Abstract
Introduction
Radiosurgery (SRS) or stereotactic fractionated radiotherapy (SFRT) is increasing in the treatment of brain metastases (BMs). Aim of the present study was to evaluate the safety and effectiveness of SRS/SFRT for BMs, using a new mono-isocenter non-coplanar solution (HyperArc™ Varian Medical System).
Methods
BMs patients with a diameter inferior to 3 cm, a life expectancy of more than 3 months and a good performance status, were eligible for Linac-based volumetric modulated arc therapy (VMAT) SFRT/SRS with HyperArc™. A retrospective analysis of patients and BMs was performed.
Results
From August 2017 to May 2018, 381 BMs in 64 patients were treated and 246 BMs (43 patients, median number of BMs: 5) of them were suitable for analysis. With a median FU time of 6 months, 244 out 246 (99%) BMs were controlled (18% complete response; 41% partial response, 40% stable disease), 2 BMs showed a progression, at the first control. No acute or late toxicities were reported. Median overall survival (OS) has not yet been achieved, while median time to progression was 5 months. In univariate analysis, statistically negative prognostic factors for OS were histology of primary tumor (p = 0.009): lung/breast cancer had better survival rates as compared to others. Cumulative intracranial volume disease ≥ 15 cc and systemic progression disease were independent prognostic factors for OS at univariate (p = 0.04; p = 0.005) and multivariate (p = 0.04; p = 0.009) analysis, respectively.
Conclusion
The present first clinical data show that SFRT/SRS with HyperArc™ is safe and effective for BMs patients. The utilization of SFRT/SRS for BMs is promising and should be further explored in randomized trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases (BMs) represent the most common intracranial tumor in adults and affect about 20–40% of cancer patients, with a 3–10 times higher incidence than primary malignant brain tumors (Davis et al. 2012). Lung cancer, breast cancer, and melanoma are the most frequent histologies of cancer that develop BMs (Nayak et al. 2012). The cornerstones of local management of BMs include surgery, whole brain radiotherapy (WBRT) and radiosurgery/stereotactic radiotherapy (SRS/SFRT) (Muller-Riemenschneider et al. 2009). On the one hand, the recent treatment strategy of WBRT with hippocampal sparing could be able to preserve neurocognitive functions (Giaj Levra et al. 2016), although no clear evidence of its effectiveness is still available. Conversely, SRS/SFRT has gained a major importance in the treatment of BMs (NCCN Central Nervous System Cancers 2018) because, in addition to the ability to deliver a high-dose per fraction to the target volume, healthy brain tissue can be better spared compared to WBRT. Nowadays, the use of SRS/SFRT is a well-recognized treatment option for patients with 1–4 BMs and a life expectancy of more than 3–6 months (NCCN Central Nervous System Cancers 2018; Scoccianti and Ricardi 2012; Aoyama et al. 2006; Kocher et al. 2011). Nevertheless, the best treatment approach for patients with multiple BMs is still a subject of debate. The prospective observational study by Yamamoto et al. (2014) found that in cases of low intracranial tumor volume (15 cc or less), patients with 5 to 10 BMs treated with SRS alone had comparable outcomes to patients treated for limited metastases (2–4 BMs). Notably, synchronous BMs irradiation of multiple BMs by means of SRS/SFRT represents a complex process in terms of dose delivery, on-board image-guidance, dedicated immobilization devices and multiple time-consuming treatment sessions corresponding to the number of isocenters (Alongi et al. 2016).
HyperArc™ (HA, Varian Medical System Inc., Palo Alto, CA, USA) represents a potential step forward for linac-based SRS/SFRT of multiple BMs. HA is a technical solution for a mono-isocenter volumetric modulated arc-therapy (VMAT) approach for SRS/SFRT which assures a largely automated optimization process, thanks to dedicated algorithms (Ruggieri et al. 2018). In August 2017, the first patients were treated with HA SRS/SFRT in our institution. Herein, we report the safety and effectiveness by means of HA SRS/SFRT in a large population of patients affected by BMs.
