Abstract
Purpose
Epithelial mesenchymal transition is a major mechanism to explain metastatic events in breast cancer. Another important aspect is that cells with stem cell properties are able to become resistant against chemotherapeutics. Our main goal was to investigate the role of the EMT marker, N-cadherin, and of the stem cell marker, CD133, in breast cancer.
Methods
The expressions of N-cadherin and CD133 were assessed by immunohistochemistry in 307 primary tumors from breast cancer patients and for 30 patients, in the related recurrences and/or metastases. We studied the correlation between both markers, their associations with known clinicopathological parameters and their role as predictive markers for survival time. Different expressions of both markers in primary tumor and recurrences or metastases were examined.
Results
N-cadherin and CD133 expressions correlated positively in the 261 primary tumor samples (p = 0.000) and in the 45 primary tumor, recurrence or metastasis samples (p = 0.010). In patients without lymph node metastases, the 10-year survival time was significantly lower when the tumor was N-cadherin-positive (p = 0.042). Expression of N-cadherin was also significantly higher in metastases than in the related primary tumors (p = 0.039).
Conclusion
N-cadherin and CD133 expressions are strongly correlated and N-cadherin appears as a potential metastases marker in a specific patient subpopulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most frequent cancer and cause of death for women worldwide (Jemal et al. 2008; Levi et al. 2005). In order to characterize the primary tumor, estrogen receptor α (ERα), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expressions are evaluated routinely at time of diagnosis or surgery. These biomarkers have prognostic and predictive relevance for individualized therapy regimes (Patani et al. 2013). But still, patients suffering from recurrences or distant metastases have a worse prognosis and a lower benefit from the known therapies (Comen 2012).
Mechanisms leading to metastases are still poorly understood. One of the events, which appears to play an important role in the development of metastases is the epithelial to mesenchymal transition (EMT), initially described for its physiological function in embryogenesis (Groger et al. 2012; Hay 1995). EMT also allows cancer cells to lose their epithelial properties and acquire mesenchymal features. They can then escape the initial cell association in the primary tumor and invade into blood vessels or into the lymphatic system, to reach distant sites in the body. There, cells are able to reactivate their epithelial properties (mesenchymal to epithelial transition or MET) to form metastases (Kalluri and Weinberg 2009).
Another major theory to explain the metastatic potential of tumor cells is that some cancer cells may have intrinsic stem cell properties, meaning that they have the ability for self-renewal, differentiation, tumor initiation and driving tumor progression. Resistance to chemotherapy may also have its origin in stem cell features (Dave et al. 2012; Schott et al. 2013).
The interplay between the two mechanisms of EMT and stem cell features has to be further evaluated (Mani et al. 2008; May et al. 2011). Many new markers may be relevant for the outcome of patients, combining the evaluation of these two mechanisms. Today, major EMT markers are N-cadherin, vimentin or fibronectin, among others (Armstrong et al. 2011; ElMoneim and Zaghloul 2011; Jeong et al. 2012; Park and Schwarzbauer 2013) and regarding cancer stem cells (CSCs), relevant markers are CD44+/CD24low, ALDH-1 or CD133 as a more recent marker (Takebe et al. 2011). In colon cancer, a connection has been demonstrated between mesenchymal and stem cell properties, which results in a highly malignant and even drug resistant phenotype of the tumor (Singh et al. 2013). In pancreatic cancer, significant correlations were observed between EMT markers and stem cell markers (Zhang et al. 2012). Also in circulating tumor cells of primary breast cancer patients, simultaneous expression of EMT and stem cell markers was described (Kasimir-Bauer et al. 2012). Comparatively, circulating tumors cells from castration-resistant prostate cancer and metastatic breast cancer patients more frequently over-expressed both groups of markers, especially the EMT marker, N-cadherin, and the stem cell marker CD133 [(Aktas et al. 2009; Armstrong et al. 2011) and unpublished data in breast cancer patients].
