Abstract
Background
Gastric cancer (GC) is one of the most common malignancies in China. B-cell translocation gene 3 (BTG3) has been identified as a tumor suppressor in several tumors, but its role in GC remains unknown. This study aimed to detect the expression of BTG3 and its prognostic value in GC tissues and determine its function in the progression of GC.
Methodology
The expression of BTG3 was detected in GC cell lines and tissues by real-time RT-PCR, Western blot or immunohistochemistry. A series of in vitro and in vivo assays were performed to evaluate the effect of BTG3 on proliferation, migration and invasion of GC cells.
Results
B-cell translocation gene 3 was obviously down-regulated in GC tissues. Its expression was positively correlated with distant metastasis (P < 0.05). Patients with lower BTG3 expression had shorter overall survival time (P = 0.015). BTG3 suppressed the proliferation of GC cells in vitro and in vivo. It also inhibited migration and invasion of GC cells in vitro.
Conclusion
Down-regulation of BTG3 is closely associated with proliferation, migration and invasion in GC. It may be a novel prognostic biomarker for GC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is one of the most common malignancies in the world (Fang et al. 2012; Catalano et al. 2009). GC is a multistep process involving progressive disruption of epithelial-cell proliferation, apoptosis, differentiation and survival mechanisms (Cheng and Fan 2013; Wadhwa et al. 2013). Early GC patients have more benefits from improvements in clinical diagnosis and treatment and have a longer-term survival rate than late GC patients. However, the prognosis for patients with GC is still poor. Traditional methods cannot precisely predict the prognosis for the patients after surgical removal of the primary tumor, so the biomarkers capable of distinguishing GC patients with poor or good prognosis are acute needed.
B-cell translocation gene 3 (BTG3) belongs to an anti-proliferative B-cell translocation gene/Transducer of ErbB2 (BTG/Tob) gene family, which also includes BTG1, BTG2/TIS21/PC3, Tob, Tob2 and PC3b in human cells (Matsuda et al. 2001). These proteins all contain two short conserved domains in their N-terminal part (box A and box B), separated by a spacer sequence of 20–25 nonconserved amino acids (Matsuda et al. 2001; Guéhenneux et al. 1997; Winkler 2010). Several membranes of this family not only inhibit cellular proliferation and differentiation, but also regulate the tumorigenic progression (Lim 2006). For example, over-expression of BTG4 suppresses colony formation in colorectal cancer cells but its expression is frequently down-regulated in primary GC (Toyota et al. 2008; Dong et al. 2009). PC3/BTG2 is highly expressed in GC cells, and its expression is related to the degree of cell differentiation (Zhang et al. 2009). TOB plays an important role in the suppression of breast cancer tumorigenesis (O’Malley et al. 2009).
Recent evidence demonstrates that BTG3 plays as a tumor suppressor in cancer progression. The expression of BTG3 is down-regulated in lung adenocarcinoma, oral squamous cell cancer or prostate cancer (Yoneda et al. 2009; Yamamoto et al. 2001; Lin et al. 2012). Aberrant epigenetic regulation of BTG3 promoter, such as by DNA hypermethylation and/or histone modification, is observed in several human cancers (Majid et al. 2009, 2010; Yu et al. 2008; Putnik et al. 2012). Until now, only two papers have discussed the function of BTG3 in tumor (Lin et al. 2012; Ou et al. 2007). BTG3 is a downstream target of p53 and also binds and inhibits E2F1. It connects functionally those two major growth-regulatory pathways (Ou et al. 2007). BTG3 triggers acute cellular senescence via the ERK-JMJD3-p16 signaling axis (Lin et al. 2012). However, the expression pattern and function of BTG3 in GC remain unknown.
In this study, we detected the expression of BTG3 in GC clinical samples and determined its prognostic value. Then, we examined the effect of BTG3 on GC cell proliferation, migration and invasion in vitro and in vivo.
