Abstract
Introduction
Tumor recurrence is the most frequent cause of death after liver transplantation for hepatocellular carcinoma. We selected ten other prognostic classifications to evaluate their potential to predict the risk of recurrence after LT for HCC as compared to the Milan classification. All of the other scores have not been compared with one another in a single cohort.
Methods
Data of 147 consecutive patients transplanted at our department between 1996 and 2014 were analyzed and staged for morphological and functional scores of underlying liver disease. For long-term follow-up, we analyzed intrahepatic (within the liver ± distant metastases) and extrahepatic (distant metastases only) recurrence separately.
Results and conclusions
The median survival time for all patients was 106 months. The 5- and 10-year observed survival rates were 61 and 43%, respectively. The observed cumulative 5- and 10-year recurrence rates were 37 and 39%, respectively, 10-year intrahepatic and extrahepatic recurrence rates were 12 and 27%, respectively. Median survival time after diagnosis of first recurrence was 7.5 (0–120) months; 2 and 18 months for all, intra- and extrahepatic recurrence, respectively. UCSF-, up to seven-, Shanghai Fudan- or Duvoux classifications can identify patients with a cumulative 10-year recurrence rate below 20%. The pre-therapeutic AFP level should be considered in addition to the geometry of the intrahepatic lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and background
Tumor recurrence is the leading cause of death after liver transplantation (LT) in patients with hepatocellular carcinoma (HCC) (Andreou et al. 2015). Early intrahepatic recurrence with poor prognosis can be distinguished from extrahepatic recurrence which develops usually post transplant and has almost always a better prognosis than intrahepatic recurrence (Roayaie et al. 2004). The risk factors and their identification before listing patients for transplantation as well as treatment of recurrence after transplantation are currently debated (Ravaioli et al. 2008; Schwartz et al. 2005; Andreou et al. 2016; Mazzaferro et al. 2009).
After the introduction of the Milan classification in 1996 (Mazzaferro et al. 1996) a group of patients with a long-term survival (and also tumor free) was identified, such as patients with benign diseases as indications for LT. It was also observed that certain patients “outside Milan” survived several years post transplant without recurrence (Duvoux et al. 2012; Chapman et al. 2008; Millonig et al. 2007; Otto et al. 2006; Herrero et al. 2008; Seehofer et al. 2012). There is currently a debate as to whether this scoring system is still appropriate for clinical decision making (Duvoux et al. 2012; Chapman et al. 2008; Millonig et al. 2007; Otto et al. 2006; Herrero et al. 2008; Seehofer et al. 2012).
We selected ten different prognostic classifications/scores to evaluate their potential to predict the risk of recurrence after LT for HCC as compared with the Milan classification (Table 1). All of the other HCC scores together have not been compared with one another in a monocenter cohort of LT patients. We also present the treatment strategies and survival in our patients with HCC recurrence.
Materials and methods
We extracted data of HCC patients who underwent liver transplantation between 1996 and 2014 from our prospectively maintained tumor register. 20 patients who died within 3 months after LT were excluded. All patients were followed up until death, or until July 1st 2016. Eighty percent of patients were followed up by our department. The remaining data were forwarded by other health care providers.
According to the German transplantation law, extrahepatic tumor and macrovascular invasion are considered contraindications of liver transplantation.
In cases of sufficient liver function, bridging procedures, such as liver resection, local ablative procedures (transarterial chemo embolization (TACE), radio frequency ablation (RFA), Yttrium90 radio embolization (Y90RE), tomotherapy), accompanied by systemic treatment with tyrosine kinase inhibitors were applied since 2004. All these measures were continued for as long as residual tumor was identified by imaging in 90-days intervals.
Patients with recurrence after LT were treated with curative intent wherever possible, otherwise with palliative intent. We performed surgical resections with curative intent for intra- and extrahepatic recurrence, applied local therapy (TACE, Y90RE, RFA) in non-resectable intrahepatic recurrences and radiation for bone metastases. Whenever possible, a systemic therapy with a tyrosine kinase inhibitor followed. mTOR-based long-term immunosuppression and sorafenib were administered in patients who had no contraindications.