Materials and methods
Study cohort
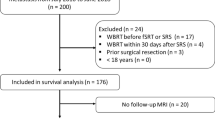
All data were collected in a mono-institutional cancer care center and analyzed retrospectively. All patients were treated for BMs using HA SRS/SFRT. Inclusion criteria for SRS/SFRT were as followed: (a) age older than 18 years; (b) diagnosis of BMs confirmed by contrast-enhanced MRI no more than 4 weeks before the procedure; (c) life expectancy > 3 months, (d) lesions diameter ≤ 30 mm, for each BMs, with a cumulative intracranial tumor volume (CITV) under 50 cc, for multiple BMs, (e) controlled primary tumor or synchronous diagnosis and (f) good performance status. Life expectancy was calculated based on DS-GPA by RTOG data analysis (Sperduto et al. 2012). Each patient had a proven histologic diagnosis of the primary tumor and underwent HA SRS/SFRT without surgical resection. Exclusion criteria for the present analysis was the absence of the first FU or MRI to evaluate the treatment response. Specific informed consent was obtained from all individual participants included in the study.
Treatment plan
Patients underwent a CT simulation without contrast media (1-mm slice thickness) for radiation therapy planning with a thermoplastic mask (QFix®, Avondale, PA–USA). A co-registration of volumetric CT and MRI-T1 sequences (3-dimensional spoiled gradient series with 1-mm slice thickness) was used to define organs at risk (OARs) and target volumes. Gross tumor volume (GTV) encompassed the macroscopic contrast enhancing lesion on T1-MRI and was assumed equal to the clinical target volume (CTV). The planning target volume (PTV) was obtained from the GTV plus an anisotropic margin of 1–2 mm in all directions. OARs, including brain (normal brain minus PTV), eyes, lenses, optic chiasm, optic nerves, brainstem and spinal cord were delineated.
The prescribed total dose and fractionation were chosen based on the size of BMs, proximity to OARs, and intent of treatment (Kocher et al. 2014) and ranged from 15 Gy in 1 fraction to 30 Gy in 5 fractions. For radiotherapy planning, a HA SRS/SFRT VMAT plan was generated with 5 no-coplanar arcs by HyperArc™ (Varian Medical System Inc., Palo Alto, CA, USA) as described in a previous publication (Ruggieri et al. 2018).
Clinical parameters and evaluation of tumor response
Patients and BMs characteristics (sex, age, KPS, tumor histology and subtypes, modality BMs presentation, previous brain treatment, systemic disease status, CITV, and number of BMs) were collected at the time of presentation for the HA SRS/SFRT treatment. At the end of treatment, all patients were clinically evaluated relating to onset of acute neurological side effects. During FU, a clinical evaluation and MRI were performed after 45–60 days from the end of the SRS/SFRT and then every 2–3 months, with the purpose to evaluate both the response at treatment that acute (within 3 months) and late (above 3 months) side effects. At each visit, neurological status and the severity of complications were scored according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC ver.4). Adverse neurological events were considered as a consequence of the treatment in the absence of progressive disease. For each treated BM, complete response (CR), partial response (PR), stable disease (SD) or progression disease (PD) were defined by an expert neuro-radiologist on MRI, according to the RECIST criteria (Schwartz et al. 2016).
Statistical analysis
To summarize the most relevant features of the clinical variables, descriptive statistical analysis was performed. OS and time to progression (TTP) rates were estimated using the Kaplan–Meier method. The OS was calculated from the date of HA SRS/SFRT treatment to the death for any cause or last follow-up date. The TTP was calculated from the date of HA SRS/SFRT treatment to the time of intracranial progression in- and out-field treatment. Neurological death was defined as event death in absence of extra-cranial disease evidence. To identify predictors for treatment activity, statistical analysis of local response related to BMs and treatment characteristics was performed using the Chi-square test for categorical and continuous variables. In line with this purpose, CR and PR have been considered result of variable activity, while SD and PD have been evaluated as “not responder”. To achieve this goal, only the BMs and treatment characteristics that might be variables strongly related to response were considered in the analysis, such as: histology, PTV volume and biological effective dose (BED). The median value both of PTV volume that of BED was considered the breakpoint in these continuous variables, for our analysis. p values < 0.05 were considered statistically significant.
To identify prognostic factors for OS and TTP, a statistical analysis of patients and disease characteristics were performed using univariate and multivariate analysis with a Cox proportional hazard regression models on all cohort of cases, while regarding breast subtypes the same analysis were performed on only patients with breast cancer. Only the factors that have been proven significant on univariate analyses were used for multivariate analyses. p values < 0.05 were considered statistically significant. Statistical analyses were carried out using STATA/SE 14.2 version.
Results
Patient and disease characteristics
From August 2017 to May 2018, 381 BMs (64 patients) were treated using HA SRS/SFRT. From this population, 246 brain lesions in 43 patients were selected for this analysis according to the inclusion/exclusion criteria of the present study. Median age was 57 years (range 38–79), lung cancer (37%) and breast cancer (33%) were the most frequent histologic types. For each patient, the median number of BMs was 5 (range 1–21) and the median CITV was 12.7 cc (range 1.1–47.7). All patients and diseases characteristics are summarized in Table 1.
