Abstract
Purpose
Tumor stem cell surface marker CD44v6, a member of the CD44 protein family, is causally involved in the metastasis of cancer. Little is known about the functions of CD44v6 in gastric cancer. The aim of this study was to evaluate the prognostic value of CD44v6 and investigate its functional roles.
Methods
The expression of CD44v6 in 208 primary gastric adenocarcinoma patient samples was examined using immunohistochemistry and its correlation with clinicopathological parameters, and 5-year patient survival was assessed. Two pairs of MGC-803 stable cells with either CD44v6 overexpression or knockdown were created. The effect of CD44v6 on cell proliferation, colony formation, migration and apoptosis was investigated using these two pairs of cells.
Results
Overexpression of CD44v6 was observed in all cancer cell lines. The 5-year survival rate of patients with positive CD44v6 expression is significantly worse compared to those with negative expression (38.8 vs. 73.6 %). CD44v6 and TNM stage are two independent prognostic factors of primary gastric adenocarcinoma. The risk factors for the positive CD44v6 expression are location of tumor, depth of invasion, lymph node metastasis, Lauren classification and TNM stage. In MGC-803 cells, CD44 stimulated proliferation and colony formation, antagonized oxaliplatin-induced apoptosis, but did not affect migration.
Conclusion
CD44v6 is an important prognosis marker in gastric cancer. Tissue specificity may affect the functions of CD44v6, and further work is needed to elucidate its regulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the fourth most common malignancy and the third leading cause of cancer-related death worldwide (Correia et al. 2009; Lozano et al. 2012). Despite the decline in global patient number, GC remains one of the most prevalent cancers in China and over 40 % of new cases in the world are diagnosed in China (Ferro et al. 2014). Although the progress in early diagnosis and adjuvant therapy has improved the prognosis of GC patients, radical surgery is still the primary approach for GC treatment. More specific diagnostic and therapeutic targets are urgently needed for better clinical application in this field.
Cancer stem cells (CSCs) in tumors are defined as a subpopulation of tumor cells that have both tumor-initiating ability and the ability to reconstitute the cellular heterogeneity typical of their tumors of origin (Schwitalla 2014). For tumors from epithelial origin including head and neck, breast, prostate and colon, isolation of CSCs is frequently based on their greater cell surface expression of CD44 (Al-Hajj et al. 2003; Collins et al. 2005; Dalerba et al. 2007; Li et al. 2007a). CD44 is a transmembrane glycoprotein participating in multiple cellular processes such as growth, survival, differentiation and mortality and plays important roles in malignant behaviors of several human cancers (Aruffo et al. 1990; Nagano et al. 2004; Orian-Rousseau and Ponta 2015; Vigetti et al. 2008; Yasui et al. 1998). It has been reported that the CD44-positive fractions in GC tumors can generate spheroid colonies under non-adherent conditions, and a small number of these cells can generate tumors in nude mice, demonstrating the potential therapeutic value for targeting CD44-positive cells in chemotherapy for GC treatment (Takaishi et al. 2009).
CD44 comprises three functional domains: the extracellular domain, the transmembrane domain and the cytoplasmic domain (Bajorath 2000). The extracellular domain is responsible for binding the extracellular matrix components such as hyaluronate, glycosaminoglycans, collagen, laminin and fibronectin. The membrane-proximal portion of the extracellular domain contains an insert from alternatively spliced exons, generating multiple CD44 isoforms (CD44v). The exons 1–5 and 16–20 of CD44 gene are present in all CD44 transcripts, whereas exons 6–15 are variably spliced (Bajorath 2000). CD44v6 is one of the CD44 isoforms and has been correlated with the malignancy of some cancer types. For example, Mikami et al. reported that CD44v6 was overexpressed in extrahepatic bile duct carcinomas and was linked with carcinoma cell differentiation (Mikami et al. 2001). In this study, we examined the expression of CD44v6 in GC patient samples using immunohistochemistry (IHC) and evaluated its prognostic value. The functional roles of CD44v6 in GC cell proliferation, colony-forming ability, migration and response to chemotherapy reagent were also investigated.