Cadherins are calcium-dependent glycoproteins, responsible for calcium-dependent cell–cell-adhesions, with an intracellular domain that allows the association with catenins and thereby with the cytoskeleton (Peinado et al. 2004; Saito et al. 2012). N-cadherin (neuronal Cadherin or Cadherin-2), is a subtype that plays an important role in gastrulation, neurulation and cardiogenesis, while in adults N-cadherin is almost only found in neural tissue and cells of mesenchymal origin (Radice et al. 1997; Stemmler 2008). Today, N-cadherin is a well-known marker for EMT as the “Cadherin switch” describes the reciprocal downregulation of E-Cadherin (epithelial) and upregulation of N-cadherin (mesenchymal) that characterizes EMT. Literature shows that N-cadherin-positive cancer cells are able to establish cell–cell-adhesion with N-cadherin-expressing cells of the stroma, promoting invasion (Christofori 2006). N-cadherin also activates Fibroblast Growth Factor Receptors (FGFRs) with downstream activation of other signaling pathways (e.g., PI(3)K), supporting cell survival, migration and invasion. Moreover, matrix metalloproteinases (MMPs) and γ-secretase-like proteases can trigger activation signals by cleaving the extracellular domain or the carboxy-terminal fragment of N-cadherin, respectively. All these features support the involvement of N-cadherin expression in tumor progression and N-cadherin is recognized as both a marker for mesenchymal cell properties (Armstrong et al. 2011; Jeong et al. 2012; Stoyianni et al. 2012) and a prognostic marker for breast cancer patients (ElMoneim and Zaghloul 2011).
CD133 (AC133 or Prominin-1) is also a transmembraneous glycoprotein, mainly localized in cellular protrusions. Its physiological functions are still unknown except that it is involved in embryogenesis by activation of the Wnt-signaling pathway resulting in an enhanced transcription activity and consequently in intensified cell proliferation (Mizrak et al. 2008). CD133 is a potential upcoming marker for stem cells, and CD133-positive cancer cells are called CSCs. Their role in cancer evolution has still to be elucidated further. Some studies already investigated the role of CD133 in breast cancer as a potential predictive marker for aggressiveness and treatment optimization (Croker et al. 2009; Ieni et al. 2011; Liu et al. 2009) and its correlation with prognosis in triple-negative breast cancer (Zhao et al. 2011). Moreover, CD133-positive tumor cells in breast cancer patients exhibit resistance to chemotherapy (Aomatsu et al. 2012; Nadal et al. 2013). This is why new targeting for anticancer therapy is required, potentially against CD133 as a marker for CSCs (May et al. 2011; Swaminathan et al. 2013; Takebe et al. 2011).
In this work, we investigated the expression of N-cadherin and/or of CD133 in 307 primary breast cancer tissues, comparing their expression in primary tumors, related recurrences and/or metastases (30 cases), and their correlation to survival and metastasis data.
Materials and methods
Characteristics of the patients
A total of 307 paraffin-embedded primary tumor tissue samples from breast cancer patients were randomly included in this study. For 30 patients, 30 primary tumors, 25 recurrences and 9 metastatic tissue samples were available for comparison of the expressions. The study has been approved by the Ethical Committee of the Ludwig Maximilians University of Munich, Germany (LMU, ethical number 048-08) and has been carried out in concordance with the guidelines of the Helsinki Declaration. Written informed consent was obtained from the patients.
The tissues we used for our investigations were sampled between 2000 and 2002 from patients of the Department of Obstetrics and Gynaecology of the LMU. The women’s average age was 68.66 years (range from 38 to 98). All the tumors were classified by the tumor-node-metastasis classification (TNM classification). Histology was determined by an experienced pathologist (Doris Mayr) of the Department of Pathology of the LMU, according to a modification of Elston and Ellis grading proposed by Bloom and Richardson (Elston and Ellis 1993). Hormone receptor status (ERα and PR) was evaluated by immunohistochemistry, where a nucleic staining of more than 10 % of the tumor cells was considered as hormone-receptor-positive tumor. HER2 expression was analyzed and evaluated also in the Department of Pathology with an automated staining system (Ventana; Roche, Mannheim, Germany). The detailed characteristics of the patients are shown in Table 1.
