubated for 4 h. The formazan crystals thus formed were solubilised by 100 µl DMSO/well and quantitated at 570 nm with reference wavelength of 630 nm. The LD50 values were calculated.
siRNA and shRNA transfection of bladder cancer cells (HT1376)
Commercially available siRNA (Mission esiRNA) was purchased from Sigma-Aldrich, USA (Supplementary Table 4), while shRNA was synthesized (Supplementary Table 2). The method used for transfection involved the cationic liposome-mediated DNA transfer with Lipofectamine™ 3000 transfection reagent (Invitrogen, ThermoFisher, USA).
For siRNA, in a six-well tissue culture plate, HT1376 cells (2 × 105) were seeded per well in 2 ml antibiotic-free medium (EMEM) supplemented with 10% FBS and kept at 37 °C in a CO2 incubator for overnight cell adherence. Cells were starved in serum-free media (SFM) after incubation and transfection mixture was prepared along with it. For a well, mixture was prepared by adding SFM and CD44 esiRNA (Sigma-Aldrich, USA) with Lipofectamine. Vials were mixed and kept at room temperature for 20 min. Meanwhile, cell culture was washed with SFM. Mixtures were added dropwise in each well and kept at 37 °C in a CO2 incubator. Complete media with 20% FBS was added after 4 h.
For silencing CD44v3 and CD44v6 individually, 63 bp shRNA was designed and cloned in pSilencer™ 5.1-U6 Retro at BamHI and HindIII sites. These vectors were then used for transfecting HT1376 cells. Briefly, DH5α strain of E. coli was used as host for performing all the transformations. Competent cells were prepared by CaCl2 treatment. The cells were transformed with the cloned vector by heat shock treatment for 90 sec. After transformation, plasmid DNA was subjected to digestion by restriction endonuclease(s) for the purpose of screening of recombinant clones or purifying a DNA fragment for sub-cloning. After confirmation of clones, 1 μg of plasmid-containing shRNA was transfected in HT1376 cells.
PI staining for assessment of apoptosis
Propidium iodide (PI) staining is used to distinguish viable cells from nonviable cells. Cells were starved overnight in EMEM with 1% FBS for synchronization following which respective treatment was given. Treated/untreated cells were washed and maintained at 1 × 106 cells per tube. Cells were fixed in 70% cold ethanol and kept in ice for 1 h. Cells were then resuspended in PBS followed by addition of RNase A and then, tubes were incubated at 37 °C for 1 h. 5μL of 0.1 mg/mL PI solution was added and kept in dark at 4 °C until analysis. Tubes were analyzed by FACS (BD FACS Canto, USA) at 488 nm.
Statistical analysis
Data were expressed as median (range) for circulatory levels and mRNA expression. Immunoreactive score (IRS) of immunohistochemistry was expressed as mean ± SD. Comparisons between patient and control groups were made using the Wilcoxon rank-sum (Mann–Whitney) test for non-parametric data. Correlation was done using Spearman correlation coefficient. Receiver-operating characteristic (ROC) curve was plotted for ELISA levels to determine optimal cut-off values with optimum sensitivity and specificity. The in vitro data were expressed in terms of fold change and Student’s t test was applied for analysis. Statistical significance was defined at a p value less than 0.05 (p < 0.05). All data analyses were conducted using GraphPad Prism 6 and SPSS 19.0.
Results
Elevated molecular expression of key members of HA family in UBC
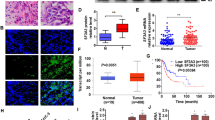
The histopathologically proven cases of urothelial carcinoma of bladder were recruited (Supplementary Table 1). As compared to HAS1, the quantitative mRNA expression of HAS2 (p ≤ 0.05) and CD44 (p < 0.0001) were significantly higher in tumor tissue than adjacent normal tissue (Fig. 1a). Immunoblotting of these proteins in tumor tissue and adjacent normal tissue and subsequent densitometry analysis demonstrated 1.3-fold and 1.6-fold higher expression of HAS2 and CD44, respectively. There was no significant difference in expression of HAS1 in tumor tissue as compared to the adjoining normal tissue (Fig. 1b, c). Quantification of immunohistochemistry by IRS scoring exhibited significant higher expression of HAS2 and CD44 in tumor tissue as compared to normal tissue. Also, there is no significant difference in HAS1 expression in tumor tissue and adjacent normal tissue (Fig. 2a, b). Upon Spearman correlation analysis, significant positive correlation (r = 0.699, p < 0.001) was found between transcript levels of CD44 and HAS2 in UBC patients.
