Abstract
Background
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a novel approach delivering intraperitoneal chemotherapy by means of a pressurized aerosol. This study was conducted to evaluate the distribution pattern of doxorubicin in the abdominal cavity after PIPAC in a postmortem swine model.
Methods
Doxorubicin was aerosolized through a Micropump© (MIP) into the peritoneal cavity of two swines at a pressure of 12 mm Hg CO2 and 32 °C. To measure the distribution of the drug, 9 different positions within the abdominal cavity were sampled. In-tissue doxorubicin penetration was evaluated using fluorescence microscopy on frozen thin sections.
Results
A maximum of drug penetration was observed in the area around the MIP. The penetration in the small intestine reached a depth of 349 ± 65 µm. Penetration depth in the right upper abdomen and left upper abdomen were 349 ± 65 and 140 µm ± 26 µm, respectively. Distant areas to the MIP showed variable penetration rates between 50 and 150 µm.
Conclusions
Doxorubicin reached all areas within the peritoneum. Highest penetration rates were measured in the area around the Micropump. Further studies are warranted to evaluate and optimize the distribution and penetration of cytotoxic agent into the tissue after PIPAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peritoneal carcinomatosis (PC) is a common manifestation of advanced gastrointestinal and gynecological malignancies. Overall survival of patients with peritoneal carcinomatosis remains very disappointing (Sadeghi et al. 2000; Sugarbaker 2003). Systemic or intraperitoneal chemotherapy in combination with surgical procedures has been extensively studied and their possible limitations have been thoroughly discussed (Chua et al. 2013; Al-Quteimat and Al-Badaineh 2014). The current anti-tumor effect of IPC is strongly limited by poor penetration (<1 mm) of anticancer drugs into peritoneal nodules, micro-metastases and peritoneal tissue (Flessner 2005; Dedrick et al. 1978). The restricted diffusion of IPC is attributed to the peritoneum–tumor barrier (Jacquet and Sugarbaker 1996; Jacquet et al. 1996), a high interstitial tumor pressure (Jain 1994) and the effect of the capillary network, which drains drugs out of the tumor. In addition, IPC shows a non-uniform distribution pattern of the drug-containing solution, leaving poorly or untreated tumor tissue behind (Reymond and Solaß 2014).
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a new, innovative approach of palliative IPC used in the treatment of peritoneal carcinomatosis. In this operative intervention, a drug-containing solution is administered into the abdominal cavity in the shape of micro-droplets generated by a Micropump© (MIP) within a “therapeutic capnoperitoneum” (Solass et al. 2014). Aerosolized chemotherapeutic applications such as PIPAC are assumed to possess superior qualities in comparison with liquid IPC applied with conventional catheters (Reymond and Solaß 2014) and show promising clinical results (Tempfer et al. 2015; Nadiradze et al. 2016; Demtröder et al. 2016). Chemotherapeutic penetration depth into tumor and peritoneal tissue is reported at 300–600 µm with high tissue concentrations observed (0.03–4.1 µmol/g) (Solass et al. 2014)—far higher than previously reported in studies analyzing IPC (0.03 µmol/g) (Jacquet et al. 1996). However, the homogeneity of drug distribution in the peritoneal cavity after PIPAC has never been evaluated in an anatomical model. This study was performed to evaluate the distribution pattern of doxorubicin in the abdominal cavity after PIPAC in a postmortem swine model.
