Abstract
Purpose
We investigated the expression status of vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF)-B and their receptors in organ-confined clear cell renal cell carcinoma (ccRCC) to evaluate their prognostic significance after radical surgery.
Methods
In 758 consecutive patients diagnosed with pT1-2N0 ccRCC between 2007 and 2012, we prospectively investigated the expression levels of VEGF, PDGF-B, VEGF receptor (VEGFR) and PDGF receptor (PDGFR)-β via immunohistochemistry. Clinicopathologic parameters and expression of the angiogenic factors were analyzed with respect to recurrence-free survival (RFS) after nephrectomy. The median follow-up was 29.5 months (IQR 21.5, 39.6) after surgery.
Results
Partial nephrectomy had been performed in 48.5 % of the patients, and tumors were staged as pT1a in 514 (67.8 %), pT1b in 192 (25.3 %) and pT2 in 52 (6.9 %). VEGF, PDGF and their receptors were identified in the cytoplasm and membranes of the tumor cells. Expression level of VEGFR inversely correlated with both tumor size (r = −0.076, p = 0.014) and nuclear grade (r = −0.297, p = 0.004). As for PDGF-B, the expression level showed an inverse correlation with tumor size (r = −0.216, p < 0.001) while PDGFR-β inversely correlated with nuclear grade (r = −0.341, p = 0.001). On multivariate analysis, age, pathologic stage, nuclear grade and PDGFR-β expression (high vs. low or none, HR 3.121 95 % CI 1.300–7.493, p = 0.011) were independently prognostic of RFS after nephrectomy.
Conclusions
In organ-confined ccRCC, high expression of PDGFR-β was independently predictive of poorer RFS after nephrectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC) is the most common malignant neoplasm of the kidney and accounts for 5 % of all adult malignancies (Jemal et al. 2009). Recent increases in the usage of abdominal imaging along with advances in the radiographic modalities have contributed to downward migration of RCC stage at diagnosis (Pantuck et al. 2001). Accordingly, up to 75 % of patients with newly diagnosed RCC are staged I (Pantuck et al. 2001). However, despite the relatively favorable prognosis after definitive surgery with a 5-year recurrence-free survival (RFS) ranging between 80 and 90 %, a substantial proportion of these pathologically organ-confined RCC recurs and demonstrates unpredictable clinical course thereafter (Delahunt et al. 2002). While tumor size, histologic subtype and nuclear grade are well-recognized prognostic factors for recurrence (Delahunt et al. 2002; Karakiewicz et al. 2008; Komai et al. 2011; Moch et al. 2000; Muramaki et al. 2011; Novara et al. 2007; Scoll et al. 2009; Tsui et al. 2000), these factors are hardly variable except for the tumor size among stage I RCCs because clear cell RCC (ccRCC) comprises up to 80 % of all RCCs (Amin et al. 2002). Thus, the need for identifying additional prognostic parameters for the localized ccRCC which accounts for majority of newly diagnosed RCC in the contemporary era remains irrefutable.
Studies on the molecular systems that control angiogenesis have provided evidence that new vessel formation promotes tumor cell growth and significantly increases the metastatic potential of RCC (Kim et al. 2004). These findings led to the development of therapeutic approaches targeting these angiogenic molecules: vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) and/or their receptors (Cao 2013; Dirim et al. 2008; Ostman and Heldin 2007). In addition to the attempts to target these molecules in metastatic RCC, prognostic significance of the expression of these proteins in the localized or advanced RCC has been the constant subject of investigation (Sulzbacher et al. 2003).
The aim of the current study was to investigate the expression status of VEGF, PDGF-B and their receptors in localized ccRCC and to evaluate their prognostic significance after radical surgery.
Methods
This study was performed with the approval and oversight of the institutional review board at our institution. Since June 2007, we have been immunostaining a set of angiogenic factors on all RCC specimens. For the present study, we reviewed the data of 758 consecutive patients with localized ccRCC who underwent radical or partial nephrectomy between June 2007 and May 2012. After surgery, patients were followed with physical examination, blood test, chest X-ray and computerized tomography of the abdomen and pelvis at 6- to 12-month intervals according to stage. Patients with inadequate follow-up records (e.g., insufficient medical records and/or a follow-up loss within 1 year among those without recurrence) were excluded from the analysis. The median follow-up duration was 29.5 months (IQR 21.5, 39.6) after surgery.
