Abstract
Background
Lung cancer concerns a worldwide health problem and the efficacy of available treatments is unsatisfactory. Recently, thromboxane A2 (TXA2) synthase (TXAS) and receptor (TXA2R) have been documented to play a role in lung cancer development. Therefore, dual TXA2R modulator (i.e., the dual blocker of TXAS and TXA2R) may be more efficacious to kill lung tumor cells than single TXAS inhibitor or TXA2R antagonism. The close relationship between cyclooxygenase (COX)-2 and TXAS also raises whether or how TXA2 contributes to the oncogenic activity of COX-2. This study is therefore conducted to answer these questions.
Methods
Various inhibitors and siRNA were used to evaluate the roles of TXA2 and COX-2 in the proliferation and apoptosis of lung adenocarcinoma cells. Cell proliferation was detected using both MTS ELISA and BrdU labeling ELISA. Cell cycle distribution and apoptosis were examined by flow cytometric analysis. TXB2 level, reflecting the biosynthesis of TXA2, was detected by peroxidase-labeled TXB2 conjugates using an enzyme immunoassay kit. Western blotting was performed to evaluate many biomarkers for cell cycles, apoptosis and proliferation. The levels of COXs were screened by reverse transcriptase and real-time quantitative PCR.
Results
We found either single TXAS inhibitor/TXA2R antagonist or the dual TXA2 modulators offered a similar inhibition on cell proliferation. Moreover, inhibition of TXA2 arrested cells at the G2/M phase and induced apoptosis. It is further demonstrated that TXA2 was able to function as a critical mediator for tumor-promoting effects of COX-2 in lung adenocarcinoma cells.
Conclusion
The present study has for the first shown that dual TXA2 modulators and the single blocker of TXAS or TXA2R offer a similar inhibitory role in lung adenocarcinoma cell proliferation and that the tumor-promoting effects of COX-2 can largely be relayed by TXA2. Thus, TXA2 should be regarded as a critical molecule in COX-2-mediated tumor growth and a valuable target against lung cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the principle cause for cancer-related death worldwide, and the survival rate of 5 years is only 8–14 % (Huang and Chen 2011; Huang et al. 2011). Small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) are two major types of lung cancer, while NSCLC accounts for approximately 85–90 % of all cases (Cheng et al. 2012). Once diagnosed, most of NSCLC cases (>60 %) are locally advanced, unresectable, or metastatic (stage III/IV) with a fatally poor prognosis (Larzabal et al. 2011). Unfortunately, there is, as yet, no effective strategy for treating this deadly disease (Larzabal et al. 2011). Therefore, to identify new prognostic factors and potential therapeutic targets for lung cancer, NSCLC in particular, is urgently needed.
Recently, there is growing evidence demonstrating the involvement of TXA2-related molecules in cancer progression (Ekambaram et al. 2011; Moussa et al. 2011; Li and Tai 2013). TXA2 is located downstream of the cyclooxygenases (COX-1 and COX-2) pathway and considered as a local hormone which acts close to the site of its synthesis through autocrine or paracrine pathways (Huang and Chen 2011; Cathcart et al. 2010). COXs catalyze the conversion of arachidonic acid (AA) into prostaglandin H2 (PGH2), an unstable intermediate from which all other prostanoids, including prostaglandin E2 (PGE2), prostacyclin (PGI), and TXA2, are finally derived by a variety of terminal synthases (Cathcart et al. 2011; Huang and Chen 2011). These terminal synthases are PGE2 synthase (PGES), prostacyclin synthase (PGIS), and TXAS (Cathcart et al. 2011; Huang and Chen 2011). The role of PGES/PGE2 in tumor progression is, to some extent, controversial since PGE2 has both pro-inflammatory and immunosuppression effects via binding with different types of receptors (Huang and Chen 2011). PGIS is generally believed to be anti-tumor, and the imbalance of PGI/TXA2 in favor of the latter is one of the critical mechanisms underlying tumor development and metastasis (Cathcart et al. 2011; Huang and Chen 2011). Therefore, TXA2-related molecules, TXAS and TXA2R, have drawn increasing attention in recent years.