Immunohistochemistry
Paraffin-embedded tissue was used for preparation of 3 µm sections. For staining procedure, tissue sections were dewaxed in xylol (Carl Roth GmbH & Co. KG, Karlsruhe, Germany) for 15 min at room temperature. For reducing endogenous peroxidase reaction, sections were immersed in a solution of 3 % hydrogen peroxide (VWR International S.A.S., Fontenay-sous-Bois, France) in methanol (Sigma-Aldrich, Steinheim, Germany) for 20 min. After rehydrating in decreasing concentrations of ethanol (100–0 %) in distilled water, slides were boiled for 5 min in a pressure cooker with sodium citrate buffer (pH 6) for epitope retrieval. Then, the sections were washed with distilled water and phosphate buffered saline (PBS), before blocking with Powerblock (Biogenex, San Ramon, CA, USA) in distilled water (1:10) for 3 min. Tissue sections were then incubated with either a polyclonal rabbit antibody against N-cadherin (Abcam, Cambridge, UK) or with a monoclonal mouse antibody against CD133 (clone 3F10; Thermo Scientific, Waltham, MA, USA) for 1 h. After incubation with a biotinylated secondary anti-rabbit or anti-mouse IgG antibody, respectively, and with the associated avidin–biotin-peroxidase-complex (both Vectastain Elite ABC Kit; Vector Laboratories, Burlingame, CA, USA), visualization was performed with substrate and chromogen 3,3′-diamino-benzidine (DAB; Dako, Glostrup, Denmark). Sections were counterstained with acidic hematoxylin and afterward dehydrated in increasing concentrations of ethanol (70–100 %). The slides were immediately mounted with Eukitt (Merck, Darmstadt, Germany) before manual analysis with a Diaplan light microscope (Leitz, Wetzlar, Germany) with 2.5×, 10× or 40× magnifications. Pictures were obtained with a digital CCD camera system (JVC, Tokio, Japan). Positive controls were carried out with heart muscle tissue for N-cadherin and kidney tissue for CD133. Negative controls were performed by replacement of the primary antibodies by species-specific isotype control antibodies (Dako, Glostrup, Denmark).
Data analysis
N-cadherin or CD133 status was classified by evaluation of the percentage of brown stained tumor cell membranes and staining intensity, allowing assessment of an immunoreactive score (IRS = percentage score × intensity score). For the percentage score of stained tumor cells, we considered no staining (score = 0), <10 % stained (score = 1), 10–50 % stained (score = 2), 51–80 % stained (score = 3) and 81–100 % stained (score = 4). Staining intensity was evaluated as no staining (score = 0), weak staining (score = 1), moderate staining (score = 2) and strong staining (score = 3). For both markers, IRS of 0 or 1 was considered as negative, 2–4 as low expression, 6–8 as moderate expression and 9–12 as high expression. In doubtful cases, slides were evaluated by two or three independent examiners (CB, UJ, CK) with consent at the end.
Statistical analysis
Statistical analyses were performed using SPSS 21 (IBM SPSS Statistics, IBM Corp., Armonk, NY, USA). Correlation analysis was performed by calculating the Spearman’s–Rho correlation coefficient or a chi-square test. To visualize the correlation, a scatter blot was performed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). For the comparison of survival times, Kaplan–Meier curves were generated. The chi-square statistic of the log-rank test was calculated to test differences between survival curves for significance. The Wilcoxon test was used to compare marker expressions in different tissues, depicted in a box plot. Multivariate analysis for prognostic value was performed using the Cox regression model. P values below 0.05 were considered statistically significant.
Results
Immunohistochemical staining
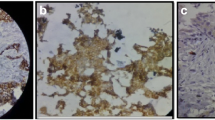
We first stained 307 primary breast cancer tissue samples. In Fig. 1, staining results are shown examplary for N-cadherin (a–c) and CD133 (d–f). Positive staining for both markers, with an IRS higher than 1, resulted in a membraneous and partially cytoplasmatic brown coloration, observed specifically in the tumor tissue. Pictures a and d show patients classified as not expressing N-cadherin or CD133, respectively, (IRS = 0). Patients with a high expression of N-cadherin or CD133 (IRS = 9 and 12) are also shown (b, c and e, f, respectively).