CD44 variant analysis and their expression in UBC
CD44 variants were observed using various combinations of primers. Agarose gel electophoretogram of PCR products using five primer combinations (Supplementary Table 2) has been shown in Fig. 3a. CD44v3 and CD44v6 have been found to be overexpessed in tumor tissue as compared to adjacent normal tissue. Further, validation of specific variant expression was performed by Q-PCR for CD44v3 and CD44v6 in tissues and twofold higher expression has been found in tumor tissue relative to normal tissue (Fig. 3b). This result was confirmed by silencing CD44 mRNA expression using CD44 siRNA in HT1376 cell line, and further quantification of CD44v3 and CD44v6 expression. Silencing CD44 significantly reduced expression of CD44 variants in bladder cancer cells (Fig. 4a, b).
Silencing of CD44v3 demonstrated potentiate effect on cell cycle progression and signaling pathway
To study the CD44v3- and CD44v6-induced alteration in signaling pathways in bladder cancer cells, it was essential to transiently silent individual CD44 variant (Supplementary Figs. 1, 2). After transient transfection of CD44v3 shRNA, quantitative expression of entire CD44 (0.4-fold; p ≤ 0.001) and CD44v3 (0.7 fold; p < 0.0001) mRNA showed significant downregulation (Fig. 5a). Immunoblotting results also explained downregulation of CD44 expression and other key signaling cascade molecules at 24 and 48 h. There was a decrease in PI3 K, pAKT, pERK, pSTAT3 and Bcl2 expressions implicating CD44v3 in tumor growth, differentiation and development as well as gain of anti-apoptotic nature (Fig. 5b, c). Analysis of cell cycle after CD44v3 silencing exhibited arrest of urothelial carcinoma cells in G0/G1 phase and apoptosis as reflected by cells in sub-G0/G1 phase (Fig. 5d; Table 1). Transient transfection of CD44v6 shRNA downregulated expression of CD44v6 mRNA (0.6-fold; p < 0.0001), but not entire CD44 mRNA (Supplementary Fig. 3A). Western blotting analysis also revealed that there is no significant outcome in expression of signaling molecules after silencing CD44v6 (Supplementary Figs. 3B, C). There was no change in the downstream effect after transiently silencing CD44v6 in urothelial carcinoma of bladder.
Fold change (2−ΔΔCt) in expression of CD44, CD44v3 and CD44v6 represented as mean ± SD. Significant difference between control, at 24 h and at 48 h, was indicated by *p < 0.05 (a). Effect of transient silencing of CD44v3 on signaling cascade of HT1376 cells. Cell lysate containing equal amount of total protein was resolved on SDS-PAGE and processed for western blotting using different antibodies (b). Fold change in expression of downstream signaling cascade molecule after CD44v3 silencing. Densitometry analysis using ImageJ software showed significant decrease in expression of CD44v3, PI3K, pAKT, pERK, pSTAT3 and Bcl2 after 48 h of transfection (c). Histogram of flow cytometry analysis after CD44v3 silencing in HT1376 cell lines. FACS analysis after PI treatment of HT1376 cells in control, after 24 h of transfection and after 48 h of transfection (d)
4-MU has chemosensitizing effect on bladder cancer cells
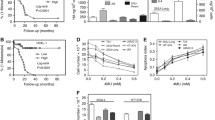
In our study, to demonstrate the effect of 4-MU treatment in Urothelial carcinoma cells, LD50 of 4-MU was determined in HT1376 cells by cell survival assay (Supplementary Fig. 4). To further assess the effect of 4-MU in combination with chemotherapeutic drugs, cells were treated with defined concentration of 4-MU (0.4 mM and 0.8 mM) with different concentrations of Cisplatin (1–10 μg/ml) and Doxorubicin (2–10 μg/ml). Evaluation of cell survival after 48 h demonstrated that combinatorial treatment of 4-MU + Cisplatin has more lethal effect on cancer cells than Cisplatin alone (Fig. 6a). Similarly, we also observed that combinatorial treatment of 4-MU + Doxorubicin is more lethal than Doxorubicin alone (Fig. 6b). The effect of combinatorial treatment on signaling cascade molecules confirmed its chemosensitizing effect by reducing expression of pAKT, pERK, STAT3, pSTAT3 and Bcl2 (Fig. 6c, d). Moreover, cell cycle analysis showed that a higher percentage of bladder cancer cells is undergoing apoptosis after combination therapy (Fig. 6e, Table 2). These findings demonstrate that effect of combinative treatment on bladder cancer cells improves chemosensitivity for these drugs, influencing important intracellular signaling pathways that promote cancer cell proliferation, differentiation and also inhibits apoptosis.