Volume and treatment characteristics
The median PTV volume and diameter were 1 cc (0.1–42) and 1 cm (0.6–3), respectively. Median BED, calculated with the linear quadratic (LQC) model using a value of alpha/beta 12 Gy (Wiggenraad et al. 2011), was 47.2 Gy, with 68% of BMs that received a dose ≥ 40 Gy, which was considered an efficient dose. The volume and treatment characteristics are reported in Table 2.
Outcomes
Regarding the local control (LC) of brain lesions, at first FU (median time of 2 months; range 1–4), 244 out of 246 BMs (99%) were locally controlled (18% CR; 41% PR, 40% SD), and 2 BMs (1%) showed a PD. At the second FU, (median time of 4 months; range 3–7), the data were available for 101 BMs out 246 (41%): CR in 28%, PR 40%, SD 16% and PD 16% of cases. For only 9% of BMs the data of the third control performed at median time of 6 months (range 5–7) were available. In this last control, 43% and 39% of BMs showed a CR or SD, respectively; while 4 BMs were in PD. At the time of analysis, acute and late toxicities were mild: no adverse events of greater than grade 2 were reported, and no case of radionecrosis or cerebral hemorrhage was radiologically identified.
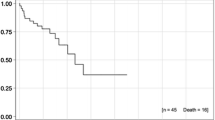
At a median follow-up time of 6 months (range 1–10), median OS has not yet been achieved, while median TTP was 5 months, as shown in Fig. 1. At the time of analysis, 27 out of 43 (63%) patients were alive and 16/43 (37%) died. A systemic progressive disease in association with an intracranial progression was observed in 10 out of 16 patients who had died (62%), while a sole systemic progression was the cause of death in 5 patients (31%), while only 1 patient died due to neurological progressive disease. An intracranial out-of-field progressive disease was observed in 23 of 43 patients (53%), including 8 patients that also developed an intracranial in-field progression. In 18 of these cases, a new RT treatment was performed: WBRT in 7 cases and HA SRS/SFRT in the other 11 cases. For 5 patients, no re-treatment was proposed due to the poor clinical conditions.
Predictive factors for Local Control
The analysis of LC profile stratified by histology, using Chi-square test, showed a correlation statistically significant with histological type (p = 0.0001): melanoma and others are predictive factors of “not response” vs lung and breast cancer that are predictive factors of “response” (Table 3). Considering the switch between “response” vs “not response” mostly related to histology of BMs as index of biological resistance, we have excluded the “not responder” from the analysis and PTV volume and BED variables were evaluated in the set of “responder” to identify possible discriminating factors in the treatment activity. The result of this analysis, showed that PTV volume ≤ 1 cc and BED ≥ 47.2 Gy were predictive factors statistically significant correlated with CR vs PR, using Chi-square test (p = 0.041 and p = 0.0001, respectively) (Table 4).
Prognostic factors for OS
The univariate and multivariate prognostic factors influencing OS are shown in Table 5, while the univariate analysis for TTP was performed without statistically significant results. In the survival analysis, the univariate and multivariate analysis about histology were performed in the entire group of patients taking into account lung and breast cancer group vs melanoma and others group. In univariate analysis, the histology of the primary tumor was significantly correlated with increased OS: lung and breast cancer had better survival rates as compared to melanoma and other histologies [hazard ratio (HR) 1.81, 95% CI 1.16–2.81, p = 0.009). Among subtypes of breast cancer, the triple-negative (TN) group was associated with worse prognosis compared to hormone receptors positive (HR+)/HER2− and HER2+ subtypes (HR 18.44, 95% CI 1.35–251.56, p = 0.02), in univariate analysis. Other factors associated with decreased OS were CITV ≥ 15 cc (HR 2.83, 95% CI 1.01–7.86, p = 0.04) and the presence of systemic progression disease (HR 18.92, 95% CI 2.48–144.07, p = 0.005). Intracranial progression, number of BMs and pre-brain treatment were not prognostic factor for survival. At multivariate analysis, the cumulative volume of intracranial disease ≥ 15 cc (HR 2.91, 95% CI 0.98–8.55, p = 0.04) and systemic progression disease (HR 16.01, 95% CI 2.02–126.84, p = 0.009) were independent factors for decreased OS. In Fig. 2, the main comparative OS curves are reported.