Table 2 presents the immunohistochemical analysis that resulted in 275 evaluable cases for N-cadherin, 278 for CD133 and 261 for both markers (in some cases, the quality and/or quantity of material did not allow a correct evaluation). 71.3 and 42.1 % of the evaluable tumors were positive for N-cadherin and CD133, respectively, with an IRS higher than 1. The two markers were co-expressed in 37.5 % of the tumors, with 50,0 % (98/196) of the N-cadherin-positive tumors were also positive for CD133, and 83,8 % (98/117) of the CD133-positive tumors were also positive for N-cadherin.
Additionally, 25 recurrences and 9 metastatic tissue samples were stained in parallel with the related primary tumors from 30 patients (Table 3). For these 30 primary tumors, 23 cases were evaluable for N-cadherin, 27 for CD133 and 21 for both. While again the majority of the primary tumor (69.6 %) were N-cadherin-positive, only 3.7 % were CD133-positive and 4.8 % were positive for both markers. For the recurrences and metastases, 47.4 and 100 % were, respectively, positive for N-cadherin, 5.6 and 33.3 % for CD133 and 6.3 and 37.5 % for both markers. It is noteworthy that all the CD133-positive primary tumors, recurrences and metastases (n = 1, 1 and 3) were N-cadherin-positive, whereas only 6.3, 11.1 and 37.5 %, respectively, of the N-cadherin-positive one (n = 16, 9 and 8) were CD133-positive, too.
Correlation between N-cadherin and CD133 expressions
We first analyzed any positive or negative correlation between N-cadherin and CD133 expressions for the 261 primary tumors that were stained for both markers. We performed a Spearman’s correlation test and could demonstrate a very high statistical significance for a positive correlation coefficient (p = 0.000), significant at the 0.01 level (2-tailed). Scatter blots showing the IRS values for N-cadherin or CD133 of each individual sample are presented in Fig. 2a, b, respectively. In the two Figures, samples expressing none or only one marker (n = 163, on the left) are compared to samples expressing both markers (n = 98, on the right).
a Scatter blot showing the IRS values for N-cadherin of each individual sample. Samples negative for both markers or positive for only one marker are shown on the left (n = 163). On the right, IRS values of N-cadherin are shown for all the samples that are positive for N-cadherin and CD133 (n = 98). b Scatter blot showing the IRS values for CD133 of each individual sample. Samples negative for both markers or positive for only one marker are shown on the left (n = 163). On the right, IRS values of CD133 are shown for all the samples that are positive for CD133 and N-cadherin (n = 98)
We performed the same test with the 30 patients, from whom the tissue samples of the primary tumor (n = 21), recurrence (n = 16) and/or metastasis (n = 8) were analyzed, with a total of 45 evaluable cases for both markers. N-cadherin and CD133 again correlated positively (p = 0.010), confirming the result obtained above on an almost 10 times smaller scale and with different tissue origins indicating the metastatic process.
Correlation of N-cadherin and/or CD133 with patient characteristics
In addition, we calculated correlations between N-cadherin and/or CD133 expressions and the clinicopathological characteristics of the patients (described in Table 1), such as hormone receptor status, HER2 status, grading or age. No chi-square correlation coefficient was found significant (Additional data 1).
Survival analysis
To determine the relevance of both markers’ expression for prognosis and survival time, we correlated the 10-year survival with the expression of N-cadherin and/or CD133. Considering the whole cohort, we found no statistically significant difference. Consequently, we performed various stratifications of the cohort, based on their clinicopathological characteristics. The stratification in patients having or not axillary lymph node metastases allowed to show that within the lymph node-negative patients strata, N-cadherin expressing patients had a significantly lower survival time than patients not expressing the EMT marker (p = 0.042). This result is presented in a Kaplan–Meier curve in Fig. 3a. Multivariate analysis for the lymph node-negative patients using the Cox regression model showed tumor staging to be the only independent prognostic marker for survival but not N-cadherin or CD133 (Additional data 2). For the lymph node-positive patients, N-cadherin expressions showed no statistical difference in the Kaplan–Meier curve (Fig. 3b). In total, we analyzed data of 150 lymph node-negative patients and 118 lymph node-positive patients, in addition we had 7 patients with unknown lymph node status. Consequently, our results strongly suggest that N-cadherin is a negative predictor for 10-year survival time in patients without lymph node metastases. Investigation of simultaneous N-cadherin and CD133 expression gave no indication for a better possibility to predict survival times or patient’s outcome (data not shown).