Materials and methods
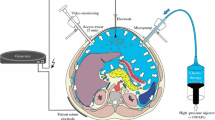
Experiments were performed 30 min postmortem in two swines (German land race pigs). All experiments and procedures were repeated to verify the results. The postmortem swine cadavers were placed in a supine position. An infra-umbilical mini-laparotomy was performed, and a 12-mm trocar (Kii®Balloon Blunt Tip System, Applied Medical, Rancho Santa Margarita, CA, USA) was inserted into the abdominal cavity (Fig. 1). A constant capnoperitoneum was established throughout the entire PIPAC experiment (Olympus UHI-3 insufflator, Olympus medical life science and industrial divisions, Olympus, Notting Hill, Australia). A five-mm camera (Karl Storz GmbH & Co KG, Tuttlingen, Germany) was introduced into the 12-mm trocar. A 5-mm trocar (Kii®Balloon Blunt Tip System, Applied Medical, Rancho Santa Margarita, CA, USA) was then placed under visual control in the right lateral hemi-abdomen. The Micropump (MIP®, Reger Medizintechnik, Rottweil, Germany) was connected to a high-pressure injection line (High Pressure Injection Line with Male/Female Luer lock 120 cm, 1200 psi, Smith Medical, Hranice, Czech Republic). Doxorubicin (Teva®, 2 mg/ml, Pharmachemie B.V., Haarlem, Netherlands) was filled in a sterile syringe (3 mg in 50 ml NaCl 0.9 %). The syringe was then tightly connected to the high-pressure line and finally brought into the injector head of the high-pressure injector (Injektron 82 M, MedTron, Saarbrücken, Germany). The MIP® was inserted into the 12-mm trocar in a perpendicular position with a maximum distance (8–10 cm) of the MIP® nozzle orifice to the small bowel serosal surface. The camera was placed and fixed in the 5-mm trocar to monitor adequate nebulization of the MIP®. After the tightness of the abdominal cavity (no CO2 flow) was confirmed, PIPAC was delivered (23 °C) with a flow rate of 30 ml/min into the abdominal cavity with a constant capnoperitoneum of 12 mmHg. The abdominal cavity was exposed to the doxorubicin aerosol for another 30 min following the injection phase. The capnoperitoneum was then evacuated via a high-efficiency particulate arrestance (HEPA) filter system. Thirty minutes after PIPAC a laparotomy was performed to retrieve the samples. Tissue samples of the peritoneum were recovered from 9 different sections, each measuring 3.0 × 3.0 × 0.5 cm. Among these were (1) right upper abdomen; (2) epigastric area; (3) small intestine; (4) left subdiaphragmal area; (5) left upper abdomen; (6) left lower abdomen; (7) right lower abdomen; and (8) stomach.
Microscopic analysis
Following the treatments, all tissue samples were rinsed with sterile NaCl 0.9 % solution in order to eliminate superficial cytostatics and immediately frozen in liquid nitrogen. A representative amount of cryo-sections (7 µm) were prepared from the 9 different areas of each specimen. Sections were mounted with VECTASHIELD containing 1.5 µg/ml 4′,6-diamidino-2-phenylindole (DAPI) to stain nuclei. Penetration depth of doxorubicin was monitored using a Leica TCS SP8 confocal laser scanning microscope at 488 nm. The distance between the luminal surface and the innermost positive staining for doxorubicin accumulation was measured and reported in micrometers (Fig. 2).
Microscopic analysis (Magnification: 20×) of penetration depth of doxorubicin in local peritoneal tissue samples of fresh of German land race pigs. Nuclei (blue) are stained with 4′,6-diamidino-2-phenylindole (DAPI). a Right upper abdomen; b epigastric area; c right subdiaphragmal area, d left subdiaphragmal area; e left upper abdomen; f left lower abdomen; g right lower abdomen; h stomach; and i small intestine
Micropump
Micropump (MIP®, Reger Medizintechnik, Rottweil, Germany): The MIP® consists of a high-pressure injector, a high-pressure connecting line, a connecting port at the shaft of the nozzle and a nozzle head with an opening of 200 µm. The drug is delivered with a pressure of up to eight bars. Doxorubicin was filled in a sterile plastic syringe and applied at the injector head of the high-pressure injector (Injektron 82M, MedTron, Saarbrücken, Germany) and then connected to the connecting port of the nozzle via a high-pressure line (High Pressure Injection Line with Male/Female Luer lock 120 cm, 1200 psi, Smith Medical, Hranice, Czech Republic).
Ethics
The swines were used and killed for a laparoscopic course at a local training center (Aesculap Akademie Bochum) prior to the experiment. An approval of the Local Board on Animal Care was obtained.