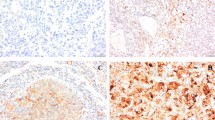
All specimens were reviewed to confirm diagnosis according to the AJCC 2009 TNM classification for tumor staging, and the Fuhrman nuclear grading system for tumor grading (Fuhrman et al. 1982) after which representative formalin-fixed as well as paraffin-embedded tumor sections were immunostained. The primary antibodies used were a purified mouse monoclonal antibody against VEGF (G153-694, 1:500 dilution, BD Biosciences Pharmingen, San Jose, CA), a rabbit monoclonal antibody against VEGF-R2 (55B11, 1:100 dilution, Cell Signaling Technology, Danvers, MA), a rabbit polyclonal antibody against PDGF-B (L48, 1:400 dilution, Bioworld Technology, Louis Park, MN) and a rabbit monoclonal antibody against PDGFR-β (Y92, 1:200 dilution, Epitomics, Burlingame, CA). Immunostaining was performed by an autostainer (OptiView DAB IHC Detection Kit, Ventana Medical System, Oro Valley, AZ) according to the protocols provided by the manufacturer. The uniform immunostained normal human placenta tissues were used as the positive control for adequate immunohistochemical application and for evaluating positive expressions of angiogenic factors and their receptors in tumor tissues. The vascular endothelial cells in normal kidney tissues were also immunostained as internal positive control. The normal kidney tissue stained in the absence of primary antibody was used as the negative control. The expression levels of VEGF, VEGF-R, PDGF-B, and PDGFR-β were quantified by counting 500 cells and estimating the proportion of immunopositive cells (%) (Fig. 1). The proportion of staining cells was graded on a four-tiered scale (0–3) by the genitourinary pathologist (YM Cho) as follows: grade 0, <5 %; grade 1, 5–33 %; grade 2, 33–66 %; grade 3, >66 %.
a–c Immunostaining patterns for VEGF (a), VEGFR (b) and PDGF-B (c) showed diffusely distributed positive cells in clear cell RCC (original magnification ×400). d Immunostaining pattern for PDGFR-β was observed in both membrane and cytoplasm of the positive cells (dashed line) (original magnification ×400). Vascular endothelial cells are also immunostained as a positive control (small box in picture d)
The relationship between angiogenic factor expression and clinicopathologic parameters was assessed using ordered logistic regression. All potential factors including the expression levels of angiogenic factors associated with disease recurrence defined as local and/or distant metastasis identified by radiologic examinations were analyzed with Kaplan–Meier analysis (univariate) and Cox proportional hazards model (multivariate). For survival analysis, angiogenic factor expression was grouped as high (grade 2 or 3) or low or none (grade 0 or 1). All tests were done using STATA version 12.0 (StataCorp, College Station, TX), and a p value <0.05 was considered to be statistically significant.
Results
The median patient age was 55 years (IQR 47,64), and 70.7 % of the patients were male. Partial nephrectomy had been performed in 48.5 % of patients, and tumors were pathologically staged as T1a in 514 (67.8 %), T1b in 192 (25.3 %) and T2 in 52 (6.9 %) patients. On immunostaining, both membranous and cytoplasmic expressions were noted for all four angiogenic proteins. The expression levels of PDGF-B and PDGFR-β were lower than those of VEGF or VEGFR, showing grade 0 in 57.6 and 49.3 % of the specimens for PDGF-B and PDGFR-β, respectively (Table 1).
We observed a weak but significant relationship between the expression level of some of the angiogenic factors and the clinical and pathological parameters (Table 2). With increasing tumor size, the expression levels of VEGFR (β = −0.0761, p = 0.014) and PDGF-B (β = −0.2166, p < 0.0001) decreased. A similar pattern was observed for pathologic stage. With respect to Fuhrman nuclear grade, an increase in nuclear grade was associated with decreasing receptor expression (VEGFR, β = −0.2971, p = 0.004; PDGFR-β, β = −0.3414, p = 0.002), while the expression levels of the respective ligand did not demonstrate any relationship (Table 2A). The result was similar when the patients were stratified by the Fuhrman nuclear grade (Table 2B). However, when the relationship was analyzed stratified by the pathologic T stage (pT stage), most relationships lost its significance (Table 2C).