Cell proliferation assay after combination treatment of 4-MU and Cisplatin: different concentrations of 4-MU (0.4 mM and 0.8 mM) along with Cisplatin (1–10 μg/ml) and Cisplatin alone were used to treat Urothelial carcinoma cell line for 48 h (a). Cell proliferation assay after combination treatment of 4-MU and Doxorubicin: different concentrations of 4-MU (0.4 mM and 0.8 mM) along with Doxorubicin (2–10 μg/ml) and Doxorubicin alone were used to treat urothelial carcinoma cell line for 48 h (b). Effect of combination treatment of 4-MU and Cisplatin/Doxorubicin on signaling cascade of HT1376 cells. Cell lysate containing equal amount of total protein were resolved on SDS-PAGE and processed for western blotting using different antibodies. The control, 0.4 mM 4-MU, 0.8 mM 4-MU, 0.8 mM 4-MU + Cisplatin and 0.8 mM 4-MU + Doxorubicin after 48 h shows expression of different molecules (c). Fold change in expression of downstream signaling cascade molecule after treatment of 4-MU and Cisplatin/Doxorubicin. Densitometry analysis using ImageJ software showed significant decrease in expressions of AKT, pAKT, pERK, STAT3, pSTAT3 and Bcl2 after 48 h of transfection (d). Cell-cycle analysis of HT1376 after combined treatment with 4-MU + Cisplatin and 4-MU + Doxorubicin. FAC analysis after PI treatment of HT1376 cells in control and in cells treated with combination of different doses of 4-MU with Cisplatin and Doxorubicin for 48 h (e)
Discussion
Tumor microenvironment plays an essential role in maintaining the sustenance of tumor cells in all the malignancies including urothelial carcinoma of bladder (UBC). This tumor microenvironment is a complex entity that includes numerous proteins, proteoglycans and growth factors. Hyaluronic acid (HA) is a non-sulfated proteoglycan present in the ECM and is responsible for maintaining tissue scaffolding. HA is synthesized by HA synthase (HAS) enzyme and then secreted in the surrounding milieu. It then binds to the CD44 receptor and triggers downstream signals to perform several functions in favor of biological homeostasis (Adamia et al. 2013; Toole and Hascall 2002). Limited literature is available showing involvement of HA signaling via CD44 in UBC. Moreover, therapeutic potential of CD44 and HAS inhibitor has not been elucidated in UBC till date. Hence, this maiden study examined the transcript and protein expression of HAS (1 and 2), CD44 in UBC patients to determine their status in this disease. Furthermore, the effect of CD44 silencing on cancer pathogenesis in vitro has been studied. Besides, the role of variant-specific CD44 was elucidated in bladder cancer in vitro along with the signaling pathways involved. Finally, the adjuvant therapy of HAS inhibition in combination with standard chemotherapeutic drugs (cisplatin and doxorubicin) has been studied.