Materials and methods
Construction of plasmids and transfection
For depletion of BTG3, the lentivirus vectors carrying two human shRNA1 or shRNA2 toward BTG3 (Genechen, Shanghai, China) were transfected into lentiviral packaging cell lines 293T cells. The sequences were shRNA1 (sense, 5′-GGU GAA GGA CUU CCU GACA dTdT-3′) and shRNA2 (sense, 5′-GGG AUA GGC UAA GGA GUAA dTdT-3′) (Invitrogen, Foster City, CA, USA). A scramble shRNA (5′-AAT CGC ATA GCG TAT GCC GTT-3′), which has no homology with the mammalian mRNA sequences, was inserted into empty lentivirus vector as described above. Then, 1 mL of viral supernatant containing 4Ag of polybrene was added into GC cell lines for stable transduction. After 14 days, puromycin-resistant cell pools were established. After 72 h, the protein level of BTG3 was detected by Western blot. For over-expression BTG3, the fragment of human BTG3 was amplified from human cDNA by PCR using the primers (5′-AAA GGT ACC ATG AAG AAT GAA ATT GCT GCC GTTG and ACT CGA GTT AGT GAG GTG CTA ACA TGT GAG GA-3′). The fragment was digested with Kpn1 and Xho1 and cloned into the Kpn1–Xho1 site of the pEGFP-C1 expression vector.
Cell proliferation assay
1 × 103 cells were seeded into 96-well plates. The number of viable cells was determined by cell counting kit-8 (CCK-8) (Dojindo, Kumamoto, Japan) for 6 days. Briefly, 10 mL CCK-8 solution was added, and absorbance at 490 nm was measured after 2 h of incubation at 37 °C. Each cell group was plated in three duplicate wells.
Cell-cycle analysis
About 1 × 106 cells were trypsinized, washed twice with PBS, and fixed in 70 % ice-cold ethanol for 1 h. The samples were then centrifuged by removing the ethanol and exposing to 100 mg/mL RNaseA (Sigma, USA) for 30 min at 37 °C. Cellular DNA was stained with propidium iodide (PI). Cell-cycle distributions were determined using flow cytometry.
In vitro apoptosis assay
Cells were all transfected with 50 nM of oligo using Lipofectamine2000 according to the instructions (Invitrogen, USA). Then, cells were harvested and stained with Annexin V-FITC and PI according to the manufacturer’s protocol (BioVision #K101–100). Annexin V-FITC/PI binding was analyzed by flow cytometry using a BD FACSCalibur system, and data were analyzed using the CellQuest software.
In vitro cell scratch assay
To quantify cell migration, a scratch was made down the center of each well in a 24-well plate using a p1000 pipette tip at 48 h after transfection. Along the scratch line, the cells were washed away and replaced with serum-free culture medium. And then, we took pictures every 12 h for 48 h. The distances between the parallel cell edges were measured at each time point. For each well, three different fields along the scratch were analyzed in triplicate. Cell motility was calculated as the percentage of the cell migration distance with respect to the initial scratch distance.
In vitro invasion assay
The invasion Boyden chambers (BD Biosciences, Foster city, USA) were rehydrated with RPMI 1640 (serum-free) for 2 h at 37 °C. RPMI 1640 with 100 ml/L fetal bovine serum was added to the lower compartment as the chemotactic factor. Then, 1.5 × 105 tumor cells in serum-free RPMI 1640 were added to the upper compartment of the chamber. Each cell group was plated in three duplicate wells. After incubation for 48 h, the noninvasive cells were removed with a cotton swab. Cells that had migrated through the membrane and stuck to the lower surface of the membrane were fixed with methanol and stained with hematoxylin. Finally, the cells in lower compartment of the chamber that had invaded the lower sides of the membrane were counted under a light microscope in five random visual fields (200×).
In vivo tumorigenesis assay
To evaluate in vivo tumor growth, 1 × 107 cells were injected subcutaneously into the left flank or right flank of nude mice (n = 5 per group). Tumors were measured with calipers to estimate volume from day 5 to day 28 after injection. BTG3 expression was detected in xenograft tumors by Western blotting. The proliferative index of Ki67 was evaluated in xenograft tumors by immunohistochemistry (IHC).
Materials of GC cell lines and clinical tissue specimens, IHC, real-time RT-PCR, Western blotting and statistical analysis were seen in Method S1.