Discussion
The present study reports the first clinical experience using the new non-coplanar monoisocenter HyperArc™ technique for linac-based VMAT SRS/SFRT in multiple BMs, reporting safety and effectiveness in a population of patients affected by BMs. In case of multiple brain metastases, cranial stereotactic radiotherapy allowed to obtain similar survival rates comparing to whole brain irradiation in combination to focal treatments (Alongi et al. 2016). Recently, the NCCN guidelines recommend the possible appropriateness of focal therapy also in case of multiple brain metastases (5). It is recognized that whole brain irradiation is affected by neurological deterioration that could impact on patients’ quality of life (Giaj Levra et al. 2016). This last issue remains crucial in the era of new molecules (e.g. immunotherapy, target therapy) that are changing the natural history of intracranial disease in several clinical scenario. In a silico study by Fiorentino et al. (2018) the hippocampal dose during Linac-based stereotactic radiotherapy for brain metastases was negligible. Last but not least, the adoption of high-dose per fraction could overcome the possible intrinsic radioresistance of specific diseases. Looking at similar published papers, the well-known and much-discussed study by Yamamoto and colleagues (Yamamoto et al. 2014) investigated the benefits of SRS for patients with 1–10 BMs (largest tumor < 10 mL in volume and < 3 cm in longest diameter; total cumulative volume ≤ 15 mL). All patients were treated using a gamma-knife platform. BMs with a volume of less than 4 mL were irradiated with 22 Gy at the lesion periphery, whereas lesions with volumes of 4–10 mL with 20 Gy. In the present study, the prescribed total dose was quite lower than Yamamoto et al. (2014) (i.e. a range of dose between 15 Gy in 1 fraction to 30 Gy in 5 fractions). This aspect was explained by the heterogeneous sample size (more than 5 BMs, re-irradiation, palliative intent in some cases, etc.). These inclusion criteria were obviously less selective due to the intent of the present analysis that is to test the safety of HyperArc™ (Ruggieri et al. 2018).
Regarding acute and late toxicity, the present analysis did not show any moderate or severe side effects. However, considering the short FU, the data about acute toxicity can be considered conclusive, but the same evidence is not completely relevant for late toxicity, especially in terms of radionecrosis, and a longer FU remains mandatory. In fact, the incidence of radionecrosis has been reported around 5% at 6 months and 17% at 12 months (Kohutek et al. 2015; Minniti et al. 2011; Moraes et al. 2018).
Regarding the effectiveness, the response rate of BMs was analyzed in detail. The crude LC rate was 99%, with only 1% of treated BMs showing a progression, at the first control. These results are in line with previous published studies, both, for those with limited intracranial disease (NCCN Central Nervous System Cancers 2018; Scoccianti and Ricardi 2012; Aoyama et al. 2006; Kocher et al. 2011; Chang et al. 2009; Brown et al. 2016; Sahgal et al. 2015) and for studies with multiple BMs (Yamamoto et al. 2014; Sahgal et al. 2017). As described in a meta-analysis of Wiggenraad et al. a BED of ≥ 40 Gy is needed to achieve a tumor control probability of ≥ 70% at 1 year (Wiggenraad et al. 2011). In the present study, this type of correlation was found with a BED ≥ 47.2 Gy (p = 0.0001). Moreover, the present data showed that the probability of achieving a greater number of CR seems to be related to the PTV volume ≤ 1 cc (p = 0.041) and the primary tumor histology (p = 0.0001). These evidence suggest that in the case of BMs from primitive biologically not strongly aggressive and radiosensitive cancer and with PTV volume around 1 cc, the use of prescription dose with BED between 40 and 47.2 Gy could be appropriate to achieve excellent local disease response.