a Kaplan–Meier survival analysis of patients without lymph node metastases. For patients without lymph node metastases (n = 150), N-cadherin negatively correlates with the survival time, p = 0.042; N-cadherin-positive in black and N-cadherin-negative in gray. b Kaplan–Meier survival analysis of patients with lymph node metastases. For patients with lymph node metastases (n = 118), N-cadherin does not correlate with the survival time, p = 0.741; N-cadherin-positive in black and N-cadherin-negative in gray
Different expressions of N-cadherin in the primary tumors and the related metastases
Focusing on the potential role of N-cadherin on the metastatic evolution, we compared the expression of N-cadherin in the primary tumors and related metastases, available for 5 patients (Wilcoxon test). The data we generated demonstrate a statistically significant higher expression of N-cadherin in the metastases in contrast to the related primary tumors (p = 0.039, Fig. 4). For CD133 expression, no significant difference was found between the primary tumors and the metastases (9 evaluable patients).
Discussion
In this study, we evaluated the expression of N-cadherin and CD133 in primary breast cancer tissue of 307 breast cancer patients randomly included. For 30 patients, we also investigated related recurrences and/or metastases for both markers.
Although the majority of breast cancer patients show no evidence of metastasis at time of diagnosis, nearly 30 % of these women will recur metastatic lesions sometimes years later (O’Shaughnessy 2005; Redig and McAllister 2013). Both EMT and stem cell properties are strongly suggested to be part of the metastatic events (May et al. 2011), while in parts being regulated via the same embryonic signaling pathways (Takebe et al. 2011). The existence of these pathways, e.g. Wnt, can support the connection between EMT and stem cell features and explain their deregulation in some cancers, also in breast cancer (Liu et al. 2010). Consequently, along such deregulations, the association of EMT and stem cell properties may gain importance in breast cancer, strongly suggesting further evaluations of EMT and CSCs markers. In our study, we found a very strong correlation between the EMT marker N-cadherin and the stem cell marker CD133 in breast cancer specimens. As already shown for pancreatic cancer, EMT markers (E-Cadherin and vimentin) correlated significantly with stem cell markers (CD24, CD44 and CD133) (Zhang et al. 2012). It is reported that EMT may induce stem cell properties and vice versa in human mammary epithelial cells (Mani et al. 2008). Here, we clearly demonstrated that a strong connection exists between EMT and stem cell features not only in cell models but also in breast tissue. We were able to show this association not only in a consequent primary tumor tissue collection (p = 0.000, n = 261), but also in their related recurrences and/or metastases, and this in a much smaller group (p = 0.010, n = 45). This connection between both markers, with 83.8–100 % of the CD133-positive samples being N-cadherin-positive too, can display that EMT is essential to trigger and/or accompany stem cell properties, reflecting the aggressive behavior of such cells. Our results obtained with CD133, a recent upcoming stem cell marker in breast cancer, have to be extended now to established stem cell markers in breast cancer, mainly CD24 and CD44.
Authors showed correlations between CD133 or N-cadherin expression and few clinicopathological parameters, but this was investigated either in small cohorts or with definite breast cancer subgroups, e.g., ERα-positive patients or mainly triple-negative patients [for N-Cad (Carvalho et al. 2011; ElMoneim and Zaghloul 2011; Jeong et al. 2012; King et al. 2008; Nagi et al. 2005; Nakagawa et al. 2011; Sarrio et al. 2008; van Nes et al. 2012), for CD133 (Ieni et al. 2011; Liu et al. 2009; Zhao et al. 2011)]. Although we found no correlation between any clinicopathological parameter and N-cadherin/CD133 expressions, N-cadherin or CD133 analysis can be of interest as marker for a definite subpopulation of patients, to study on a larger scale. The risk of recurrences, lymph node metastases or distant metastases may be decreased by targeting N-cadherin and/or CD133-related pathways. Swaminathan et al. (2013) recently showed that CD133-targeted nanoparticles loaded with paclitaxel obtained a significant decrease in tumor initiating cell populations compared to free paclitaxel treatment (Swaminathan et al. 2013). Knowing this, N-cadherin and CD133 may be further used as novel targets for new targeted and individualized therapies in breast cancer to limit tumor spread and aggressiveness (Creighton et al. 2010).