Results
A maximum of drug penetration was observed in the area around and opposite to the MIP. Penetration reached in the small intestine 349 ± 65 µm (swine one) and 311 ± 59 µm (swine two). Penetration depth in the right upper abdomen and left upper abdomen were 349 ± 65 µm (311 ± 59 µm in the second swine) and 140 ± 26 µm (104 ± 38 µm in the second swine), respectively. Other locations showed variable penetration rates (Fig. 3). Doxorubicin penetration at the right subdiaphragmal area was 35 ± 26 µm for swine one and 121 ± 36 µm for swine two. The lowest penetration depth was measured for swine one at the stomach with 17 ± 17 and 37 ± 28 µm in the swine two at the left subdiaphragmal area.
Discussion
One of the assumed advantages of PIPAC is the homogeneous drug distribution inside the intraperitoneal cavity. The results of our study demonstrate that the staining of all samples, which were collected from different areas of the abdomen, shows a notable distribution capability of the doxorubicin aerosol. However, the differences in the penetration depth values of the samples indicate the complexity of the distribution and penetration pattern of PIPAC. Some authors report a homogenous spatial methylene blue distribution pattern in the abdominal cavity after PIPAC-like procedures in animal experiments (Reymond and Solaß 2014; Kakchekeeva et al. 2016). Our data show a more complex picture concerning the penetration depth of cytotoxic agent after PIPAC. In both swines, a remarkable difference of penetration depth between different regions within each swine was measured. The highest penetration rates were measured in proximity of the small intestine directly opposite and around the MIP. This indicates the high penetration capability of PIPAC. This is in line with the report of other studies, which report a high uptake of the drug into the tumor. In the study of Solaß et al., three end-stage patients with advanced PC were treated with PIPAC (doxorubicin 1.5 mg/m2 and cisplatin 7.5 mg/m2). The authors report a drug uptake into the tumor nodules up to 500 µm and throughout the whole peritoneal layer into the preperitoneal fatty tissue up to 600 µm (Solass et al. 2014). Our results indicate the notable distribution pattern of PIPAC. In the staining of all samples, which were collected from different areas of the abdomen, a notable degree of doxorubicin uptake was observed. Some authors report that the capability of drug uptake might be associated with the distance to MIP, drug concentration (Khosrawipour et al. 2016a) and intraperitoneal pressure (Jacquet et al. 1996; Solaß et al. 2012; Solass et al. 2012; Esquis et al. 2006).
We observed the highest drug uptake opposite and around the MIP with a penetration depth of >311 µg. However, the penetration depth of doxorubicin in the samples of left lower abdomen was about 40 µm. This is in line with our previous studies (Khosrawipour et al. 2016a), which indicate that the proximity to the MIP might be a crucial factor affecting the uptake of the cytotoxic agent. We previously demonstrated in an ex vivo model that higher drug dosage and a closer positioning of the MIP toward the target lead to a higher penetration of doxorubicin after PIPAC (Khosrawipour et al. 2016a). In that study, doxorubicin was aerosolized in an ex vivo PIPAC model using a hermetic container system mimicking the abdominal cavity. Fresh postmortem swine peritoneum pieces were spatially placed at four different spots within the box. The highest drug penetration depth was observed in the tissue placed on the distributing surface directly opposite to the MIP with 351 µm penetration depth compared to 34 µm penetration depth in the sample which was placed within the box in a distant area to the MIP. The results of the present study confirm our former results in ex vivo models. The advantage of the ex vivo model in the present study compared to our other ex vivo model mentioned above (using a hermetic container system mimicking the abdominal cavity) (Khosrawipour et al. 2016a, b, c) is that the anatomical boundaries and peritoneal surface are more similar to the clinical setting and allow a more precise evaluation of the impact of anatomy on the distribution and penetration pattern of cytotoxic agent following PIPAC. One should note that the area around and opposite to the MIP is exposed longer time to the maximal concentration of the drug. In fact, the concentration of the aerosol drug might be lower at distant areas due to the thinning with the normal intraperitoneal fluid through the way. Indeed, inhomogeneous drug distribution and absorption is a challenge for IPC. To overcome this disadvantage for hyperthermic intraperitoneal chemotherapy (HIPEC), numerous technical variations have been developed such as open and laparoscopic techniques (Lotti et al. 2016; Gesson-Paute et al. 2008). There are several hypothetical approaches, which might lead to a better drug uptake at distant areas to MIP.