The analysis of prognostic factors for OS has shown that also the survival was strongly correlated with the tumor histology (lung/breast vs melanoma/other type) (p = 0.009). In metastatic melanoma, targeted agents such as BRAF/MEK kinase inhibitors (e.g. Dabrafenib and Trametinib), and immunotherapeutic agents (e.g. Nivolumab and Ipilimumab) have revolutionized the outcomes of these patients and prolonged their survival with a median OS ranging between 2 and 3 years (Weber et al. 2015; Long et al. 2017; Margolin et al. 2012). Considering that the main cause of failure in patients affected by metastatic melanoma with BMs remains neurological death, achieving intracranial disease control is of high importance to further improve OS. As suggested in the most recent NCCN guidelines (2018), SRS/SFRT is a treatment option also indicated in the case of multiple BMs. Furthermore, in the present analysis, the breast cancer subtypes (TN vs HER2+ and HR+/HER2−) is prognostic factor statistically significant for OS (p = 0.02). Thus, in TN patients, probably the role of brain radiotherapy remains important as a supportive therapy, more oriented towards WBRT due to the poor OS (Lin et al. 2008). In contrast, the 75% of patients with HER2+ metastatic disease were still alive at the time of our analysis. The HER2 overexpression associated with the young age, in which this disease occurs, are important predictive factors for BMs development (Kennecke et al. 2010). The success of the first-generation HER2− directed therapies (e.g. Trastuzumab) has brought to improve OS and systemic disease control changing the natural history of this breast cancer subtype. Nevertheless, approximately half of the patients with BMs die due to intracranial disease progression 24 months after BMs diagnosis (O’Sullivan et al. 2017). Probably, the intracranial disease progression is the result of the aggressive biological behavior and the genetic heterogeneity and actionable mutations in HER2+ breast cancer interposed between primary tumor and its BMs (De Mattos-Arruda et al. 2018), in a framework of selective resistance to the drug and/or the poor or non-penetration of the same, across the blood–brain barrier. New drug or old drug with new escape action mechanism would seem to achieve the control of brain micrometastatic or low-volume disease with a response rate of 65.9% and 1-year OS of 70% (Bachelot et al. 2013; Regina et al. 2008). Considering the long-life expectancy and young age of these patients, to avoid WBRT-related cognitive impairment and to improve intracranial disease control, SRS/SFRT also for multiple BMs (Yamamoto et al. 2014; Halasz et al. 2016) could be considered the successful treatment strategy.
A similar clinical argument can be made for the NSCLC oncogene-addicted metastatic disease. In these cases, with a median OS of 3 years, SRS/SFRT repeatable over time, also for extended intracranial disease, guarantees excellent local control emphasizing the importance of minimizing toxicities (Robin et al. 2018). Furthermore, a recent retrospective multi-institutional analysis showed that SRS followed by EGFR-tyrosine kinase inhibitors resulted in the longest OS (Magnuson et al. 2017). Therefore, for HER2+ breast cancer and oncogene-addicted NSCLC, the control of arising intracranial disease through SRS/SFRT (possibility repeatable over the time) could be a victorious choice, also for multiple BMs, leaving that new drugs have an effect similar to the old idea of the “WBRT”. In this direction, a prospective randomized trial is needed.
The weakness of our study is the retrospective nature, the limited sample size and a relatively short FU time; as such, inherent selection bias and high population heterogeneity might have skewed the results.
In summary, SRS-SFRT with HA seems a safe and effective treatment modality for multiple BMs and could serve as an alternative treatment option to WBRT in selected patients. Main advantages are the ability to improve the probability of local control, which in some cases could translate into an increased survival, or reduced neuro-cognitive impairment. The HyperArc technique is a fast and simple tool to plan and deliver these highly complicated and sophisticated local-ablative brain treatments. In the future, a better definition of intracranial disease burden, understood as magnitude of volume and not as a plain number of BMs is necessary to facilitate the decision-making process by taking into account tumor biology, oncogenetic mutations and patient history.