Among the 758 RCC patients, 25 (3.3 %) developed recurrence during the follow-up period, all of whom had distant metastasis. One patient with recurrence had concomitant distant metastasis and local recurrence. Recurrence-free survival (RFS) rates for stage T1a, T1b and T2 tumors were 97.9 % (95 % CI 95.7–99.0), 83.7 % (95 % CI 70.5–91.3) and 70.0 % (95 % CI 49.0–83.7), respectively (p < 0.0001). Patient age, tumor size and Fuhrman nuclear grade were associated with RFS (p = 0.001, <0.001, 0.002, respectively). Among the angiogenic factors, PDGF-B and PDGFR-β expression were associated with RFS (Fig. 2). Patients with high expression levels (grade 2 or 3) of PDGF-B but low or no expression of PDGFR-β demonstrated significantly better RFS after surgery. In multivariate analysis, in addition to age, pT stage, and Fuhrman nuclear grade, high PDGFR-β expression was independently associated with poor RFS (HR 3.121, 95 % CI 1.300–7.493, p = 0.011; Table 3). We tested combinations of ligand and receptor expression statuses for each angiogenic factor on RFS but found no significant relationships (data not shown).
Discussion
In the normal human development early in the embryonic period, VEGF regulates vasculogenesis, after which period the expression gradually declines eventually becoming minimal in most normal adult tissues (Nicol et al. 1997). However, VEGF expression is re-induced under pathologic conditions such as during tumorigenesis promoting neo-angiogenesis, thereby playing a major role in tumor migration and metastasis (Nicol et al. 1997). On the other hand, PDGF is primarily found in alpha granules of platelets and is physiologically secreted by mesenchymal cells as well as epithelial cell. Specifically, PDGF-B promotes the survival of pericytes, which cover the surface of blood vessels, contributing to the maintenance of vascular structures (Bergers and Benjamin 2003). Overexpression of PDGF in various tumor cells has also been reported previously (Heldin and Westermark 1999). Identification of these angiogenic factors has vastly improved not only the insights into cancer cell biology but also the development of therapeutic targets for metastatic RCC (Kim and Kaelin 2006). And accordingly the value of expression status of such molecular markers in determining prognosis after treatment, especially in pathologically organ-confined RCC, has been constantly investigated (Cao 2013; Dirim et al. 2008; Sulzbacher et al. 2003). Similarly, in addition to VEGF and PDGF, expression status and their correlation with prognosis of hypoxia-inducible factor, carbonic anhydrase 9 and proteins in the mammalian target of rapamycin pathway as well as their receptors have been studied extensively (Darwish et al. 2013; Gilbert et al. 2006; Lidgren et al. 2005).
However, most preexisting studies on the molecular markers as potential predictors of oncological outcome of RCC are limited by their retrospective nature with a relatively small sample size, insufficient follow-up, lack of reliability in the assay used for marker detection and limited availability of reference values (Gerber et al. 1999). Contributing to the inconsistent results across the previous studies was the inclusion of samples with various pathologic characteristics at various stages, sizes and histologic subtypes. There is insufficient knowledge as to what stage in the tumorigenesis and propagation the genes for the expressions of these proteins are activated. Expression status may reflect the initial, active or terminal phase after feedback in the process (Ostman and Heldin 2007). Furthermore, tumor necrosis at the center of the tumor and the pseudocapsule formation at the periphery are all related to the increase in the tumor size, and the possibly differential activity of these molecules still remains to be discovered (Rajandram et al. 2014). Coupled with frequent sarcomatoid differentiation and lymphovascular invasion found in RCC tissues, choice of the area within the tumor mass for immunohistochemical investigation should affect the outcome greatly (Rioux-Leclercq et al. 2007). All these factors contributed to inconsistent results across the studies, preventing direct comparison, and hence, few molecular markers are employed in actual clinical practice, most requiring further investigation.
To minimize these shortcomings of the previous studies, we limited our analysis to size <7 cm, organ-confined RCC with clear cell histology, the patient group which constitutes more than 70 % of the contemporary RCC population. Larger tumors had been included for comparison purposes. Also due to the association with the von Hippel–Lindau gene, aberration of which is frequently observed in ccRCC (Park et al. 2013), we hypothesized that VEGF and/or its receptor expression could be altered at earlier stage which could convey its metastatic potential. Using the same protocol, we prospectively stained representative areas in the tumor without gross necrosis or hemorrhage, confirmed microscopically and scored. Because the staining intensities were relatively weak and similar in most of the subjects, we could quantify the staining result only by estimating the proportion (%) of immunopositive cells rather than scoring the staining intensity. In the current study, we found increasing tumor size and increasing nuclear grade were associated with decreasing VEGFR expression. On the other hand, similar correlation was observed between the tumor size, nuclear grade and the PDGF-B. With respect to RFS, we found only PDGFR-β was of an independently prognostic value. There are two previous studies which evaluated the prognostic value of PDGF-A and/or its receptor (Sulzbacher et al. 2003; Tawfik et al. 2007). Results are in accordance with our study, but still inconclusive most probably owing to the aforementioned differences in study population. And more importantly, the markers investigated were PDGF-AA or PDGFR-α in these studies. While these receptors share similar structures and form homo- or heterodimers to exert various downstream effects, PDGFR-α is mainly expressed by malignant cells whereas PDGFR-β is expressed in stromal and perivascular cells (Ostman and Heldin 2007). For this reason, most previous studies in RCC had focused on PDGFR-α. However, with the more recent insight into the tumor microenvironment and its importance in the control and promotion of tumor proliferation and migration, we were led to investigate the role of PDGF-B and PDGFR-β.