Materials and methods
Clinical samples
Gastric cancer (GC) tumor samples were obtained from 208 patients at Fujian Medical University Union Hospital, and the detailed clinicopathological parameters were assessed. All GC patients were diagnosed and gastrectomized with lymph node dissection in the Department of Gastric Surgery of the Union Hospital from 2006 to 2008. All patients had a well-documented clinical history and detailed follow-up information. None of the patients underwent preoperative chemotherapy and radiation therapy. The ethics committee of Fujian Medical University Union Hospital approved this study, and written consent was obtained from all patients involved.
IHC analysis
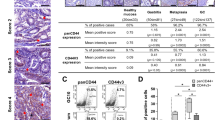
Immunohistochemistry (IHC) staining for CD44v6 was performed on formalin-fixed, paraffin-embedded gastric tissue sections (3-μm-thick tumor or normal). The CD44v6 protein expression was immunohistochemically demonstrated as yellowish to brown staining in the cytoplasm and membrane of gastric glandular cells. Two pathologists, blinded to the clinical data, reviewed the immunoreactivity for CD44v6 protein under a light microscope. The protein expression was scored independently according to the intensity of cellular staining and the proportion of stained tumor cells. The staining intensity was scored as 0 (no staining), 1 (weak staining, light yellow), 2 (moderate staining, yellow brown) and 3 (strong staining, brown). The proportions of stained tumor cells were classified as 0 (≤5 % positive cells), 1 (6–25 % positive cells), 2 (26–50 % positive cells) and 3 (≥51 % positive cells). The total scores for intensity and proportion were used to signify the level of protein expression. A score of 3 or less was considered negative CD44v6 expression, and a score of 4 or more was considered positive CD44v6 expression.
Cell culture
Human GC cell lines MGC-803, BGC-823, SGC-7901 and MKN-45, and the normal gastric epithelial cell line GES-1 were purchased from the Institute of Biochemistry and Cell Biology at the Chinese Academy of Sciences (Shanghai, China). These cell lines were maintained in RPMI 1640 (Invitrogen Corp., Carlsbad, California) containing 10 % fetal bovine serum (FBS) (Equitech-Bio, Ingram, Texas), 100 units/ml penicillin G and 100 μg/ml streptomycin (Invitrogen Corp.). Human CD44v6 was cloned from human brain cDNA library (Invitrogen) and subcloned into the pcDNA3 plasmid containing enhanced green fluorescent protein (EGFP) to generate EGFP-CD44v6 construct. The scramble and CD44v6 shRNA were purchased from GeneChem Corporation (Shanghai, China). MGC-803 cells were transfected with plasmids or shRNA using Lipofectamine 2000 (Invitrogen). The stable clonal cell lines were selected with media containing 1 mg/ml G418 and maintained in media with 0.2 mg/ml G418.
Cell proliferation and migration analysis
In a typical proliferation experiment, 20,000 cells were seeded on a 48-well plate in 200 µl medium and incubated for indicated time. Cell growth was monitored by the classical 2-(4,5-dimethyltriazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay using the cell proliferation kit (Roche, Mannheim, Germany). All assays were performed in triplicate. For the comparisons of growth rate, one-way ANOVAs were used.
Cell migration was measured with scratch wound-healing assay. Cells were seeded in a six-well plate and grown overnight to confluence. The monolayer cells were then scratched with a sterile pipette tip to create a wound, washed twice with serum-free media to remove floating cells, and left to grow in serum-free media. The cells migrating from the leading edge were photographed at 0, 12 and 24 h. Multiple views of each well were documented, and three independent experiments were performed.