The molecular expression of HA family members was investigated in the study subjects. We observed that overexpression of HAS2 than HAS1 in UBC patients also correlated with disease severity. These findings are in concordance with reports in breast and pancreatic cancer (Cheng et al. 2013; Li et al. 2015). An earlier report suggested that the different stages of malignant tumor progression were due to the involvement of HAS isoforms (Bernert et al. 2011). Rika Kosaki et al. showed that HAS2 promotes anchorage-independent growth and tumorigenicity of human fibrosarcoma cells by increasing HA production (Kosaki et al. 1999). Accrued data strengthen the concept that HAS2 overexpression often accounts for the augmented hyaluronan synthesis in many tumors, correlating poor outcome (Li et al. 2015; Sato et al. 2004). Our observation also supports previous findings where higher HA synthesis is linked to bladder cancer in study subjects which might be due to the increased expression of HAS2 enzyme.
The primary receptor of HA for mediating its downstream effect is CD44 and we determined the expression of CD44 in study subjects and observed significantly elevated levels in UBC patients as compared to controls, demonstrating similarity with previous reports in colorectal and colon cancer (Amirghofran et al. 2008; Subramaniam et al. 2007). Overexpression of CD44 in tumor emphasizes about its various biological roles such as cell–cell, cell–ECM interactions, tumor cell migration and in chemoresistance (Bánky et al. 2012; Mayr et al. 2017). We also observed a significant positive correlation between CD44 and HAS2. Our observation unravels for the first time that in Indian UBC patients, there is an enhanced expression of HAS2 which is accountable for increased HA synthesis that in turn mediates its downstream effect by augmented expression of CD44 (Li et al. 2015).
In addition to our analysis, we further tried to identify the plausible variants of CD44 in UBC that may indicate about the CD44 isoforms involved in this cancer. It has been reported that different tumor cell types express a different set of CD44 variants (Bánky et al. 2012). In a recent study by Morath et al., the contribution of CD44v6 in stimulating downstream signaling pathways associated with mammary carcinoma has been reported (Morath et al. 2018). Using five different primer-pair spanning variable regions, for qualitative and quantitative PCR, we observed splice pattern of CD44 and found that CD44v3 and CD44v6 exons are overexpressed in tumor tissue of UBC patients. This was further validated by silencing CD44 by siRNA treatment in HT1376 bladder cancer cells. We observed reduced expression of CD44v3 and CD44v6 mRNA in HT1376 cells after silencing CD44. Taken together, these results suggest that CD44v3 and CD44v6 play a key role in mediating HA-induced downstream effects in UBC which is supported by previous report in colorectal cancer (Bánky et al. 2012). Kobayashi et al. have reported the importance of CD44 variant 9 in bladder cancer and stated CD44v9 as a useful predictive biomarker for basal-type muscle and high-risk non-muscle invasive bladder cancer (Kobayashi et al. 2016). Moreover, to corroborate our findings with the downstream effect on cancer cells, we exclusively silenced CD44v3 and CD44v6 by designing variant-specific shRNA and using silencing vector (pSilencer 5.1-U6 retro). We observed significant role of CD44v3 as compared to CD44v6 in bladder cancer cells by its effect on essential cellular pathways and cell cycle. Silencing CD44v3 reduced expression of PI3 K, pAKT, pERK, pSTAT3 and Bcl2 proteins also triggered arrest of cells in G0/G1 and sub-G0/G1 phase of cell cycle implicating its plausible role in tumor growth, differentiation, development and apoptosis in UBC. Silencing CD44v6 did not show any significant effect. These evidences confirm variant-specific expression of CD44 in different tumor types (Williams et al. 2013). The similar conclusion had also been drawn by Van et al. in head and neck squamous cell carcinoma in which they reported that soluble CD44v6 could not be exploited as a marker for the cancer (Van Hal et al. 1999).