References
Alongi F, Fiorentino A, Mancosu P et al (2016) Stereotactic radiosurgery for intracranial metastases: linac-based and gamma-dedicated unit approach. Expert Rev Anticancer Ther 16(7):731–740
Aoyama H, Shirato H, Tago M et al (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491
Bachelot T, Romieu G, Campone M et al (2013) Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 14(1):64–71
Brown PD, Jaeckle K, Ballman KV et al (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 316(4):401–409
Chang EL, Wefel JS, Hess KR et al (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037–1044
Davis FG, Dolecek TA, McCarthy BJ et al (2012) Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol 14:1171–1177
De Mattos-Arruda L, Nag C, Piscuoglio S et al (2018) Genetic heterogeneity and actionable mutations in HER2-positive primary breast cancers and their brain metastases. Oncotarget 9(29):20617–20630
Fiorentino A, Tebano U, Sicignano G et al (2018) Hippocampal dose during Linac-based stereotactic radiotherapy for brain metastases: an observational study. Phys Med 49:135–138
Giaj Levra N, Sicignano G, Fiorentino A et al (2016) Whole brain radiotherapy with hippocampal avoidance and simultaneous integrated boost for brain metastases: a dosimetric volumetric-modulated arc therapy study. Radiol Med 121(1):60–69
Halasz LM, Uno H, Hughes M et al (2016) Comparative effectiveness of stereotactic radiosurgery versus whole-brain radiation therapy for patients with brain metastases from breast or nonsmall cell lung cancer. Cancer 122(13):2091–2100
Kennecke H, Yerushalmi R, Woods R et al (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28(20):3271–3277
Kocher M, Soffietti R, Abacioglu U et al (2011) Adjuvant whole brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol 29:134–141
Kocher M, Wittig A, Piroth MD et al (2014) Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol 190(6):521–532
Kohutek ZA, Yamada Y, Chan TA et al (2015) Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol 125(1):149–156
Lin NU, Claus E, Sohl J et al (2008) Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 113(10):2638–2645
Long GV, Flaherty KT, Stroyakovskiy D et al (2017) Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol 28(7):1631–1639
Magnuson WJ, Lester-Coll NH, Wu AJ et al (2017) Management of brain metastases in tyrosine kinase inhibitor-naive epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol 35(10):1070–1077
Margolin K, Ernstoff MS, Hamid O et al (2012) Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 13(5):459–465
Minniti G, Clarke E, Lanzetta G et al (2011) Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 6:48
Moraes FY, Winter J, Atenafu EG et al (2018) Outcomes following SRS for small- to medium-sized brain metastases are exceptionally dependent upon tumor size and prescribed dose. Neuro Oncol. https://doi.org/10.1093/neuonc/noy159
Muller-Riemenschneider F, Bockelbrink A, Ernst I et al (2009) Stereotactic radiosurgery for the treatment of brain metastases. Radiother Oncol 91:67–74
Nayak L, Lee EQ, Wen PY et al (2012) Epidemiology of brain metastases. Curr Oncol Rep 14(1):48–54
NCCN Central Nervous System Cancers (2018) Version 1.2018 guidelines. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed Sept 2018
O’Sullivan CC, Davarpanah N, Abraham J, Bates SE (2017) Current challenges in the management of breast cancer brain metastases. Semin Oncol 44:85–100
Regina A, Demeule M, Che C et al (2008) Anti tumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br J Pharmacol 155(2):185–197
Robin TP, Camidge DR, Stuhr K et al (2018) Excellent outcomes with radiosurgery for multiple brain metastases in ALK and EGFR driven non-small cell lung cancer. J Thorac Oncol 13(5):715–720
Ruggieri R, Naccarato S, Mazzola R et al (2018) Linac-based VMAT radiosurgery for multiple brain lesions: comparison between a conventional multi-isocenter approach and a new dedicated mono-isocenter technique. Radiat Oncol 13(1):38
Sahgal A, Aoyama H, Kocher M et al (2015) Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol 91(4):710–717
Sahgal A, Ruschin M, Ma L et al (2017) Stereotactic radiosurgery alone for multiple brain metastases? A review of clinical and technical issues. Neuro Oncol 19(suppl_2):ii2–ii15
Schwartz LH, Seymour L, Litière S et al (2016) RECIST 1.1—standardisation and disease-specific adaptations: perspectives from the RECIST working group. Eur J Cancer 62:138–145
Scoccianti S, Ricardi U (2012) Treatment of brain metastases: review of phase III randomized controlled trials. Radiother Oncol 102:168–179
Sperduto PW, Kased N, Roberge D et al (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30(4):419–425
Weber JS, D’Angelo SP, Minor D et al (2015) Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16:375–384
Wiggenraad R, Verbeek-de Kanter A, Kal HB, et (2011) Dose–effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother Oncol 98:292–297
Yamamoto M, Serizawa T, Shuto T et al (2014) Results of stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective study. Lancet Oncol 15:387–395
Funding
No funding or financial interests for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
For this type of study formal consent is not required. However, all procedures were in accordance with the ethical standards and the Helsinki declaration.
Informed consent
A specific informed consent was obtained from all individual participants included in the study.
Conflict of interest
FA acts as Varian Consultant and he had Speacker Onoraria. Remaining authors (AF, FG, SC, NGL, LR, MR, FR, AB, GL, RM, RR) declare no conflict of interests.
Rights and permissions
About this article
Cite this article
Alongi, F., Fiorentino, A., Gregucci, F. et al. First experience and clinical results using a new non-coplanar mono-isocenter technique (HyperArc™) for Linac-based VMAT radiosurgery in brain metastases. J Cancer Res Clin Oncol 145, 193–200 (2019). https://doi.org/10.1007/s00432-018-2781-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2781-7