Western blot
Cells were homogenized in 100–200 μl of radioimmunoprecipitation assay (RIPA) lysis buffer containing protease inhibitors at 4 °C for 30 min, followed by centrifugation at 16,000g for 15 min at 4 °C. The protein concentration of the supernatant was measured using the BCA Protein Assay Kit (Thermo). A total of 40 mg of protein of each sample was denatured and separated via SDS-PAGE and transferred to a nitrocellulose membrane (Millipore, Billerica, MA). Subsequently, the membrane was blocked with 5 % nonfat milk at room temperature for 1 h. The membrane was then incubated with mouse anti-CD44v6 (1:300; ab78960, Abcam, Cambridge, MA) or rabbit anti-GAPDH (Cell Signaling Technology) primary antibodies overnight at 4 °C. After washing with wash buffer (10 mM Tris–HCl, 150 mM NaCl, and 0.1 % Tween 20), the membrane was further incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (Sigma, St. Louis, MO) at a 1:3000 dilution for 1 h at room temperature. In the end, the membrane was washed for 30 min with wash buffer and detected using enhanced chemiluminescence (Amersham Corporation, Arlin-gton Heights, IL, USA).
Colony formation assay
Cells were seeded into six-well plates at a density of 1,000 cells/well and incubated for 14 days. The plates were then washed with phosphate-buffered saline (PBS) for three times, fixed with 70 % methanol for 15 min, and stained with Giemsa (Sigma) for 30 min. After five washes with PBS, the plates were left to dry and the number of colonies was counted using optical microscope. Each experiment was repeated three times.
Statistical analysis
All statistical analyses were performed using the software “Statistical Package for Social Science” (SPSS) version 9.0 for Windows (SPSS, Inc, Chicago, Illinois). Intergroup comparisons of the clinical and pathologic variables were analyzed using the Student t test for continuous variables and two-tailed two tests for discrete variables. The Kaplan–Meier method was used for calculating the survival rate, and the difference between the curves was assessed using the log-rank test.
Results
Overexpression of CD44v6 in gastric cancer is associated with worse patient survival
In order to investigate whether CD44v6 may have an important role in GC, we first compared the protein expression of CD44v6 in four GC cell lines with one normal gastric epithelial cell line GES-1. CD44v6 protein was significantly higher in all four cancer cell lines in comparison with GES-1 (Fig. 1a), suggesting CD44v6 may be overexpressed in GC. Next, we set out to evaluate CD44v6 expression in 208 paraffin-embedded primary gastric adenocarcinoma samples using IHC. The samples were divided into CD44v6-negative or CD44v6-positive based on the intensity of the CD44v6 protein staining and the proportion of CD44v6-expressing cells (see “Method”). Nearly 60 % of patients were CD44v6-positive (Table 1), supporting our hypothesis of common overexpression of CD44v6 in GC. In addition, CD44v6 overexpression and later TNM stages (Table 1) were the only two clinicopathological parameters significantly associated with worse patient survival (Fig. 1b), indicating CD44v6 may be a useful prognostic marker and pose important oncogenic functions in GC. The risk factors associated with CD44v6 overexpression were also assessed. Location of tumor, depth of invasion, lymph node metastasis, Lauren classification and TNM stage are significantly associated with CD44v6-positive expression (Table 2).
Investigation of the functional roles of CD44v6 in gastric cancer
In order to investigate the functional roles of CD44v6 in GC, we first created two MGC-803 cell lines stably expressing GFP or GFP-CD44v6 (Fig. 2a). Next, we compared the proliferation of these two cell lines using MTT assay. The growth of cells expressing GFP-CD44v6 is faster than those expressing GFP, suggesting CD44v6 is capable of stimulating cell proliferation (Fig. 2b). Similar results were observed in colony formation assays as GFP-CD44v6-overexpressing cells displayed a higher colony-forming ability (Fig. 2c). Afify et al. have shown that CD44v6 can promote breast cancer cell migration in wound-healing assays (Afify et al. 2009). However, overexpression of CD44v6 did not affect cell migration in MGC-803 cells (Fig. 2d), indicating that the migration-promoting ability of CD44v6 may be tissue specific. In addition, CD44v6 was reported to antagonize Fas ligand-mediated apoptosis (Mielgo et al. 2006). Therefore, we investigated the effect of CD44v6 overexpression on the apoptosis induced by the routine chemotherapy reagent for GC, oxaliplatin. The cells expressing GFP-CD44v6 displayed a weaker apoptosis in response to oxaliplatin (Fig. 2e), supporting the anti-apoptotic role of CD44v6 in GC.