Results
BTG3 is down-regulated in human GC tissues
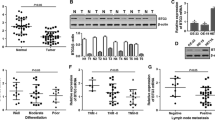
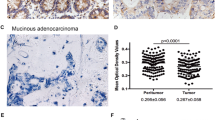
To explore the expression pattern of BTG3 and its clinicopathologic features in GC patients, we first detected BTG3 expression in 18 paired samples of patients by real-Time PCR and Western blot. We found that the mRNA and protein levels of BTG3 in GC tissues were down-regulated compared to adjacent normal gastric tissue (Fig. 1a–c, P = 0.031). After that, we analyzed BTG3 expression in 131 cases of paraffin-embedded archived GC tissues using IHC. Strong staining for BTG3 protein was frequently observed in adjacent gastric mucosa (Fig. 1d), especially in the cytoplasms of mucous glandular epithelia in cardiac or pyloric glands, parietal and chief cells in fundic glands (Fig. 1h, i). Interestingly, the positive signal of BTG3 was mainly observed in two-thirds of glands distributed in the lamina propria mucosa of the fundus, body or pylorus, but not in the superficial epithelia and the crypt (Fig. 1a, h, i). However, negative or very week BTG3 staining was observed in GC tissues and lymphatic metastatic cancer tissues (Fig. 1e, f, g). The expression of BTG3 was obviously lower in GC tissues than adjacent gastric mucosa (P < 0.001, Table S1). No obvious difference of BTG3 expression was observed between GC tissues with metastasis and those without metastasis (P = 0.908, Table S1). Clinicopathological analyses showed that BTG3 expression was correlated strongly with distant metastasis (P = 0.004, Table 1). These above results indicate that down-regulation of BTG3 may be associated with the progression of GC.
Down-regulation of BTG3 in GC tissues. a BTG3 expression in 18 paired human GC tissues and the corresponding normal mucosa by real-time PCR. Each bar represents the mean of three independent experiments. b Real-time PCR analysis of BTG3 expression in GC tissues compared to the corresponding normal mucosa. P = 0.031. c Western blot analyses of BTG3 expression in the same 8 paired GC tissues. d Strong positive expression of BTG3 in adjacent normal gastric mucosa epithelium. BTG3 was mainly localized in the cytoplasm of normal cells. e Week expression of BTG3 in GC tissues. f Loss of BTG3 in metastatic GC tissues in lymph nodes. g Strong positive expression of BTG3 in adjacent noncancerous areas and negative expression of BTG3 in GC tissues. h and i Strong positive expression of BTG3 in two-thirds of glands distributed in the lamina propria mucosa of the fundus, body or pylorus, but not in the superficial epithelia and the crypt. For all micrographs, scale bars represent 20 μm
Correlation between BTG3 expression and patients’ survival
The prognostic effect of BTG3 on GC patients’ overall survival was compared between patients with high and low BTG3 protein levels. By Kaplan–Meier curve assessment, patients with high BTG3 protein level had a better outcome than those with low BTG3 protein level (P = 0.001, Fig. 2a). From univariate analysis, the significant prognostic factors were BTG3 expression (P = 0.015), serosal invasion (P = 0.010) and remote metastasis (P = 0.001, Table 2). Multivariate analysis results showed that BTG3 expression (P = 0.040) and remote metastasis (P = 0.023) might play a role in predicting the overall survival in GC patients (Table 2). These results demonstrate that BTG3 expression is an independent prognostic marker for survival of GC patients.
Survival analysis of BTG3 expression in GC patients and expression of BTG3 in GC cell lines. a Kaplan–Meier survival analysis of primary GC patients with high and low BTG3 expressions, P = 0.001. b Real-time RT-PCR analysis of BTG3 in five GC cell lines. c Western blotting analysis of BTG3 in five cell lines. Real-time RT-PCR d and Western blotting e analyses of BTG3 expression in BTG3-depleting AGS and BGC823 cells and BTG3 over-expressing MGC803 and SGC7901 cells. Error bars represent mean ± SD from three independent experiments. *P < 0.05
Effect of BTG3 on the proliferation of GC cells in vitro and in vivo
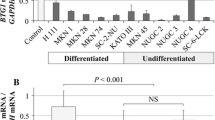
To explore the role of BTG3 in the progression of GC, we first detected the endogenous expression of BTG3 in five GC cell lines by real-time PCR and Western blotting. Among five GC cell lines, BTG3 expression was highest in AGS cells and gradually decreased in BGC823, MGC803, MNK45 and SGC7901 cells (Fig. 2b). In addition, the level of BTG3 protein expression in each cell line by Western blot coincided precisely with that of the mRNA level (Fig. 2c). Next, we performed loss of function and gain of function to examine the effect of BTG3 on the proliferation of GC cells. We chose AGS and BGC823 cell lines for stable transfection with shRNA lentivirus vectors toward BTG3, and SGC7901 and MGC803 cell lines for transient transfection with BTG-expressing vector. High transfection efficiency was validated by real-time PCR or Western blot (Fig. 2d, e). Results of