Using higher drug concentration to overwhelm the thinning effect in the peritoneal cavity might be contra-productive. First, a higher drug concentration increases the possibility of local toxicity and perforation. Second, our previous results indicate that higher doxorubicin concentrations lead to a significant increase in drug penetration only in the area around the MIP but only a lower increase in more distant areas, and thus, there might be no balance between risks and benefits of this approach. Thus, the application technique of PIPAC has to be optimized. In clinical practice, the MIP might be placed at the most possible outlying position to the tumor-bearing tissues for the application. This could be combined with rotation of the MIP and changing its spray direction. The application device could be optimized as well by adding several (rotating) heads and nozzles to the MIP. These changes might reduce the thinning of the doxorubicin aerosol through intraperitoneal fluid and thus enable higher penetration of the applied doxorubicin into distant areas to the MIP. An important limitation of our study resides in the fact that our experiments were performed in a postmortem model, where the blood irrigation is absent. Although peritoneum is not a shock organ like heart, brain or liver, its response to PIPAC may differ in a living organism with regular blood circulation. However, the result of this study helps to establish the preamble for further studies using in vivo models.
Conclusion
Improving technical features of PIPAC and its clinical application should be further evaluated to optimize the distribution pattern and penetration depth of the cytotoxic agents without increasing the toxicity. During PIPAC therapy, an intense penetration of the small intestine can be achieved, a finding which may be of therapeutic benefit in the clinical setting. Until today, PIPAC therapy has shown a good clinical outcome. However, further studies must be conducted to determine whether an increase in chemotherapeutic application dosage may improve the clinical outcome by increasing drug penetration especially in the small intestine.
Abbreviations
- CO2 :
-
Carbon dioxide
- CRS:
-
Cytoreductive surgery
- HIPEC:
-
Hyperthermic intraperitoneal chemotherapy
- IAP:
-
Intra-abdominal pressure
- IPC:
-
Intraperitoneal chemotherapy
- PC:
-
Peritoneal carcinomatosis
- PCI:
-
Sugarbaker’s peritoneal cancer index
- PIPAC:
-
Pressurized intraperitoneal aerosol chemotherapy
- MIP® :
-
Micropump (Reger Medizintechnik, Rottweil, Germany)
References
Al-Quteimat OM, Al-Badaineh MA (2014) Intraperitoneal chemotherapy: rationale, applications, and limitations. J Oncol Pharm Pract 20(5):369–380
Chua TC, Esquivel J, Pelz JO, Morris DL (2013) Summary of current therapeutic options for peritoneal metastases from colorectal cancer. J Surg Oncol 107(6):566–573
Dedrick RL, Myers CE, Bungay PM, De Vita VT Jr. (1978) Pharmacokinetic rational for the peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 62(1):1–11
Demtröder C, Solass W, Zieren J, Strumberg D, Giger-Pabst U, Reymond MA (2016) Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Colorectal Dis 18(4):364–371
Esquis P, Consolo D, Magnin G et al (2006) High intra-abdominal pressure enhances the penetration and antitumor effect of intraperitoneal cisplatin on experimental peritoneal carcinomatosis. Ann Surg 244(1):106–112
Flessner MF (2005) The transport barrier in intraperitoneal therapy. Am J Physiol Renal Physiol 288(3):433–442
Gesson-Paute A, Ferron G, Thomas F, de Lara EC, Chatelut E, Querleu D (2008) Pharmacokinetics of oxaliplatin during open versus laparoscopically assisted heated intraoperative intraperitoneal chemotherapy (HIPEC): an experimental study. Ann Surg Oncol 15(1):339–344
Jacquet P, Sugarbaker PH (1996) Peritoneal-plasma barrier. Cancer Treat Res 82(1):53–63
Jacquet P, Stuart OA, Chang D, Sugarbaker PH (1996) Effects of intraabdominal pressure on pharmacokinetics and tissue distribution of doxorubicin after intraperitoneal administration. Anticancer Drugs 7(5):596–603
Jain RK (1994) Barriers to drug delivery in solid tumors. Sci Am 271(1):58–65
Kakchekeeva T, Demtröder C, Herath NI et al (2016) In vivo feasibility of electrostatic precipitation as an adjunct to pressurized intraperitoneal aerosol chemotherapy (ePIPAC). Ann Surg Oncol (Epub ahead of print)