Investigation of the oncogenic functions of CD44v6 overexpression. a Cell lysates were extracted from MGC-803 cell lines stably expressing GFP or GFP-CD44v6, and the expression of GFP-tagged proteins and GAPDH was detected by Western blot. b Proliferation of MGC-803 cell lines stably expressing GFP or GFP-CD44v6 over 5 days was examined using MTT assay. The readings at day 0 were used as 1 for fold change calculation. c Colony-forming ability of MGC-803 cell lines stably expressing GFP or GFP-CD44v6 was investigated by colony formation assay. The upper panel was the representative result images of the relative cell line. The lower panel was the quantitative comparison. The MGC-803 cell lines stably expressing GFP were used as 1 for fold change calculation. d MGC-803 cell lines stably expressing GFP or GFP-CD44v6 were subject to scratch wound-healing assays. The wound status was recorded at 0-, 12- and 24-h post-scratch, and the representative images were presented. e MGC-803 cell lines stably expressing GFP or GFP-CD44v6 were exposed to oxaliplatin at indicated concentrations, and the cell lysates were collected 24-h post-treatment. The expression of cleaved-PARP (c-PARP) and GAPDH was detected by Western blot. The experiments in (b–e) have been repeated three times
To further confirm the functional roles of CD44v6, we created another pair of MGC-803 cells stably expressing scramble RNA or CD44v6 shRNA (Fig. 3a). Consistent with the results observed in the CD44v6-overexpressing cell line, knockdown of CD44v6 reduced the proliferation rate (Fig. 3b) and suppressed the colony-forming ability (Fig. 3c) of MGC-803 cells. Moreover, the cell migration was not affected by CD44v6 knockdown (Fig. 3d), while the apoptosis induced by oxaliplatin was enhanced (Fig. 3e).
Investigation of the effects of CD44v6 knockdown. a Cell lysates were extracted from MGC-803 cell lines stably expressing scramble RNA or CD44v6-shRNA, and the expression of CD44v6 and GAPDH was detected by Western blot. b Proliferation of MGC-803 cell lines stably expressing scramble RNA or CD44v6-shRNA over 5 days was examined using MTT assay. The readings at day 0 were used as 1 for fold change calculation. c Colony-forming ability of MGC-803 cell lines stably expressing scramble RNA or CD44v6-shRNA was investigated by colony formation assay. The upper panel was the representative result images of the relative cell line. The lower panel was the quantitative comparison. The MGC-803 cell lines stably expressing scramble RNA were used as 1 for fold change calculation. d MGC-803 cell lines stably expressing scramble RNA or CD44v6-shRNA were subject to scratch wound-healing assays. The wound status was recorded at 0-, 12- and 24-h post-scratch, and the representative images were presented. e MGC-803 cell lines stably expressing scramble RNA or CD44v6-shRNA were exposed to oxaliplatin at indicated concentrations, and the cell lysates were collected 24-h post-treatment. The expression of cleaved-PARP (c-PARP) and GAPDH was detected by Western blot. The experiments in (b–e) have been repeated three times
Discussion
As a CSC marker, CD44 is widely studied in various cancer types. For example, CD44+/CD24− is commonly used as a surface marker to sort out CSCs in breast cancer (Li et al. 2007b). In GC, Xin et al. first discovered that CD44v6-positive expression was associated with poorer patient survival in advanced gastric adenocarcinoma (Xin et al. 2001). Since then, multiple studies have investigated the correlation between CD44v6 overexpression and prognosis of GC patients. In 2014, two meta-analysis studies using the published data both confirmed the association between positive CD44v6 expression and worse overall patient survival (Chen et al. 2014a, b). In this work, we evaluated the correlation between CD44v6 overexpression and clinicopathological parameters in 208 primary gastric carcinoma patient samples, providing extra evidence supporting the role of CD44v6 as an important independent prognosis factor for GC. Zlobec et al. have shown that loss of membranous CD44v6 in colorectal cancer was associated with multiple clinicopathological factors, but not as an independent prognosis factor (Zlobec et al. 2009). Of note, the cytoplasmic expression of CD44v6 was only observed together with membranous CD44v6 staining in very few samples (3 out of 208) in this study. Therefore, the discrepancy of the roles of CD44v6 in colorectal and GCs is probably due to tissue specificity of colon and stomach, but not stratification of the protein localization.