CCK8 assay showed that BTG3 depletion increased the proliferative abilities of AGS and BGC823 cell lines (P < 0.05, Fig. 3a), while forced expression of BTG3 caused a significant decrease of the proliferation rate in SGC7901 and MGC803 cell lines (P < 0.05, Fig. 3b). Furthermore, SGC7901 and MGC803 cells treated with forced BTG3 showed a significant increase in the percentage of cells in the G1/G0 peak and a decrease in the percentage of cells in the S and G2/M peak (P < 0.05, Fig. 3c). However, AGS and BGC823 cells treated with BTG3 shRNA showed the opposite effect (P < 0.05, Fig. 3d). These results suggest that BTG3 inhibits GC cell proliferation by eliciting G1/G0 phase arrest. Compared to control cells, SGC7901 cells over-expressing BTG3 showed increased rate of apoptosis (P < 0.05, Fig. 3e), while the rate of apoptosis was remarkably decreased when BTG3 was depleted in AGS cells (P < 0.05, Fig. 3f). We also implanted BTG3-depleting cells and control cells subcutaneously into nude mice, respectively, and then monitored the growth of the resultant primary tumors. Tumors in mice injected with BTG3-depleting cells grew more rapidly than those with control cells (Fig. 3g). BTG3 was down-regulated in BTG3-depleting subcutaneous tumors (Fig. 3h). IHC staining confirmed that the tumors of BTG3-depleting group displayed much higher Ki67 index than those of control group (Fig. 3i). All the above results demonstrate that BTG3 suppresses GC cell proliferation by down-regulating cell-cycle progression or accelerating apoptosis.
Effect of BTG3 on the proliferation of GC cells in vitro and in vivo. a Effect of BTG3 knockdown on cell proliferation in vitro by MTT assay. b Effect of ectopic BTG3 on cell proliferation in vitro by MTT assay. c Representative histograms depicting cell-cycle profiles of BTG-expressing MGC803 and SGC7901 cells. Cells were stained with PI and analyzed by flow cytometry. d Representative histograms depicting cell-cycle profiles of BTG3-depleting AGS and BGC823 cells. e Percent of apoptotic cells of transfected with BTG-expressing SGC7901 cells. f Percent of apoptotic cells of transfected with BTG3-depleting AGS cells. g Subcutaneous tumors of mice injected with stable BGC823/NC and BGC823/ShRNA1 cells. Histopathological analyses of subcutaneous tumors injected with stable BGC823/ShRNA1 cells or control cells. h Western blotting analysis of BTG3 expression in subcutaneous tumors of mice injected with stable BGC823/ShRNA1 cells or control cells. i The tumor sections were stained with H&E or subjected to IHC staining using an antibody against Ki-67. Error bars represent mean ± SD from three independent experiments. Scale bars 20 μm. *P < 0.05
Effect of BTG3 on migration and invasion of GC cells in vitro
We assessed the effect of BTG3 on cell migration and invasion in vitro. Results of Boyden Chamber assay showed that BTG3-depleting cells displayed a marked increase of invasive ability in AGS and BGC823 cell lines (P < 0.05, Fig. 4a, c), while forced expression of BTG3 showed the opposite effect (P < 0.05, Fig. 4b, d). Cell scratch assays showed that BTG3-depleting AGS and BGC823 cells displayed a marked increase of migration ability (P < 0.05, Fig. 4e, g). However, forced expression of BTG3 markedly blocked the migration abilities of SGC7901 cells (P < 0.05, Fig. 4f, h). The results make it evident that BTG3 suppresses migration and invasion of GC cells.
Effect of BTG3 on the migration and invasion of GC cells in vitro. a, c Effect of BTG3 knockdown on cell invasion in vitro. Morphological comparison of cells penetrating the artificial basement membrane was also shown. b, d Effect of ectopic BTG3 on cell invasion in vitro. Morphological comparison of cells penetrating the artificial basement membrane was also shown. e and f Image examples of migrated cells. The dotted straight lines indicate the dimensions of the scratch. The solid irregular lines indicate the cell edges. Effect of down-regulated BTG3 or ectopic BTG3 on cell migration in vitro by cell scratch assay. g, h Quantitative measurements of the cell motility. After the scratch was made, cell motility was evaluated after 24 h. Error bars represent mean ± SD from three independent experiments. *P < 0.05
Discussion
Using BTG1 and BTG2/TIS21 probes, BTG3 was isolated by low-stringency screening of a cDNA library (Guéhenneux et al. 1997). As follows, its physiological functions began to be discovered, such as neuron phylogenesis, the control of muscle cell development and bone formation (Putnik et al. 2012), especially, for the control of cell cycle (Winkler 2010; Lim 2006; Yamamoto et al. 2001; Lin et al. 2012). Recently, several studies have shown that BTG3 was associated with tumorigenesis. It has been reported as a tumor suppressor in several tumor types (Majid et al. 2009, 2010; Yu et al. 2008; Lv et al. 2013; Chen et al. 2013). However, the expression pattern and function of BTG3 in the progression of GC remain elusive.