Follow-up consultations were standardized (monthly during the first year, subsequently four times per year). As long as laboratory tests including AFP were within the normal range, a CT scan was performed every 3 months. If tumor recurrence was suspected or confirmed, therapeutic options were discussed on the interdisciplinary hepatobiliary tumor board.
We analyzed the morphological data of the tumor load in pre-transplant contrast computed tomography (CT) or magnetic resonance imaging (MRI) scans, α-Fetoprotein (AFP) (ng/ml) level, stage of underlying liver disease (Child Stage) (Child and Turcotte 1964), use of locoregional therapy, and type of LT. Patients were classified according to 11 different scores, 8 of which designed to identify patients for LT, Milan classification (Mazzaferro et al. 1996), University of California, San Francisco (UCSF) classification (Yao et al. 2001), the Okuda classification (Okuda et al. 1985), Shanghai Fudan criteria (Fan et al. 2006), Hangzhou criteria (Zheng et al. 2008), Asan criteria (Lee et al. 2008), up-to-seven criteria (Mazzaferro et al. 2009), Duvoux Score (Duvoux et al. 2012), and three established HCC prognostic scores: Barcelona Clinic Liver Cancer (BCLC) Score (Llovet et al. 1999), Cancer of the Liver Italian Program (CLIP) Score (Capuano et al. 1998) and Union for International Cancer Control (UICC) stage 2010 (Sobin et al. 2010). All tumors have been re-classified according to the 7th edition tumor-node-metastases (TNM) staging of 2010 (Sobin et al. 2010).
The univariate analysis was performed with SPSS software version 19. Differences in the distribution of variables have been tested with Fisher’s exact test or with χ 2-test for statistically significant differences. Survival rates were calculated with the Kaplan–Meier procedure and significance testing was performed with the log-rank test. Before analysis, the original 3-tier or 4-tier scales of the above-mentioned scores were reduced to a 2-tier system (low risk or high risk of HCC recurrence). The cumulative recurrence rates for HCC were calculated with adjustment for death before HCC recurrence as a competing risk. The competing risk analyses were performed with SPSS using a specially designated macro to calculate the cumulative incidence functions. This macro is freely available at the website http://www.msbi.nl/lecessie (Verduijn et al. 2011).
Results
147 patients underwent LT for HCC between 1996 and 2014. Three patients were diagnosed with incidental carcinoma which had not been detected during the evaluation procedure. In two patients, one positive lymph node metastasis was detected in the resected specimen by the pathologist. There were 120 men and 27 women with a median age of 59 years (35–71 years). The median AFP level before transplantation was 13(1–56,139) ng/ml. The median waiting time was 136 (0–2505) days for all patients. The median waiting time was 170 (0–2505) days for deceased donor transplant and 36 (2–388) days for living donor LT, respectively.
The median follow-up time after LT was 48 (3–238) months for the entire group and 72 (10–238) months for the patients still alive. The cumulative 10-year death rates for death of recurrence, death of other malignancies (three lung cancers, one cancer of the urinary bladder), and death of other causes were 29, 6, and 22%, respectively (Fig. 1).
The median survival time for all patients was 106 months. The 5- and 10-year observed survival rates were 61 and 43%, respectively. Patients treated before 2004 had nearly identical survival rates as compared to patients who underwent transplantation 2004 and later (p = 0.869).
Recurrence rates
At the end of the study, 48 patients had developed a recurrence. The median time from transplantation to recurrence was 12.5 (3–62) months. Extrahepatic recurrence was observed in 32 cases and intrahepatic recurrence with or without distant metastases in 16 cases. The median time from transplantation to recurrence was 12.5 (3–62) months for all, 9.5 (3–32) months for intrahepatic recurrence only, and 18 (3–62) months for extrahepatic and combined recurrence. The difference is of marginal statistical significance (p = 0.05; Mann–Whitney U test). In the univariate analysis, the time to recurrence was related to number of tumors, Milan criteria, CLIP Score, Okuda stage, and UCSF classification, but only the difference between CLIP 0-1 and CLIP > 1 reached statistical significance (p = 0.014).