Despite the studies on the CD44v6 expression, little is known about the mechanisms of how it exerts its oncogenic function in GC. Although numerous studies have discovered the cellular process and signaling pathways CD44v6 is involved in (Orian-Rousseau 2010), it is important to validate them in GC. Therefore, we created two pairs of MGC-803 cells with either GFP-CD44v6 overexpression or knockdown of endogenous CD44v6 to investigate the functional roles of CD44v6 in GC. Consistent with previous studies in other caner types (Orian-Rousseau 2010), CD44v6 positively stimulated cell proliferation and colony formation and inhibits oxaliplatin-induced apoptosis in MGC-803 cells. However, CD44v6 did not activate cell migration in the scratch wound-healing assays, suggesting tissue specificity may affect the functions of CD44v6. The mechanisms on why CD44v6 lost its ability to stimulate GC cell migration are unclear, and further studies will be needed to elucidate this question.
Taken together, our data demonstrated some similarity and difference of the functions of CD44v6 in GC compared with previous reports from other cancer types. Much more work needs to be done for a better knowledge of the signaling pathways CD44v6 participates in GC. For example, CD44v6 has been reported to rescue a mouse thymoma cell from apoptosis by persistently activating MAP kinase pathway (Marhaba et al. 2005), which has been found to antagonize apoptosis in GC cell lines (Kim et al. 2014). It will be interesting to explore whether the activation of the MAP kinase pathway is responsible for the anti-apoptotic effect of CD44v6 in response to oxaliplatin in GC. These works may shed light on understanding the metastasis of GC and provide further information on the molecular regulation of CSCs.
References
Afify A, Purnell P, Nguyen L (2009) Role of CD44 s and CD44v6 on human breast cancer cell adhesion, migration, and invasion. Exp Mol Pathol 86:95–100. doi:10.1016/j.yexmp.2008.12.003
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 100:3983–3988. doi:10.1073/pnas.05302911000530291100
Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B (1990) CD44 is the principal cell surface receptor for hyaluronate. Cell 61:1303–1313. doi:10.1016/0092-8674(90)90694-A
Bajorath J (2000) Molecular organization, structural features, and ligand binding characteristics of CD44, a highly variable cell surface glycoprotein with multiple functions. Proteins 39:103–111. doi:10.1002/(SICI)1097-0134(20000501)39:2<103:AID-PROT1>3.0.CO;2-G
Chen J, Li T, Liu Q, Jiao H, Yang W, Liu X, Huo Z (2014a) Clinical and prognostic significance of HIF-1alpha, PTEN, CD44v6, and survivin for gastric cancer: a meta-analysis. PLoS One 9:e91842. doi:10.1371/journal.pone.0091842PONE-D-13-38378
Chen Y, Fu Z, Xu S, Xu Y, Xu P (2014b) The prognostic value of CD44 expression in gastric cancer: a metaanalysis. Biomed Pharmacother 68:693–697. doi:10.1016/j.biopha.2014.08.001S0753-3322(14)00095-X
Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ (2005) Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 65:10946–10951. doi:10.1158/0008-5472.CAN-05-2018
Correia M, Machado JC, Ristimaki A (2009) Basic aspects of gastric cancer. Helicobacter 14(Suppl 1):36–40. doi:10.1111/j.1523-5378.2009.00696.xHEL696
Dalerba P et al (2007) Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A 104:10158–10163. doi:10.1073/pnas.0703478104
Ferro A et al (2014) Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer 50:1330–1344