Focusing on the survival analyses, N-cadherin and CD133 do not appear as predictors for survival time for every breast cancer patient. Splitting the cohort in lymph node metastasis-negative and -positive patients, we demonstrated a statistically significant difference for N-cadherin expression. In the subgroup of our cohort having no lymph node metastasis, a significant lower 10-year survival rate characterized the patients expressing N-cadherin compared to the patients expressing not the EMT marker. This suggests that N-cadherin could be a potential marker for distant metastases. Even among patients having no afflicted lymph nodes, the majority dies because of distant metastases. Consequently, tumor cells must have taken their way to distant organs not via the lymphatic system, but via the blood circulation and N-cadherin could be a predictor for this scenario. Indeed, preliminary results of our group show that circulating tumor cells in the blood circulation of breast cancer patients express different amounts of N-cadherin (unpublished data). By investigating the primary tumors and the related metastatic tissue samples available for 5 patients, a significant higher expression of N-cadherin was observed in metastases than in the primary tumors. Previously published data of our group show that 80 % of the metastases also express E-Cadherin (Jeschke et al. 2007), meaning that a co-expression of mesenchymal and epithelial markers exists in metastases. Due to the very limited number of samples in our study, this promising result has to be validated in larger scale studies.
Conclusions
Altogether, we could demonstrate that N-cadherin and CD133 expressions are highly correlated in breast cancer tissue, meaning that EMT and stem cell properties may be intricate. Further studies have to be conducted on larger cohorts to investigate correlations between both markers and relevant clinicopathological parameters in definite subpopulations of patients. By examining specifically N-cadherin expression, we clearly elucidated that it can be a negative predictor for 10-year survival time in patients without lymph node metastases. Moreover, we demonstrated a significantly higher expression of N-cadherin in metastatic tissues than in the primary tumors. All these observations highlight the aggressive behavior and the metastatic potential of tumor cells expressing especially N-cadherin, and potentially co-expressing CD133.
Abbreviations
- CSC:
-
Cancer stem cell
- DAB:
-
3, 3′-Diaminobenzidine
- EMT:
-
Epithelial to mesenchymal transition
- ERα:
-
Estrogen receptor α
- FGFR:
-
Fibroblast growth factor receptor)
- HER2:
-
Human epidermal growth factor receptor 2
- IRS:
-
Immunoreactive score
- MET:
-
Mesenchymal to epithelial transition
- MMP:
-
Matrix metalloproteinase
- PBS:
-
Phosphate buffered saline
- PI(3)K:
-
Phosphatidylinositide 3-kinase
- PR:
-
Progesterone receptor
- TNM classification:
-
Tumor-node-metastasis classification
References
Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S (2009) Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res BCR 11:R46. doi:10.1186/bcr2333
Aomatsu N et al (2012) CD133 is a useful surrogate marker for predicting chemosensitivity to neoadjuvant chemotherapy in breast cancer. PLoS ONE 7:e45865. doi:10.1371/journal.pone.0045865
Armstrong AJ et al (2011) Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res MCR 9:997–1007. doi:10.1158/1541-7786.MCR-10-0490
Carvalho ST, Stiepcich MM, Fregnani JH, Nonogaki S, Rocha R, Soares FA (2011) Evaluation of prognostic factors in stage IIA breast tumors and their correlation with mortality risk. Clinics (Sao Paulo) 66:607–612
Christofori G (2006) New signals from the invasive front. Nature 441:444–450. doi:10.1038/nature04872
Comen EA (2012) Tracking the seed and tending the soil: evolving concepts in metastatic breast cancer. Discov Med 14:97–104