To authenticate our hypothesis for involvement of HA and its family molecules in tumor progression, we used 4-MU (4-methylumbelliferone), an HA synthesis inhibitor in our study. 4-MU has no serum or organ toxicity, therefore, its role in controling tumor progression needs to be examined (Lokeshwar et al. 2014). In this study, we have observed that 4-MU abrogated in vitro growth of bladder cancer cells with reduction in anti-apoptotic marker, Bcl-2. These findings were in concordance with a study by Morera et al. who had also reported the inhibition of proliferation and other cancer hallmarks of bladder cancer cells by 4-MU (Morera et al. 2017). In addition to explore the therapeutic potential of 4-MU alone, we have observed for the first time in this maiden attempt, that 4-MU in combination with lower doses of chemotherapeutic drugs, cisplatin and doxorubicin, inhibits proliferation of bladder cancer cells suggesting its chemosensitization effect against these cells. In addition, combinatorial treatment reduced expression of key signaling cascade molecules, pAKT, pERK, pSTAT3 and Bcl2, suggesting its inhibitory effect in tumor development. Moreover, cell-cycle analysis also revealed that 4-MU in conjunction with cisplatin and doxorubicin promotes apoptosis. Our finding advances the knowledge to earlier studies where anti-tumor effect of 4-MU, cisplatin and doxorubicin has been reported independently in other cancers (Park et al. 2006; Saito et al. 2013).
Overall, this study demonstrates for the first time in Indian UBC patients that there is a higher expression of HA, HAS2, and CD44. Higher CD44 expression in UBC promotes the concept of its functional role in the tumor progression. Our results also demonstrate that specific CD44 isoform-containing splice variant CD44v3 may have distinct role in bladder cancer development/growth and might have significant potential for therapeutic intervention. Furthermore, we also observed that 4-MU complements effect of Cisplatin or Doxorubicin by enhancing the chemosensitivity to bladder cancer cells that could be utilized as potential therapeutic approach for better management (Fig. 6). CD44 splice-variant inhibition could be employed as an adjuvant therapy along with standard regimen in clinical settings after validation in future (Fig. 7).
Proposed mechanism of hyaluronic acid (HA)-CD44 signaling and their involvement in bladder cancer progression. HA–CD44 interaction activates downstream signaling through PI3 K followed by activation of pAkt, pERK and pSTAT3 contributing to tumor cell survival and progression. This signaling could be hampered by either inhibiting HA synthesis by 4-methylumbelliferone or by variant-specific silencing of CD44v3. Solid lines indicate direct regulatory mechanism, while broken lines specify indirect regulation
References
Adamia S, Pilarski PM, Belch AR, Pilarski LM (2013) Aberrant splicing, hyaluronan synthases and intracellular hyaluronan as drivers of oncogenesis and potential drug targets. Curr Cancer Drug Targets 13(4):347–361
Akisik E, Bavbek S, Dalay N (2002) CD44 variant exons in leukemia and lymphoma. Pathol Oncol Res 8(1):36–40
Amirghofran Z, Jalali SA, Hosseini SV, Vasei M, Sabayan B, Ghaderi A (2008) Evaluation of CD44 and CD44v6 in colorectal carcinoma patients: soluble forms in relation to tumor tissue expression and metastasis. J Gastrointest Cancer 39(1–4):73–78
Bánky B, Rásó-Barnett L, Barbai T, Tímár J, Becságh P, Rásó E (2012) Characteristics of CD44 alternative splice pattern in the course of human colorectal adenocarcinoma progression. Mol. Cancer 11:83
Basakran NS (2015) CD44 as a potential diagnostic tumor marker. Saudi Med J 36(3):273–279