It is found that TXAS is over-expressed in NSCLC and inhibition of this enzyme suppresses the proliferation of the tumor cells via inducing apoptosis (Cathcart et al. 2011; Huang et al. 2011; Moussa et al. 2008b), which strongly suggests a positive role of TXA2 in lung tumor progression. TXA2 acts through its signature receptor TXA2R, which is a member of the G protein-coupled receptor family (Ekambaram et al. 2011; Huang and Chen 2011). Of note, TXA2R is a common receptor for both TXA2 and PGH2 (Nakahata 2008; Patscheke 1990). Nevertheless, TXA2 is the main ligand binding with TXA2R, as PGH2 is an unstable intermediate (Nakahata 2008; Cathcart et al. 2011; Huang and Chen 2011). Importantly, it is reported that TXA2R has an important role in pathology of many types of cancers, such as prostate and bladder cancers (Moussa et al. 2008a; Nie et al. 2008). Two alternatively spliced isoforms of TXA2R, termed α and β, have been identified in human beings (Ekambaram et al. 2011; Huang and Chen 2011). In A549 lung adenocarcinoma cells, Tai HH’s team has demonstrated the involvement of α isoform of TXA2R in tumor proliferation, angiogenesis, and invasion (Li and Tai 2012; Wei et al. 2010). Moreover, we have documented that the single TXAS inhibitor or the single TXA2R antagonist could abrogate cell proliferation induced by 4-methylnitrosamino-1-3-pyridyl-1-butanone (NNK), the strongest tobacco carcinogen (Huang et al. 2011). However, the mechanisms underlying the role of TXA2 in tumor development are still largely unknown. In addition, though TXAS inhibitors can suppress TXA2 biosynthesis and increase PGI formation, PGH2 can accumulate instead of TXA2 and substitute for TXA2 at TXA2R (Nakahata 2008; Patscheke 1990). TXA2R antagonists can block the activity of both TXA2 and PGH2. Therefore, it is possible that the dual actions of the TXAS inhibitor to increase PGI2 formation and of the TXA2R antagonist to antagonize PGH2 and TXA2 can be more efficacious in tumor prevention or treatment than the function of either a single synthase inhibitor or a receptor antagonist. This possibility was, for the first time, examined in the present study.
Materials and methods
Cell lines and cell culture
Human lung cell lines CCL-202, CCL-75.1, NCI-H23, A549, and NCI-H460 were bought from American Type Culture Collection (ATCC). CCL-202 and CCL-75.1, both of which are immortalized lung fibroblast, were cultured in Dulbecco’s modified Eagle’s medium (DMEM). NCI-H23, A549, and NCI-H460 belong to NSCLC. Both NCI-H23 and A549 are lung adenocarcinoma cell lines, and NCI-H460 is a large cell line. NCI-H23 was cultured in DMEM, while A549 and NCI-H460 were cultured in Roswell park memorial institute (RPMI) 1,640 medium. All cell lines were cultured in the medium supplemented with 10 % fetal bovine serum (FBS) and propagated at 37 °C in an atmosphere of 5 % CO2.
Chemicals and drug treatment
Dual TXA2 modulators BM567 and PTXA2, which can block TXA2 synthesis and action, were purchased from Cayman Chemical (Ann Arbor, MI). Selective COX-1 inhibitor SC560, specific COX-2 inhibitor NS398, single TXAS inhibitor furegrelate, single TXA2R antagonist SQ29548, as well as TXA2 mimetic U46619 (also known as TXA2R agonist) were bought from Santa Cruz Biotechnology (Santa Cruz, Santa Cruz, CA). NNK was obtained from Toronto Research Chemicals Inc. (Toronto, North York, Canada). Before treatment with aforementioned chemicals, cells were changed into serum-free medium for 24 h to obtain synchronization (Brodskii et al. 2012). Chemicals used were dissolved in dimethyl sulfoxide (DMSO), and the final DMSO concentration in culture medium did not exceed 0.01 %.
Detection of cell proliferation
Cell proliferation was detected using a bromodeoxyuridine (BrdU) labeling ELISA kit (Roche Applied Science, Penzberg, Germany) and CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS assay, Promega, Madison, WI), respectively.
BrdU cell proliferation assay is to quantitate BrdU incorporated into the newly synthesized DNA strands of actively proliferating cells. Briefly, cells were seeded into a 96-well plate at the same density and incubated overnight to allow cells to attach to the plate. Cells were labeled with 20 μL/well BrdU for 12 h prior to the end of the experiment. After the proper treatment, the cells were probed with anti-BrdU monoclonal antibody and its substrate and then fixed for 30 min. The absorbance at 450 nm was measured using a reference wavelength of 595 nm with an ELISA reader (Bio-Rad, Philadelphia, PA). The results were normalized as percent of control.
MTS assay was performed according to the manufacturer’s instruction. Briefly, after proper treatments as described in the result section, 20 μL of MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] was added in each micro-well, and plate was read using a microplate reader (Bio-Rad).
Flow cytometric analysis
Cell cycle progression was determined using flow cytometric analysis. In brief, live cells were collected, washed with PBS, and stained with 5 μL presidium iodide (PI, 1 mg/mL) using cell cycle staining kit (Liankebio, Hangzhou, China). Cells were incubated at room temperature for 30 min in dark. For apoptosis analysis, cell pellets were obtained and washed twice by ice-cold PBS. Cells were stained with Annexin V fluorescein dye and PI at room temperature in dark for 15 min and then resuspended in 400 μL Annexin-binding buffer. The stained cells were analyzed by Beckman Flow Cytometers (Beckman Coulter, Inc., Brea, CA) for cell cycle distribution and apoptosis.