We found that expression of PDGFR-β correlated inversely with RCC tumor differentiation, but on multivariate analysis, high PDGFR-β expression was observed to be prognostic of increased risk of recurrence following radical surgery. In order to minimize the impact of tumor size, we had stratified the cohort according to the pathologic stage (Table 2B). In this stratified analysis, the correlation between PDGFR-β expression status and Fuhrman grade lost its statistical significance although the inverse direction of the relationship remained unchanged. Poorly differentiated tumors are by definition tumors with increased mitotic figure, disrupted cellular architecture with aberrant microvasculature (Fuhrman et al. 1982). Our finding that PDGFR-β expression was in inverse relationship with the nuclear grade irrespective of tumor stage can thus be explained. The inverse correlations of VEGFR, PDGF-B/PDGFR-β and pathological grade are unique and interesting finding of this study. Most previous studies analyzed the expression status of angiogenic factors and their receptors in RCC patients which included locally advanced RCCs (e.g., pT3 RCCs), whereas our data only included pT1-2 N0M0 ccRCCs. Since the exact timing of PDGF and VEGF expression increase is unknown throughout the tumorigenesis, we presumed that our finding is probably due to different tumor stages of the patients and thus can be limited to only localized ccRCCs. However, the finding that high PDGFR-β expression in localized RCC has independent prognostic value in a multivariate model is novel and worth note. Considering that all of our recurrences were distant metastases, this finding could imply that while poorly differentiated tumors lose the ability to maintain a well-formed vascular structure, those that retain the ability possess increased potential for subsequent recurrence. On the other hand, since the timing of the initiation of metastasis in a pathologically organ-confined cancer is unknown, this could also imply that elevated PDGFR-β expression in organ-confined RCC is part of the early events. Further research of the molecules that function in concert with or at the downstream of these vasculogenic proteins could help elucidate the mechanism. Moreover, to be more generalizable, confirming the prognostic role of PDGFR-β expression may require additional validations.
Conclusions
Although high tumor expression level of PDGFR-β was associated with low nuclear grade, it was independently predictive of poorer RFS after nephrectomy in patients with organ-confined ccRCC. While the mechanism of PDGFR-β contributing to the development of subsequent metastasis and its relations with other vascular markers needs further to be determined, our finding provides additional prognostic information to aid in the following of patients with stage I ccRCC.
References
Amin MB, Amin MB, Tamboli P et al (2002) Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol 26(3):281–291
Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3(6):401–410
Cao Y (2013) Multifarious functions of PDGFs and PDGFRs in tumor growth and metastasis. Trends Mol Med 19(8):460–473
Darwish OM, Kapur P, Youssef RF et al (2013) Cumulative number of altered biomarkers in mammalian target of rapamycin pathway is an independent predictor of outcome in patients with clear cell renal cell carcinoma. Urology 81(3):581–586
Delahunt B, Kittelson JM, McCredie MR, Reeve AE, Stewart JH, Bilous AM (2002) Prognostic importance of tumor size for localized conventional (clear cell) renal cell carcinoma: assessment of TNM T1 and T2 tumor categories and comparison with other prognostic parameters. Cancer 94(3):658–664
Dirim A, Haberal AN, Goren MR et al (2008) VEGF, COX-2, and PCNA expression in renal cell carcinoma subtypes and their prognostic value. Int Urol Nephrol 40(4):861–868. doi:10.1007/s11255-008-9362-7
Fuhrman SA, Lasky LC, Limas C (1982) Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 6(7):655–663
Gerber HP, Hillan KJ, Ryan AM et al (1999) VEGF is required for growth and survival in neonatal mice. Development 126(6):1149–1159