In this study, we detected the expression of BTG3 in GC tissues and then explored the clinical prognostic values of BTG3 by using complete long-term follow-up data of a large cohort of GC samples. Our results clearly showed that BTG3 expression was significantly down-regulated in GC tissues or lymphatic metastatic tissues. Aberrant epigenetic regulation of BTG3 promoter, such as by DNA hypermethylation and/or histone modification, may represent a key mechanism of down-regulation of BTG3 in renal cancer, prostate cancer and several human cancers (Majid et al. 2009, 2010; Yu et al. 2008; Lv et al. 2013). Moreover, BTG3 expression was correlated strongly with distant metastasis, suggesting that BTG3 may be associated with the progression of GC. Kaplan–Meier survival analysis revealed that patients with low BTG3 protein level had a significantly lower 5-year survival rate. BTG3 is also regarded as a independent predictor of prognosis for ovarian cancer patients (Deng et al. 2013). BTG3 expression, serosal invasion and distant metastasis were associated with an increased risk of death from GC. Several clinicopathological parameters, such as serosal invasion and metastasis, have been found to contribute to poor prognosis of GC patients (Yasuda et al. 2007; Junfeng et al. 2013). Thus, our data clearly demonstrate that the decreased expression of BTG3 is associated with the progression of GC and an independent prognostic marker for survival of GC patients.
Having established the expression pattern of BTG3 in GC, we next investigated the function of BTG3 in the progression of GC. Our results showed that BTG3 inhibited GC cell proliferation by down-regulating cell-cycle progression or accelerating apoptosis. BTG3 has been reported to be involved in negative control of the cell cycle (Guéhenneux et al. 1997). One possible mechanism for BTG3-induced cell-cycle arrest is that BTG3 is a transcriptional target of p53 that inhibits E2F1 (Ou et al. 2007). Our data also indicate that the suppression of BTG3 on GC cell proliferation may be associated with apoptosis. In addition, we demonstrated that BTG3 obviously decreased the migratory and invasive abilities of GC cells in vitro. Decreased expression of BTG3 is linked to carcinogenesis and aggressiveness of ovarian cancer and hepatocellular cancer (Lv et al. 2013; Deng et al. 2013). Therefore, the above results provide evidence that BTG3 can function as a suppressor in the progression of GC. The molecular mechanisms of BTG3 in GC progression need to be further investigated.
In summary, our study demonstrates that down-regulation of BTG3 in GC tissues correlates with poor survival of patients. All of the functional experiments confirm BTG3 as a tumor suppressor in the progression of GC. In combination with other biomarkers of GC, BTG3 expression status may be useful to stratify patients for novel therapeutic strategies, such as those of adjuvant chemotherapy, radiosensitization, or the establishment of rational treatment selection criteria for patients with this disease.