Khosrawipour V, Khosrawipour T, Falkenstein TA et al (2016a) Evaluating the effect of Micropump© position, internal pressure and doxorubicin dosage on efficacy of pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in an ex vivo model. Anticancer Res (Accepted for publication on 12 July 2016)
Khosrawipour V, Bellendorf A, Khosrawipour T et al (2016b) Irradiation does not increase the penetration depth of doxorubicin in normal tissue after pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in an ex vivo model. In Vivo 30(5):593–597
Khosrawipour V, Giger-Pabst U, Khosrawipour T et al (2016c) Effect of irradiation on tissue penetration depth of doxorubicin after pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in a novel ex vivo model. J Cancer 7(8):910–914
Lotti M, Capponi MG, Piazzalunga D, Poisasina E, Pisano M, Manfredi R, Anssloni L (2016) Laparoscopic HIPEC: a bridge between open and closed-techniques. J Minim Access Surg 12(1):86–89
Nadiradze G, Giger-Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA (2016) Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg 20(2):367–373
Reymond MA, Solaß W (2014) Pressurized intraperitoneal aerosol chemotherapy—cancer under pressure, 1st edn. De Gruyter, Berlin
Sadeghi M, Arvieux C, Glehen O et al (2000) Peritoneal carcinomatosis from non-gynecological malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 88(2):358–363
Solaß W, Hetzel A, Nadiradze G, Sagynaliev E, Reymond MA (2012a) Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc 26:1849–1855
Solass W, Herbette A, Schwarz T, Hetzel A, Sun JS, Dutreix M, Reymond MA (2012b) Therapeutic approach of human peritoneal carcinomatosis with Dbait in combination with capnoperitoneum: proof of concept. Surg Endosc 26(3):847–852
Solass W, Kerb R, Mürdter T et al (2014) Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 21(2):553–559
Sugarbaker PH (2003) Peritoneal carcinomatosis: Is cure an option? J Clin Oncol 21(5):762–764
Tempfer CB, Rezniczek GA, Ende P, Solass W, Reymond MA (2015) Pressurized intraperitoneal aerosol chemotherapy with cisplatin and doxorubicin in women with peritoneal carcinomatosis: a cohort study. Anticancer Res 35(12):6723–6729
Funding
This study was funded by institutional funds (Department of Surgery, Marien Hospital Herne, Ruhr University Bochum).
Authors’ contribution
Veria Khosrawipour contributed to study design, laboratory analysis, data acquisition and drafting of the manuscript; Tanja Khosrawipour contributed to laboratory analysis, data acquisition and drafting of the manuscript; Alexander Jens Peter Kern contributed to laboratory analysis, data acquisition and drafting of the manuscript; Aras Osma contributed to laboratory analysis and data acquisition; Burak Kabakci contributed to experimental and study design, laboratory analysis and data acquisition; David Diaz-Carballo contributed to supervision of the experiments and critical revision for important intellectual content of the manuscript; Eckart Förster contributed to study design, drafting and critical revision for important intellectual content of the manuscript; Jürgen Zieren contributed to supervision of the study, drafting and critical revision for important intellectual content of the manuscript; Khashayar Fakhrian contributed to study design, supervision of the study, data interpretation, drafting and critical revision for important intellectual content of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Human and animals rights
The swines were used and killed for a laparoscopic course at a local training center (Aesculap Akademie Bochum) prior to the experiment. This article does not contain any studies with human participants performed by any of the authors.
Ethical approval
An approval of the Local Board on Animal Care was obtained.
Rights and permissions
About this article
Cite this article
Khosrawipour, V., Khosrawipour, T., Kern, A.J.P. et al. Distribution pattern and penetration depth of doxorubicin after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a postmortem swine model. J Cancer Res Clin Oncol 142, 2275–2280 (2016). https://doi.org/10.1007/s00432-016-2234-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2234-0