We saw no statistically significant differences in frequencies of recurrence for sex, locoregional therapy, type of LT (LDLT vs DDLT), diameter of lesions (maximum), alpha-fetoprotein level, portal vein thrombosis without tumor invasion in imaging studies pre LT without tumor invasion, child stage, underlying liver disease, and BCLC Score. Details and p values are shown in Table 1.
Prognostic scores
The observed cumulative 5- and 10-year recurrence rates for all patients were 37 and 39%, respectively. The 10-year cumulative recurrence rate corrected for competing risk of death from other causes was 34%.
We analyzed the cumulative 10-year recurrence rate as related to Milan classification, UCSF classification, Up to seven criteria, Shanghai Fudan criteria, Asan criteria, Hangzhou criteria, CLIP Score, Okuda stage, Duvoux Score, UICC stage, and BCLC stage. The first four classifications consider only number and diameter of lesions, the others include gross vascular invasion (Asan criteria), grading (Hangzhou criteria), Underlying Liver Disease Score, laboratory findings (Okuda stage), pre-therapeutic AFP level (Duvoux Score), microvascular invasion (UICC stage), or performance status (BCLC stage). The percentage of patients assigned to the respective low risk groups ranged from 38% (Milan classification) to 91% (Okuda classification). Ten-year cumulative recurrence rates were strongly related to the subgroup under consideration, ranging from 14% in the low risk group of the Duvoux Score to 75% in Okuda A stage III. Only the BCLC Score did not reflect recurrence rates statistically significant. Recurrence rates below 20% in the low risk group were observed only with the up to seven criteria, Shanghai Fudan criteria, Hangzhou criteria, and Duvoux Score. For all groups, the 10-year cumulative recurrence rate corrected for competing risk of death from other causes was slightly lower than the uncorrected rate (Table 2). Two examples for the effect of the different classifications on the 10-year cumulative recurrence rate corrected for competing risk of death from other causes is shown in Fig. 2.
All scores besides BCLC reflected statistically significant differences in intrahepatic recurrence rates. The number of patients with recurrence assigned to the low risk group ranged from 9/48 (19%, Milan classification) to 33/48 (69%, Hangzhou criteria). Except for the Duvoux Score, scores that combined number and diameter of lesions with other criteria tended to underestimate the risk of intrahepatic recurrence.
Extrahepatic recurrences were not statistically significantly reflected by the Milan classification, Hangzhou criteria, and BCLC Score. Considering the results for total, intrahepatic, and extrahepatic recurrence rates, the Duvoux Score provides the most accurate prediction. The application of the Duvoux Score identified only 9/48 recurrences in the pre-operative low risk group, three of those were intrahepatic and six were extrahepatic (Table 3).
Treatment of recurrences
Fifteen of 48 recurrences (31%) were treated with curative intent. Of 16 patients with intrahepatic recurrence, 14 had diffuse intrahepatic disease which was accompanied by pulmonary or skeletal metastases in nine cases. These patients were given best supportive care only in six cases and systemic therapy in the remaining eight cases. Two patients underwent treatment with curative intent, one partial liver resection followed by TACE, the other radiofrequency ablation followed by RE and percutaneous radiation therapy. One patient died 6 months after the diagnosis of recurrence; the second is still alive with disease 32 months after the diagnosis of recurrence.
Two patients with pulmonary metastases received systemic treatment with a tyrosine kinase inhibitor, five best supportive care only. Seven patients with bone metastases were treated with radiation, one patient received systemic treatment with a tyrosine kinase inhibitor, one best supportive care. In 13 patients the initial extrahepatic recurrence was resected with curative intent (Table 4).