Kim MC, Lee HJ, Lim B, Ha KT, Kim SY, So I, Kim BJ (2014) Quercetin induces apoptosis by inhibiting MAPKs and TRPM7 channels in AGS cells. Int J Mol Med 33:1657–1663. doi:10.3892/ijmm.2014.1704
Li C et al (2007a) Identification of pancreatic cancer stem cells. Cancer Res 67:1030–1037. doi:10.1158/0008-5472.CAN-06-2030
Li F, Tiede B, Massague J, Kang Y (2007b) Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res 17:3–14. doi:10.1038/sj.cr.7310118
Lozano R et al (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. doi:10.1016/S0140-6736(12)61728-0S0140-6736(12)61728-0
Marhaba R, Bourouba M, Zoller M (2005) CD44v6 promotes proliferation by persisting activation of MAP kinases. Cell Signal 17:961–973. doi:10.1016/j.cellsig.2004.11.017
Mielgo A, van Driel M, Bloem A, Landmann L, Gunthert U (2006) A novel antiapoptotic mechanism based on interference of Fas signaling by CD44 variant isoforms. Cell Death Differ 13:465–477. doi:10.1038/sj.cdd.4401763
Mikami T, Saegusa M, Mitomi H, Yanagisawa N, Ichinoe M, Okayasu I (2001) Significant correlations of Ecadherin, catenin, and CD44 variant form expression with carcinoma cell differentiation and prognosis of extrahepatic bile duct carcinomas. Am J Clin Pathol 116:369–376. doi:10.1309/VV6D-3GAH-VEJM-DUJT
Nagano O et al (2004) Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca(2+) influx and PKC activation. J Cell Biol 165:893–902. doi:10.1083/jcb.200310024jcb.200310024
Orian-Rousseau V (2010) CD44, a therapeutic target for metastasising tumours. Eur J Cancer 46:1271–1277. doi:10.1016/j.ejca.2010.02.024
Orian-Rousseau V, Ponta H (2015) Perspectives of CD44 targeting therapies. Arch Toxicol 89:3–14. doi:10.1007/s00204-014-1424-2
Schwitalla S (2014) Tumor cell plasticity: the challenge to catch a moving target. J Gastroenterol 49:618–627. doi:10.1007/s00535-014-0943-1
Takaishi S et al (2009) Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 27:1006–1020. doi:10.1002/stem.30
Vigetti D et al (2008) Hyaluronan-CD44-ERK1/2 regulate human aortic smooth muscle cell motility during aging. J Biol Chem 283:4448–4458. doi:10.1074/jbc.M709051200
Xin Y, Grace A, Gallagher MM, Curran BT, Leader MB, Kay EW (2001) CD44V6 in gastric carcinoma: a marker of tumor progression. Appl Immunohistochem Mol Morphol 9:138–142
Yasui W, Kudo Y, Naka K, Fujimoto J, Ue T, Yokozaki H, Tahara E (1998) Expression of CD44 containing variant exon 9 (CD44v9) in gastric adenomas and adenocarcinomas: relation to the proliferation and progression. Int J Oncol 12:1253–1258
Zlobec I, Gunthert U, Tornillo L, Iezzi G, Baumhoer D, Terracciano L, Lugli A (2009) Systematic assessment of the prognostic impact of membranous CD44v6 protein expression in colorectal cancer. Histopathology 55:564–575. doi:10.1111/j.1365-2559.2009.03421.x
Acknowledgments
This work was supported by the National Key Clinical Specialty Discipline Construction Program of China ([2012] 649), National Natural Science Foundation of China for Young Scholar (31301172) and Emergency Management (81441123), Key Project of Science and Technology Plan of Fujian Province (2014Y0025) and the natural science foundation of Fujian Province (2014J01122 and 13141078).
Conflict of interest
The authors claim no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xie, JW., Chen, PC., Zheng, CH. et al. Evaluation of the prognostic value and functional roles of CD44v6 in gastric cancer. J Cancer Res Clin Oncol 141, 1809–1817 (2015). https://doi.org/10.1007/s00432-015-1964-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-1964-8