Bernert B, Porsch H, Heldin P (2011) Hyaluronan synthase 2 (HAS2) promotes breast cancer cell invasion by suppression of tissue metalloproteinase inhibitor 1 (TIMP-1). J Biol Chem 286(49):42349–42359
Chen C, Zhao S, Karnad A, Freeman JW (2018) The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol 11(1):64
Cheng XB, Sato N, Kohi S, Yamaguchi K (2013) Prognostic impact of hyaluronan and its regulators in pancreatic ductal adenocarcinoma. PLoS One 8(11):e80765
Gee K, Kryworuchko M, Kumar A (2004) Recent advances in the regulation of CD44 expression and its role in inflammation and autoimmune diseases. Arch Immunol Ther Exp (Warsz) 52(1):13–26
Ghatak S, Misra S, Toole BP (2005) Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. J Biol Chem 280(10):8875–8883
Hamilton SR, Fard SF, Paiwand FF, Tolg C, Veiseh M, Wang C, McCarthy JB, Bissell MJ, Koropatnick J, Turley EA (2007) The hyaluronan receptors CD44 and Rhamm (CD168) form complexes with ERK1,2 that sustain high basal motility in breast cancer cells. J Biol Chem 282(22):16667–16680
He Q, Lesley J, Hyman R, Ishihara K, Kincade PW (1992) Molecular isoforms of murine CD44 and evidence that the membrane proximal domain is not critical for hyaluronate recognition. J Cell Biol 119(6):1711–1719
Herishanu Y, Gibellini F, Njuguna N, Hazan-Halevy I, Farooqui M, Bern S, Keyvanfar K, Lee E, Wilson W, Wiestner A (2011) Activation of CD44, a receptor for extracellular matrix components, protects chronic lymphocytic leukemia cells from spontaneous and drug induced apoptosis through MCL-1. Leuk Lymphoma 52(9):1758–1769
Ilangumaran S, Briol A, Hoessli DC (1998) CD44 selectively associates with active Src family protein tyrosine kinases Lck and Fyn in glycosphingolipid-rich plasma membrane domains of human peripheral blood lymphocytes. Blood 91(10):3901–3908
Jamshidian H, Hashemi M, Nowroozi MR, Ayati M, Bonyadi M, Najjaran Tousi V (2014) Sensitivity and specificity of urinary hyaluronic acid and hyaluronidase in detection of bladder transitional cell carcinoma. Urol J 11(1):1232–1237
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ (2005) Cancer statistics, 2005. CA Cancer J Clin 55(1):10–30
Knudsen LM, Rasmussen T, Jensen L, Johnsen HE (1999) Reduced bone marrow stem cell pool and progenitor mobilisation in multiple myeloma after melphalan treatment. Med Oncol 16(4):245–254
Kobayashi K, Matsumoto H, Matsuyama H, Fujii N, Inoue R, Yamamoto Y, Nagao K (2016) Clinical significance of CD44 variant 9 expression as a prognostic indicator in bladder cancer. Oncol Rep 36(5):2852–2860
Kosaki R, Watanabe K, Yamaguchi Y (1999) Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res 59(5):1141–1145
Kouvidi K, Berdiaki A, Nikitovic D, Katonis P, Afratis N, Hascall VC, Karamanos NK, Tzanakakis GN (2011) Role of receptor for hyaluronic acid-mediated motility (RHAMM) in low molecular weight hyaluronan (LMWHA)-mediated fibrosarcoma cell adhesion. J Biol Chem 286(44):38509–38520
Kramer MW, Escudero DO, Lokeshwar SD, Golshani R, Ekwenna OO, Acosta K, Merseburger AS, Soloway M, Lokeshwar VB (2011) Association of hyaluronic acid family members (HAS1, HAS2, and HYAL-1) with bladder cancer diagnosis and prognosis. Cancer 117(6):1197–1209
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15(3):178–196
Li P, Xiang T, Li H, Li Q, Yang B, Huang J, Zhang X, Shi Y, Tan J, Ren G (2015) Hyaluronan synthase 2 overexpression is correlated with the tumorigenesis and metastasis of human breast cancer. Int J Clin Exp Pathol 8(10):12101–12114
Lokeshwar VB, Mirza S, Jordan A (2014) Targeting hyaluronic acid family for cancer chemoprevention and therapy. Adv Cancer Res 123:35–65
Mayr L, Pirker C, Lötsch D, Van Schoonhoven S, Windhager R, Englinger B, Berger W, Kubista B (2017) CD44 drives aggressiveness and chemoresistance of a metastatic human osteosarcoma xenograft model. Oncotarget 8(69):114095–114108