Survival after recurrence
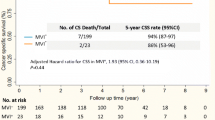
The median survival time after the diagnosis of first recurrence was 7.5- (0–120) month, 2 and 18 months for all, intra- and extrahepatic recurrence, respectively. As yet, none of the patients with intrahepatic recurrence survived 10 years compared with two patients who had developed extrahepatic recurrence. The 10 years survivors are one with eight bilateral lung metastases which were resected 33 months after transplantation and one with metastasis to the adrenal gland which was R0-resected 55 months after transplantation (Fig. 3). The median survival time for patients treated with curative intent was 38 months, compared to 6 months for patients treated with palliative intent. The corresponding 5-year survival rates are 31 and 0%, respectively (Fig. 4).
Discussion
Recurrence is the leading cause of death after curatively intended treatment for patients with solid tumors. We addressed the question as to whether different scoring systems may adequately predict the outcome of LT for HCC. We evaluated the ability of different classifications to predict the risk of recurrence. We choose 10-years recurrence rate to detect late recurrence. Moreover, we checked the ability of the different classifications to predict intra- and extrahepatic recurrences. For patients with intra- or extrahepatic recurrences, we evaluated different therapeutic procedures. We saw the best results after surgical treatment with curative intent and here especially for extrahepatic recurrence.
To our best knowledge, we are the first to investigate the power of predicting the risk of recurrence in a monocenter German study for a variety of classifications. We compared Milan classification, CLIP Score, BCLC Score, UCSF, Shanghai Fudan criteria, Hangzhou criteria, Asan criteria, up-to-seven criteria, UICC stage 2010, and Duvoux Score with respect to their ability to predict cumulative recurrence.
In agreement with other reports, we found different survival with intra- and extrahepatic recurrences and we believe that they are caused by different biological mechanisms. We would, therefore, recommend that they should be analyzed individually.
The Milan classification and the Duvoux Score showed the lowest number of false-positive predictions in the respective low risk groups for all recurrences. All classifications considering only number and diameter of intrahepatic lesions, CLIP, and Duvoux Score were able to predict intrahepatic recurrences adequately. On the other hand, all but the Milan classification and Duvoux Score had shortcomings in predicting extrahepatic recurrences. Thus, we assume that the pre-therapeutic AFP level can provide some information about the risk of extrahepatic recurrence after LT. Other authors found that elevated AFP prior to LT is associated with higher recurrence rates (Ravaioli et al. 2008; Hameed et al. 2014; Vibert et al. 2010; Toso et al. 2008).
In our study, one-third of the first recurrences occurred in the liver only. Exactly the same percentage is reported by de’Angelis et al. (2015). Other authors found percentages of intrahepatic recurrences between 14 and 53% (Sotiropoulos et al. 2007; Croome et al. 2015).
There are numerous studies in the literature investigating the prediction of the frequency of recurrence after transplantation for HCC (Table 5). But only five of them (Andreou et al. 2015; Sotiropoulos et al. 2007; Croome et al. 2015; Agopian et al. 2015; Sapisochin et al. 2016) give cumulative predictions of the 5 year recurrence rate and only two (Croome et al. 2015; Agopian et al. 2015) consider the competing risk of death from other causes before the diagnosis of recurrence can be made. Moreover, in some of the studies, patients dying during the first 2 months after transplantation (before recurrence can occur) are included or—more frequently—no information whether these patients are excluded is given. Therefore, not unexpectedly, reported recurrence rates vary from 7.3 to 37.8% (Chaiteerakij et al. 2015; Varona et al. 2015).
In our series, the median time from transplantation to recurrence was 12.5 months. This is in agreement with a recently published review where de’ Angelis and colleagues found a median time from LT to HCC recurrence of 13 months (range 2–132 months) (de’Angelis et al. 2015). Some authors distinguish between early recurrence (<2 years after transplantation) and late recurrence (≥2 years after transplantation) (Chok et al. 2011; Pecchi et al. 2015). Others define late recurrence >12 months after transplantation (Escartin et al. 2007; Zhang et al. 2015) or >1000 days after transplantation (Schlitt et al. 1999). In addition, very late recurrences up to 10 years after LT are reported (Decaens et al. 2006).