References
Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F et al (2009) Gastric cancer. Crit Rev Oncol Hematol 71:127–164
Chen X, Chen G, Cao X, Zhou Y, Yang T et al (2013) Downregulation of BTG3 in non-small cell lung cancer. Biochem Biophys Res Commun 437:173–178
Cheng J, Fan XM (2013) Role of cyclooxygenase-2 in gastric cancer development and progression. World J Gastroenterol 19:7361–7368
Deng B, Zhao Y, Gou W, Chen S, Mao X et al (2013) Decreased expression of BTG3 was linked to carcinogenesis, aggressiveness, and prognosis of ovarian carcinoma. Tumour Biol 34:2617–2624
Dong W, Tu S, Xie J, Sun P, Wu Y et al (2009) Frequent promoter hypermethylation and transcriptional downregulation of BTG4 gene in gastric cancer. Biochem Biophys Res Commun 387:132–138
Fang CL, Hseu YC, Lin YF, Hung ST, Tai C et al (2012) Clinical and prognostic association of transcription factor SOX4 in gastric cancer. PLoS One 7:e52804
Guéhenneux F, Duret L, Callanan MB, Bouhas R, Hayette S et al (1997) Cloning of the mouse BTG3 gene and definition of a new gene family (the BTG family) involved in the negative control of the cell cycle. Leukemia 11:370–375
Junfeng Z, Yingxue H, Peiwu Y (2013) Systematic review of risk factors for metastasis to para-aortic lymph nodes in gastric cancer. Surg Oncol 22:210–216
Lim IK (2006) TIS21 (/BTG2/PC3) as a link between ageing and cancer: cell cycle regulator and endogenous cell death molecule. J Cancer Res Clin Oncol 132:417–426
Lin TY, Cheng YC, Yang HC, Lin WC, Wang CC et al (2012) Loss of the candidate tumor suppressor BTG3 triggers acute cellular senescence via the ERK-JMJD3-p16(INK4a) signaling axis. Oncogene 31:3287–3297
Lv Z, Zou H, Peng K, Wang J, Ding Y et al (2013) The suppressive role and aberrant promoter methylation of BTG3 in the progression of hepatocellular carcinoma. PLoS One 8:e77473
Majid S, Dar AA, Ahmad AE, Hirata H, Kawakami K et al (2009) BTG3 tumor suppressor gene promoter demethylation, histone modification and cell cycle arrest by genistein in renal cancer. Carcinogenesis 30:662–670
Majid S, Dar AA, Shahryari V, Hirata H, Ahmad A et al (2010) Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-Cell translocation gene 3 in prostate cancer. Cancer 116:66–76
Matsuda S, Rouault J, Magaud J, Berthet C (2001) In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS Lett 497:67–72
O’Malley S, Su H, Zhang T, Ng C, Ge H et al (2009) TOB suppresses breast cancer tumorigenesis. Int J Cancer 125:1805–1813
Ou YH, Chung PH, Hsu FF, Sun TP, Chang WY et al (2007) The candidate tumor suppressor BTG3 is a transcriptional target of p53 that inhibits E2F1. EMBO J 26:3968–3980
Putnik M, Zhao C, Gustafsson JÅ, Dahlman-Wright K (2012) Global identification of genes regulated by estrogen signaling and demethylation in MCF-7 breast cancer cells. Biochem Biophys Res Commun 14:26–32
Toyota M, Suzuki H, Sasaki Y, Maruyama R, Imai K et al (2008) Epigenetic silencing of microRNA-34b/c and B-cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer Res 68:4123–4132
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q et al (2013) Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol 10:643–655
Winkler GS (2010) The mammalian anti-proliferative BTG/Tob protein family. J Cell Physiol 222:66–72
Yamamoto N, Uzawa K, Yakushiji T, Shibahara T, Noma H et al (2001) Analysis of the ANA gene as a candidate for the chromosome 21q oral cancer susceptibility locus. Br J Cancer 84:754–759
Yasuda K, Shiraishi N, Inomata M, Shiroshita H, Izumi K et al (2007) Prognostic significance of macroscopic serosal invasion in advanced gastric cancer. Hepatogastroenterology 54:2028–2031
Yoneda M, Suzuki T, Nakamura T, Ajima R, Yoshida Y et al (2009) Deficiency of antiproliferative family protein Ana correlates with development of lung adenocarcinoma. Cancer Sci 100:225–232
Yu J, Zhang Y, Qi Z, Kurtycz D, Vacano G et al (2008) Methylation-mediated downregulation of the B-cell translocation gene 3 (BTG3) in breast cancer cells. Gene Expr 14:173–182
Zhang ZM, Wang G, Chen C, Yang ZX, Jin F et al (2009) Rapid induction of PC3/BTG2 gene by hepatopoietin or partial hepatectomy and its mRNA expression in hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 8:288–293
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81272759, 81172382); Natural Science Foundation of Guangdong Province (S2012010009669, S2013010014544); Research Fund for the Science and technology Star of Zhujiang City of Guangzhou Province (2011J2200074). We thank for Professor Reddy for editing the English writing.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
X. L. Ren and X. H. Zhu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ren, X.L., Zhu, X.H., Li, X.M. et al. Down-regulation of BTG3 promotes cell proliferation, migration and invasion and predicts survival in gastric cancer. J Cancer Res Clin Oncol 141, 397–405 (2015). https://doi.org/10.1007/s00432-014-1826-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1826-9