Meran S, Luo DD, Simpson R, Martin J, Wells A, Steadman R, Phillips AO (2011) Hyaluronan facilitates transforming growth factor-β1-dependent proliferation via CD44 and epidermal growth factor receptor interaction. J Biol Chem 286(20):17618–17630
Misra S, Toole BP, Ghatak S (2006) Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J Biol Chem 281(46):34936–34941
Morath I, Jung C, Lévêque R, Linfeng C, Toillon RA, Warth A, Orian-Rousseau V (2018) Differential recruitment of CD44 isoforms by ErbB ligands reveals an involvement of CD44 in breast cancer. Oncogene 37(11):1472–1484
Morera DS, Hennig MS, Talukder A, Lokeshwar SD, Wang J, Garcia-Roig M, Ortiz N, Yates TJ, Lopez LE, Kallifatidis G et al (2017) Hyaluronic acid family in bladder cancer: potential prognostic biomarkers and therapeutic targets. Br J Cancer 117(10):1507–1517
Olsson E, Honeth G, Bendahl PO, Saal LH, Gruvberger-Saal S, Ringnér M, Vallon-Christersson J, Jönsson G, Holm K, Lövgren K et al (2011) CD44 isoforms are heterogeneously expressed in breast cancer and correlate with tumor subtypes and cancer stem cell markers. BMC Cancer 11:418
Park SH, Lee Y, Han SH, Kwon SY, Kwon OS, Kim SS, Kim JH, Park YH, Lee JN, Bang SM et al (2006) Systemic chemotherapy with doxorubicin, cisplatin and capecitabine for metastatic hepatocellular carcinoma. BMC Cancer 6:3
Parkin DM (2008) The global burden of urinary bladder cancer. Scand J Urol Nephrol Suppl 218:12–20
Ploeg M, Aben KKH, Kiemeney LA (2009) The present and future burden of urinary bladder cancer in the world. World J Urol 27(3):289–293
Saito T, Tamura D, Nakamura T, Makita Y, Ariyama H, Komiyama K, Yoshihara T, Asano R (2013) 4-methylumbelliferone leads to growth arrest and apoptosis in canine mammary tumor cells. Oncol Rep 29(1):335–342
Sato N, Maehara N, Goggins M (2004) Gene expression profiling of tumor-stromal interactions between pancreatic cancer cells and stromal fibroblasts. Cancer Res 64(19):6950–6956
Stern R, Asari AA, Sugahara KN (2006) Hyaluronan fragments: an information-rich system. Eur J Cell Biol 85(8):699–715
Subramaniam V, Gardner H, Jothy S (2007) Soluble CD44 secretion contributes to the acquisition of aggressive tumor phenotype in human colon cancer cells. Exp Mol Pathol 83(3):341–346
Toole BP (2004) Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4(7):528–539
Toole BP, Hascall VC (2002) Hyaluronan and tumor growth. Am J Pathol 161(3):745–747
Turley EA, Noble PW, Bourguignon LYW (2002) Signaling properties of hyaluronan receptors. J Biol Chem 277(7):4589–4592
Urakawa H, Nishida Y, Knudson W, Knudson CB, Arai E, Kozawa E, Futamura N, Wasa J, Ishiguro N (2012) Therapeutic potential of hyaluronan oligosaccharides for bone metastasis of breast cancer. J Orthop Res 30(4):662–672
Van Hal NL, Van Dongen GA, Ten Brink CB, Heider KH, Rech-Weichselbraun I, Snow GB, Brakenhoff RH (1999) Evaluation of soluble CD44v6 as a potential serum marker for head and neck squamous cell carcinoma. Clin Cancer Res 5(11):3534–3541
Williams K, Motiani K, Giridhar PV, Kasper S (2013) CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Exp Biol Med (Maywood) 238(3):324–338
Acknowledgements
We acknowledge Indian Council for Medical Research (ICMR) for the grant to carry out research work. ICMR Grant No.: 5/13/50/10/NCD-III. All the authors have read the journal’s authorship agreement and policy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Anand, V., Khandelwal, M., Appunni, S. et al. CD44 splice variant (CD44v3) promotes progression of urothelial carcinoma of bladder through Akt/ERK/STAT3 pathways: novel therapeutic approach. J Cancer Res Clin Oncol 145, 2649–2661 (2019). https://doi.org/10.1007/s00432-019-03024-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-03024-9