The median survival time after diagnosis of first recurrence was 7.5 (0–120) months, 2 and 18 months for all, intra- and extrahepatic recurrence, respectively. Bodzin et al. (2016) and Na et al. (2016) report similar findings for recurrences after DDLT and LDLT (10.6 and 6.6 months, respectively). de’Angeilis et al. (2015) found in a multicenter study a median survival of 13 months after the diagnosis of recurrence.
According to the consensus conference, surgical treatment with curative intent should be considered in all cases of recurrence (Clavien et al. 2012). Particularly patients with single intra- or extrahepatic deposit benefit from surgical treatment (Na et al. 2016; Bodzin et al. 2016; Kornberg et al. 2010; Marangoni et al. 2008; Sapisochin et al. 2015; Taketomi et al. 2010; Valdivieso et al. 2010; Hwang et al. 2012).
In summary, tumor recurrence is the leading cause of death for transplanted patients with HCC. With the aim to offer more patients the curative option of LT, UCSF-, up to seven-, Shanghai Fudan- or Duvoux classifications can be applied. All of them can identify a group of patients with a cumulative 10-year recurrence rate below 20%. Most scores are able to estimate the risk of intrahepatic recurrence. In our patients—besides the Milan classification—only the Duvoux classification was able to identify patients with a low risk of extrahepatic recurrence. Therefore, we believe that the pre-therapeutic AFP level should be considered in addition to the geometry of the intrahepatic lesions. Surgical treatment of recurrent tumor can offer 10-year tumor-free survival to patients with resectable recurrence in individual cases. In non-resectable cases, local ablative procedures with or without mTOR-based immunosuppression and systemic therapy with a tyrosine kinase inhibitor may prolong survival.
References
Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, Finn RS, Tong M, Hiatt JR, Busuttil RW (2015) A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg 220(4):416–427. doi:10.1016/j.jamcollsurg.2014.12.025
Andreou A, Gul S, Pascher A, Schoning W, Al-Abadi H, Bahra M, Klein F, Denecke T, Strucker B, Puhl G, Pratschke J, Seehofer D (2015) Patient and tumour biology predict survival beyond the Milan criteria in liver transplantation for hepatocellular carcinoma. HPB 17(2):168–175. doi:10.1111/hpb.12345
Andreou A, Bahra M, Schmelzle M, Sucher R, Sauer IM, Guel-Klein S, Struecker B, Eurich D, Klein F, Pascher A, Pratschke J, Seehofer D (2016) Predictive factors for extrahepatic recurrence of hepatocellular carcinoma following liver transplantation. Clin Transplant. doi:10.1111/ctr.12755
Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG (2016) Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation: impact of treatment modality and recurrence characteristics. Ann Surg. doi:10.1097/SLA.0000000000001894
Capuano G, Daniele B, Gaeta GB, Gallo C, Perrone F (1998) A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 28(3):751–755. doi:10.1002/hep.510280322
Chaiteerakij R, Zhang X, Addissie BD, Mohamed EA, Harmsen WS, Theobald PJ, Peters BE, Balsanek JG, Ward MM, Giama NH, Moser CD, Oseini AM, Umeda N, Venkatesh S, Harnois DM, Charlton MR, Yamada H, Satomura S, Algeciras-Schimnich A, Snyder MR, Therneau TM, Roberts LR (2015) Combinations of biomarkers and Milan criteria for predicting hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl 21(5):599–606. doi:10.1002/lt.24117
Chan KM, Yu MC, Chou HS, Wu TJ, Lee CF, Lee WC (2011) Significance of tumor necrosis for outcome of patients withhepatocellular carcinoma receiving locoregional therapy prior to liver transplantation. Ann Surg Oncol 18(9):2638–2646. doi:10.1245/s10434-011-1779-z
Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, Lowell JA, Shenoy S, Darcy MD, Brown DB (2008) Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg 248(4):617–625. doi:10.1097/SLA.0b013e31818a07d4
Child CG, Turcotte JG (1964) Surgery and portal hypertension. Major Probl Clin Surg 1:1–85
Chok KS, Chan SC, Cheung TT, Chan AC, Fan ST, Lo CM (2011) Late recurrence of hepatocellular carcinoma after liver transplantation. World J Surg 35(9):2058–2062. doi:10.1007/s00268-011-1146-z
Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A (2012) Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 13(1):e11–e22. doi:10.1016/S1470-2045(11)70175-9
Croome KP, Lee DD, Burns JM, Musto K, Paz D, Nguyen JH, Perry DK, Harnois DM, Taner CB (2015) The use of donation after cardiac death allografts does not increase recurrence of hepatocellular carcinoma. Am J Transplant 15(10):2704–2711. doi:10.1111/ajt.13306
de’Angelis N, Landi F, Carra MC, Azoulay D (2015) Managements of recurrent hepatocellular carcinoma after liver transplantation: a systematic review. World J Gastroenterol 21(39):11185–11198. doi:10.3748/wjg.v21.i39.11185
Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F, Bernard PH, Boillot O, Compagnon P, Calmus Y, Hardwigsen J, Ducerf C, Pageaux GP, Dharancy S, Chazouilleres O, Cherqui D, Duvoux C (2006) Role of immunosuppression and tumor differentiation in predicting recurrence after liver transplantation for hepatocellular carcinoma: a multicenter study of 412 patients. World J Gastroenterol 12(45):7319–7325
Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J, Dharancy S, Gugenheim J, Bernard PH, Adam R, Radenne S, Muscari F, Conti F, Hardwigsen J, Pageaux GP, Chazouilleres O, Salame E, Hilleret MN, Lebray P, Abergel A, Debette-Gratien M, Kluger MD, Mallat A, Azoulay D, Cherqui D (2012) Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 143(4):986–994. doi:10.1053/j.gastro.2012.05.052 (quiz e914-985)
Escartin A, Sapisochin G, Bilbao I, Vilallonga R, Bueno J, Castells L, Dopazo C, Castro E, Caralt M, Balsells J (2007) Recurrence of hepatocellular carcinoma after liver transplantation. Transpl Proc 39(7):2308–2310. doi:10.1016/j.transproceed.2007.06.042
Fan J, Zhou J, Xu Y, Qiu SJ, Wu ZQ, Yu Y, Huang XW, Tang ZY, Wang YQ (2006) Indication of liver transplantation for hepatocellular carcinoma: Shanghai Fudan Criteria. Zhonghua yi Xue Za Zhi 86(18):1227–1231
Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY (2014) Alpha-fetoprotein level >1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 20(8):945–951. doi:10.1002/lt.23904
Herrero JI, Sangro B, Pardo F, Quiroga J, Inarrairaegui M, Rotellar F, Montiel C, Alegre F, Prieto J (2008) Liver transplantation in patients with hepatocellular carcinoma across Milan criteria. Liver Transpl 14(3):272–278. doi:10.1002/lt.21368
Hwang S, Kim YH, Kim DK, Ahn CS, Moon DB, Kim KH, Ha TY, Song GW, Jung DH, Kim HR, Park GC, Namgoong JM, Yoon SY, Jung SW, Park SI, Lee SG (2012) Resection of pulmonary metastases from hepatocellular carcinoma following liver transplantation. World J Surg 36(7):1592–1602. doi:10.1007/s00268-012-1533-0
Kornberg A, Kupper B, Tannapfel A, Katenkamp K, Thrum K, Habrecht O, Wilberg J (2010) Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: clinical patterns and outcome variables. Eur J Surg Oncol 36(3):275–280. doi:10.1016/j.ejso.2009.10.001
Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, Ko GY, Park KM, Ha TY, Song GW (2008) Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl 14(7):935–945. doi:10.1002/lt.21445
Llovet JM, Bru C, Bruix J (1999) Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 19(3):329–338. doi:10.1055/s-2007-1007122