Creighton CJ, Chang JC, Rosen JM (2010) Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neoplasia 15:253–260. doi:10.1007/s10911-010-9173-1
Croker AK, Goodale D, Chu J, Postenka C, Hedley BD, Hess DA, Allan AL (2009) High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J Cell Mol Med 13:2236–2252. doi:10.1111/j.1582-4934.2008.00455.x
Dave B, Mittal V, Tan NM, Chang JC (2012) Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. Breast Cancer Res BCR 14:202. doi:10.1186/bcr2938
ElMoneim HM, Zaghloul NM (2011) Expression of E-cadherin, N-cadherin and snail and their correlation with clinicopathological variants: an immunohistochemical study of 132 invasive ductal breast carcinomas in Egypt. Clinics (Sao Paulo) 66:1765–1771
Elston EW, Ellis IO (1993) Method for grading breast cancer. J Clin Pathol 46:189–190
Groger CJ, Grubinger M, Waldhor T, Vierlinger K, Mikulits W (2012) Meta-analysis of gene expression signatures defining the epithelial to mesenchymal transition during cancer progression. PLoS ONE 7:e51136. doi:10.1371/journal.pone.0051136
Hay ED (1995) An overview of epithelio-mesenchymal transformation. Acta Anat 154:8–20
Ieni A, Giuffre G, Adamo V, Tuccari G (2011) Prognostic impact of CD133 immunoexpression in node-negative invasive breast carcinomas. Anticancer Res 31:1315–1320
Jemal A et al (2008) Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 100:1672–1694. doi:10.1093/jnci/djn389
Jeong H, Ryu YJ, An J, Lee Y, Kim A (2012) Epithelial-mesenchymal transition in breast cancer correlates with high histological grade and triple-negative phenotype. Histopathology 60:E87–E95. doi:10.1111/j.1365-2559.2012.04195.x
Jeschke U et al (2007) Expression of E-cadherin in human ductal breast cancer carcinoma in situ, invasive carcinomas, their lymph node metastases, their distant metastases, carcinomas with recurrence and in recurrence. Anticancer Res 27:1969–1974
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Investig 119:1420–1428. doi:10.1172/JCI39104
Kasimir-Bauer S, Hoffmann O, Wallwiener D, Kimmig R, Fehm T (2012) Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res BCR 14:R15. doi:10.1186/bcr3099
King JA, Shevde LA, Ofori-Acquah S, Watkins G, Jiang WG (2008) Is N-cadherin expression important in ductal carcinoma? South Med J 101:470–475. doi:10.1097/SMJ.0b013e31816c0112
Levi F, Bosetti C, Lucchini F, Negri E, La Vecchia C (2005) Monitoring the decrease in breast cancer mortality in Europe. Eur J Cancer Prev 14:497–502. doi:10.1097/00008469-200512000-00002
Liu Q, Li JG, Zheng XY, Jin F, Dong HT (2009) Expression of CD133, PAX2, ESA, and GPR30 in invasive ductal breast carcinomas. Chin Med J 122:2763–2769
Liu CC, Prior J, Piwnica-Worms D, Bu G (2010) LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc Natl Acad Sci USA 107:5136–5141. doi:10.1073/pnas.0911220107
Mani SA et al (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133:704–715. doi:10.1016/j.cell.2008.03.027
May CD, Sphyris N, Evans KW, Werden SJ, Guo W, Mani SA (2011) Epithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast Cancer Res BCR 13:202. doi:10.1186/bcr2789
Mizrak D, Brittan M, Alison M (2008) CD133: molecule of the moment. J Pathol 214:3–9. doi:10.1002/path.2283
Nadal R et al (2013) CD133 expression in circulating tumor cells from breast cancer patients: potential role in resistance to chemotherapy. Int J Cancer 133:2398–2407. doi:10.1002/ijc.28263
Nagi C et al (2005) N-cadherin expression in breast cancer: correlation with an aggressive histologic variant–invasive micropapillary carcinoma. Breast Cancer Res Treat 94:225–235. doi:10.1007/s10549-005-7727-5
Nakagawa M et al (2011) Expression of p53, Ki-67, E-cadherin, N-cadherin and TOP2A in triple-negative breast cancer. Anticancer Res 31:2389–2393
O’Shaughnessy J (2005) Extending survival with chemotherapy in metastatic breast cancer. Oncologist 10(Suppl 3):20–29. doi:10.1634/theoncologist.10-90003-20