Gilbert SM, Whitson JM, Mansukhani M et al (2006) Detection of carbonic anhydrase-9 gene expression in peripheral blood cells predicts risk of disease recurrence in patients with renal cortical tumors. Urology 67(5):942–945
Heldin CH, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79(4):1283–1316
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59(4):225–249. doi:10.3322/caac.20006
Karakiewicz PI, Jeldres C, Suardi N et al (2008) Age at diagnosis is a determinant factor of renal cell carcinoma-specific survival in patients treated with nephrectomy. Can Urol Assoc J 2(6):610–617
Kim WY, Kaelin WG (2006) Molecular pathways in renal cell carcinoma–rationale for targeted treatment. Semin Oncol 33(5):588–595
Kim HL, Seligson D, Liu X et al (2004) Using protein expressions to predict survival in clear cell renal carcinoma. Clin Cancer Res 10(16):5464–5471
Komai Y, Fujii Y, Iimura Y et al (2011) Young age as favorable prognostic factor for cancer-specific survival in localized renal cell carcinoma. Urology 77(4):842–847
Lidgren A, Hedberg Y, Grankvist K, Rasmuson T, Vasko J, Ljungberg B (2005) The expression of hypoxia-inducible factor 1alpha is a favorable independent prognostic factor in renal cell carcinoma. Clin Cancer Res 11(3):1129–1135
Moch H, Gasser T, Amin MB, Torhorst J, Sauter G, Mihatsch MJ (2000) Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer 89(3):604–614
Muramaki M, Miyake H, Sakai I et al (2011) Age at diagnosis as a powerful predictor for disease recurrence after radical nephrectomy in Japanese patients with pT1 renal cell carcinoma. Int J Urol 18(2):121–125. doi:10.1111/j.1442-2042.2010.02683.x
Nicol D, Hii SI, Walsh M et al (1997) Vascular endothelial growth factor expression is increased in renal cell carcinoma. J Urol 157(4):1482–1486
Novara G, Martignoni G, Artibani W, Ficarra V (2007) Grading systems in renal cell carcinoma. J Urol 177(2):430–436. doi:10.1016/j.juro.2006.09.034
Ostman A, Heldin CH (2007) PDGF receptors as targets in tumor treatment. Adv Cancer Res 97:247–274
Pantuck AJ, Zisman A, Belldegrun AS (2001) The changing natural history of renal cell carcinoma. J Urol 166(5):1611–1623
Park BK, Kim CK, Park SY, Shen SH (2013) Percutaneous radiofrequency ablation of renal cell carcinomas in patients with von Hippel Lindau disease: indications, techniques, complications, and outcomes. Acta Radiol 54(4):418–427
Rajandram R, Yap NY, Pailoor J et al (2014) Tumour necrosis factor receptor-associated factor-1 (TRAF-1) expression is increased in renal cell carcinoma patient serum but decreased in cancer tissue compared with normal: potential biomarker significance. Pathology 46(6):518–522. doi:10.1097/PAT.0000000000000145
Rioux-Leclercq N, Fergelot P, Zerrouki S et al (2007) Plasma level and tissue expression of vascular endothelial growth factor in renal cell carcinoma: a prospective study of 50 cases. Hum Pathol 38(10):1489–1495
Scoll BJ, Wong YN, Egleston BL, Kunkle DA, Saad IR, Uzzo RG (2009) Age, tumor size and relative survival of patients with localized renal cell carcinoma: a surveillance, epidemiology and end results analysis. J Urol 181(2):506–511
Sulzbacher I, Birner P, Traxler M, Marberger M, Haitel A (2003) Expression of platelet-derived growth factor-alpha alpha receptor is associated with tumor progression in clear cell renal cell carcinoma. Am J Clin Pathol 120(1):107–112
Tawfik OW, Kramer B, Shideler B, Danley M, Kimler BF, Holzbeierlein J (2007) Prognostic significance of CD44, platelet-derived growth factor receptor alpha, and cyclooxygenase 2 expression in renal cell carcinoma. Arch Pathol Lab Med 131(2):261–267
Tsui KH, Shvarts O, Smith RB, Figlin RA, deKernion JB, Belldegrun A (2000) Prognostic indicators for renal cell carcinoma: a multivariate analysis of 643 patients using the revised 1997 TNM staging criteria. J Urol 163(4):1090–1095 quiz 1295
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors made no disclosures.
Rights and permissions
About this article
Cite this article
Shim, M., Song, C., Park, S. et al. Prognostic significance of platelet-derived growth factor receptor-β expression in localized clear cell renal cell carcinoma. J Cancer Res Clin Oncol 141, 2213–2220 (2015). https://doi.org/10.1007/s00432-015-2019-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-2019-x