Marangoni G, Faraj W, Sethi H, Rela M, Muiesan P, Heaton N (2008) Liver resection in liver transplant recipients. Hepatobiliary Pancreat Dis Int HBPD INT 7(6):590–594
Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334(11):693–699. doi:10.1056/NEJM199603143341104
Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P (2009) Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 10(1):35–43. doi:10.1016/S1470-2045(08)70284-5
Millonig G, Graziadei IW, Freund MC, Jaschke W, Stadlmann S, Ladurner R, Margreiter R, Vogel W (2007) Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl 13(2):272–279. doi:10.1002/lt.21033
Na GH, Hong TH, You YK, Kim DG (2016) Clinical analysis of patients with hepatocellular carcinoma recurrence after living-donor liver transplantation. World J Gastroenterol 22(25):5790–5799. doi:10.3748/wjg.v22.i25.5790
Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K (1985) Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 56(4):918–928
Otto G, Herber S, Heise M, Lohse AW, Monch C, Bittinger F, Hoppe-Lotichius M, Schuchmann M, Victor A, Pitton M (2006) Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl 12(8):1260–1267. doi:10.1002/lt.20837
Parfitt JR, Marotta P, Alghamdi M, Wall W, Khakhar A, Suskin NG, Quan D, McAllister V, Ghent C, Levstik M, McLean C, Chakrabarti S, Garcia B, Driman DK (2007) Recurrent hepatocellular carcinoma after transplantation: use of apathological score on explanted livers to predict recurrence. Liver Transpl 13(4):543–551. doi:10.1002/lt.21078
Pecchi A, Besutti G, De Santis M, Del Giovane C, Nosseir S, Tarantino G, Di Benedetto F, Torricelli P (2015) Post-transplantation hepatocellular carcinoma recurrence: patterns and relation between vascularity and differentiation degree. World J Hepatol 7(2):276–284. doi:10.4254/wjh.v7.i2.276
Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, Vivarelli M, Golfieri R, D’Errico Grigioni A, Panzini I, Morelli C, Bernardi M, Bolondi L, Pinna AD (2008) Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant 8(12):2547–2557. doi:10.1111/j.1600-6143.2008.02409.x
Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME (2004) Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl 10(4):534–540. doi:10.1002/lt.20128
Sapisochin G, Goldaracena N, Astete S, Laurence JM, Davidson D, Rafael E, Castells L, Sandroussi C, Bilbao I, Dopazo C, Grant DR, Lazaro JL, Caralt M, Ghanekar A, McGilvray ID, Lilly L, Cattral MS, Selzner M, Charco R, Greig PD (2015) Benefit of treating hepatocellular carcinoma recurrence after liver transplantation and analysis of prognostic factors for survival in a large Euro-American series. Ann Surg Oncol 22(7):2286–2294. doi:10.1245/s10434-014-4273-6
Sapisochin G, Goldaracena N, Laurence JM, Dib M, Barbas A, Ghanekar A, Cleary SP, Lilly L, Cattral MS, Marquez M, Selzner M, Renner E, Selzner N, McGilvray ID, Greig PD, Grant DR (2016) The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: a prospective validation study. Hepatology. doi:10.1002/hep.28643
Schlitt HJ, Neipp M, Weimann A, Oldhafer KJ, Schmoll E, Boeker K, Nashan B, Kubicka S, Maschek H, Tusch G, Raab R, Ringe B, Manns MP, Pichlmayr R (1999) Recurrence patterns of hepatocellular and fibrolamellar carcinoma after liver transplantation. J Clin Oncol 17(1):324–331
Schraiber LS, de Mattos AA, Zanotelli ML, Cantisani GP, Brandao AB, Marroni CA, Kiss G, Ernani L, Marcon Pdos S (2016) Alpha-fetoprotein Level predicts recurrence after transplantation in hepatocellular carcinoma. Medicine 95(3):e2478. doi:10.1097/MD.0000000000002478
Schwartz M, Roayaie S, Llovet J (2005) How should patients with hepatocellular carcinoma recurrence after liver transplantation be treated? J Hepatol 43(4):584–589. doi:10.1016/j.jhep.2005.07.019