Park J, Schwarzbauer JE (2013) Mammary epithelial cell interactions with fibronectin stimulate epithelial-mesenchymal transition. Oncogene. doi:10.1038/onc.2013.11810.1038/onc.2013.118
Patani N, Martin LA, Dowsett M (2013) Biomarkers for the clinical management of breast cancer: international perspective. Int J Cancer 133:1–13. doi:10.1002/ijc.27997
Peinado H, Portillo F, Cano A (2004) Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol 48:365–375. doi:10.1387/ijdb.041794hp
Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO (1997) Developmental defects in mouse embryos lacking N-cadherin. Dev Biol 181:64–78. doi:10.1006/dbio.1996.8443
Redig AJ, McAllister SS (2013) Breast cancer as a systemic disease: a view of metastasis. J Intern Med 274:113–126. doi:10.1111/joim.12084
Saito M, Tucker DK, Kohlhorst D, Niessen CM, Kowalczyk AP (2012) Classical and desmosomal cadherins at a glance. J Cell Sci 125:2547–2552. doi:10.1242/jcs.066654
Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J (2008) Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res 68:989–997. doi:10.1158/0008-5472.CAN-07-2017
Schott AF et al (2013) Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin Cancer Res Off J Am Assoc Cancer Res 19:1512–1524. doi:10.1158/1078-0432.CCR-11-3326
Singh N, Liu G, Chakrabarty S (2013) Isolation and characterization of calcium sensing receptor null cells: a highly malignant and drug resistant phenotype of colon cancer. Int J Cancer 132:1996–2005. doi:10.1002/ijc.27902
Stemmler MP (2008) Cadherins in development and cancer. Mol BioSyst 4:835–850. doi:10.1039/b719215k
Stoyianni A et al (2012) Immunohistochemical study of the epithelial-mesenchymal transition phenotype in cancer of unknown primary: incidence, correlations and prognostic utility. Anticancer Res 32:1273–1281
Swaminathan SK, Roger E, Toti U, Niu L, Ohlfest JR, Panyam J (2013) CD133-targeted paclitaxel delivery inhibits local tumor recurrence in a mouse model of breast cancer. J Control Release Off J Control Release Soc 171:280–287. doi:10.1016/j.jconrel.2013.07.014
Takebe N, Warren RQ, Ivy SP (2011) Breast cancer growth and metastasis: interplay between cancer stem cells, embryonic signaling pathways and epithelial-to-mesenchymal transition. Breast Cancer Res BCR 13:211. doi:10.1186/bcr2876
van Nes JG et al (2012) Co-expression of SNAIL and TWIST determines prognosis in estrogen receptor-positive early breast cancer patients. Breast Cancer Res Treat 133:49–59. doi:10.1007/s10549-011-1684-y
Zhang Y et al (2012) Epithelial mesenchymal transition correlates with CD24+ CD44+ and CD133+ cells in pancreatic cancer. Oncol Rep 27:1599–1605. doi:10.3892/or.2012.1681
Zhao P, Lu Y, Jiang X, Li X (2011) Clinicopathological significance and prognostic value of CD133 expression in triple-negative breast carcinoma. Cancer Sci 102:1107–1111. doi:10.1111/j.1349-7006.2011.01894.x
Acknowledgments
Thanks are due to Sandra Schulze for assistance in the protocol optimization and to Christoph Freier for assistance in creating the scatter blots.
Conflict of interest
The authors declare that they have no competing interests. We confirm that the authors have full control of all primary data and agree that the journal is allowed to review these data if requested.
Ethical standards
The study has been approved by the Ethical Committee of the Ludwig Maximilians University of Munich, Germany (LMU, ethical number 048-08) and has been carried out in concordance with the guidelines of the Helsinki Declaration. Written informed consent was obtained from the patients.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sophie Doisneau-Sixou and Udo Jeschke are co-last authors of this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bock, C., Kuhn, C., Ditsch, N. et al. Strong correlation between N-cadherin and CD133 in breast cancer: role of both markers in metastatic events. J Cancer Res Clin Oncol 140, 1873–1881 (2014). https://doi.org/10.1007/s00432-014-1750-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1750-z