Seehofer D, Nebrig M, Denecke T, Kroencke T, Weichert W, Stockmann M, Somasundaram R, Schott E, Puhl G, Neuhaus P (2012) Impact of neoadjuvant transarterial chemoembolization on tumor recurrence and patient survival after liver transplantation for hepatocellular carcinoma: a retrospective analysis. Clin Transplant 26(5):764–774. doi:10.1111/j.1399-0012.2012.01609.x
Sobin LH, Gospodarowicz MK, Wittekind C, International Union against Cancer (2010) TNM classification of malignant tumours, 7th edn. Wiley, Chichester, Hoboken
Sotiropoulos GC, Lang H, Nadalin S, Neuhauser M, Molmenti EP, Baba HA, Paul A, Saner FH, Weber F, Hilgard P, Frilling A, Broelsch CE, Malago M (2007) Liver transplantation for hepatocellular carcinoma: University Hospital Essen experience and metaanalysis of prognostic factors. J Am Coll Surg 205(5):661–675. doi:10.1016/j.jamcollsurg.2007.05.023
Taketomi A, Fukuhara T, Morita K, Kayashima H, Ninomiya M, Yamashita Y, Ikegami T, Uchiyama H, Yoshizumi T, Soejima Y, Shirabe K, Maehara Y (2010) Improved results of a surgical resection for the recurrence of hepatocellular carcinoma after living donor liver transplantation. Ann Surg Oncol 17(9):2283–2289. doi:10.1245/s10434-010-0999-y
Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, Grant DR, Greig PD, Shapiro AM, Kneteman NM (2008) Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl 14(8):1107–1115. doi:10.1002/lt.21484
Valdivieso A, Bustamante J, Gastaca M, Uriarte JG, Ventoso A, Ruiz P, Fernandez JR, Pijoan I, Testillano M, Suarez MJ, Montejo M, Ortiz de Urbina J (2010) Management of hepatocellular carcinoma recurrence after liver transplantation. Transpl Proc 42(2):660–662. doi:10.1016/j.transproceed.2010.02.014
Varona MA, Soriano A, Aguirre-Jaime A, Garrido S, Oton E, Diaz D, Portero J, Bravo P, Barrera MA, Perera A (2015) Risk factors of hepatocellular carcinoma recurrence after liver transplantation: accuracy of the alpha-fetoprotein model in a single-center experience. Transpl Proc 47(1):84–89. doi:10.1016/j.transproceed.2014.12.013
Verduijn M, Grootendorst DC, Dekker FW, Jager KJ, le Cessie S (2011) The analysis of competing events like cause-specific mortality–beware of the Kaplan–Meier method. Nephrol Dial Transplant 26(1):56–61. doi:10.1093/ndt/gfq661
Vibert E, Azoulay D, Hoti E, Iacopinelli S, Samuel D, Salloum C, Lemoine A, Bismuth H, Castaing D, Adam R (2010) Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant 10(1):129–137. doi:10.1111/J.1600-6143.2009.02750.X
Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP (2001) Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 33(6):1394–1403. doi:10.1053/jhep.2001.24563
Zhang X, Li C, Wen T, Yan L, Li B, Yang J, Wang W, Xu M, Lu W, Jiang L (2015) Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: according to the recurrence pattern. Eur J Gastroenterol Hepatol 27(8):933–940. doi:10.1097/MEG.0000000000000383
Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM (2008) Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 85(12):1726–1732. doi:10.1097/TP.0b013e31816b67e4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical standard
The retrospective study was in accordance with the ethical standards of the Helsinki Declaration.
Informed consent
All patients give their consent for registration in the tumor registry. We have only used data from the clinical data registry.
Rights and permissions
About this article
Cite this article
Bauschke, A., Altendorf-Hofmann, A., Kissler, H. et al. Validity of eleven prognostic scores with respect to intra- and extrahepatic recurrence of hepatocellular carcinoma after liver transplantation. J Cancer Res Clin Oncol 143, 2595–2605 (2017). https://doi.org/10.1007/s00432-017-2507-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-017-2507-2