Transient transfections
Cells were seeded at the same density into a 6-well culture plate and incubated overnight to allow cells to attach to the plate. 100 pmol COX-2 small interference RNA (siRNA) and non-target siRNA (control siRNA) (Santa Cruz Biotechnology) were transfected into cells using the FuGENE® HD Transfection reagent (Roche, Basel, Switzerland), according to the manufacturer’s instruction.
TXB2 measurement
TXA2 is extremely unstable in aqueous solution, being rapidly and non-enzymatically degraded into an inactive form of thromboxane B2 (TXB2). Therefore, TXB2 level reflects the biosynthesis of TXA2 (Cathcart et al. 2011; Huang et al. 2011; Moussa et al. 2008b). Cells were seeded at the same density into a 6-well culture plate and incubated overnight to allow cells to attach to the plate. The culture supernatant was collected and centrifuged after the proper treatment. TXB2 was detected by peroxidase-labeled TXB2 conjugates using an enzyme immunoassay kit (Cayman Chemical), as previously described (Leung et al. 2009).
Reverse transcriptase and real-time quantitative PCR
Total RNA was extracted from cells using Trizol reagent (Invitrogen, Grand Island, NY) according to the manufacturer’s instruction. 2 μg of total RNA was reverse transcribed using a high capacity cDNA reverse transcription kit (Promega). Aliquots of cDNA were employed as template for PCR and real-time quantitative PCR using gene-specific primers and SYBR Green qPCR SuperMix (Invitrogen). The following primer sequences were used: β-actin, (forward) 5′-GGAAATCGTGCGTGACATT-3′ and (reverse) 5′-CAGGCAGCTCGTAGCTCTT-3′; COX-1 (forward) 5′-ATCCAGAACAGTGGCTCG-3′ and (reverse) 5′-GTCCCAAGAAGAACACCC-3′; COX-2 (forward) 5′-TTCCTCCTGTGCCTGATG-3′ and (reverse) 5′-CTGATGCGTGAAGTGCTG-3′; TXAS (forward) 5′-AATAAGAACCGAGACGAACT-3′ and (reverse) 5′-GGCTTGCACCCAGTAGAG-3′; TXA2R (forward) 5′-ACGGAGAAGGAGCTGCTCATCT-3′ and (reverse) 5′-CCAGCCCCTGAATCCTCA-3′ (Moussa et al. 2008a). Real-time PCR was performed using the ABI Prism 7,900 detection system (Applied Biosystems, Carlsbad, CA) and followed the following steps: 50 °C 2 min, 95 °C 10 min, 40 cycles at 95 °C 15 s and 60 °C 60 s. The expression of target genes in the treatment and control groups was normalized using the house-keeping gene β-actin and the fold change in the expression of each target gene was calculated by the 2-ΔΔCT method.
Western blot analysis
Total protein was prepared, and equal amounts of protein (30 μg) were subjected to Western blot analysis as previously described (Huang et al. 2011). The following antibodies were used for the Western blotting: rabbit polyclonal antibody against TXAS or TXA2R detecting both α and β isoforms (1:200) was purchased from Cayman chemical. Rabbit polyclonal antibodies against COX-1 (1:1,000), COX-2 (1:1,000), cyclinB1 (1:1,000), CDK1 (1:1,000), and β-actin (1:1,000); rabbit polyclonal antibody against PARP (1:1,000); and rabbit monoclonal antibody against Survivin (1:3,000) were all bought from Cell Signaling Technology (Beverly, MA). To ensure equal protein loading, membranes were stripped and then probed with anti-β-actin antibodies. Data shown were from three independent experiments. Densitometry was performed using Image lab 4.1 software (Bio-Rad).
Statistical analysis
The statistical analyses were performed using SPSS 11.6 statistical software (SPSS, Chicago, IL). Student’s t test was used to analyze the difference between two groups, and one-way ANOVA followed by Dunnett’s test was employed for the comparisons among three or more groups. Data are presented as the mean ± SD for all statistical tests. A two-side p value of <0.05 was used to reject the null hypothesis.
Results
Dual TXA2 modulators and single TXAS inhibitor/TXA2R antagonist had similar effects on cell proliferation
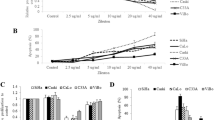
Both NCI-H23 and A549, two lung adenocarcinoma cell lines, were used in the present study, and the data from NCI-H23 model will be shown when the similar results were obtained in two cell lines. To assess the effects of single TXAS inhibitor/TXA2R antagonist and dual TXA2R modulators on cell proliferation, we first performed BrdU cell proliferation assay to select the optimal concentration of chemicals used. The concentrations of all chemicals used were based on the product datasheets and previous publications (Moussa et al. 2008b; de Leval et al. 2006). It was found that treatment cells with single TXAS inhibitor furegrelate at 1 mM and single TXA2R antagonist SQ29548 at 5 μM for 24 h could suppress cell proliferation up to nearly 50 % (Fig. 1a, b). Likewise, treatment of dual TXA2 modulators BM567 at 10 μM and PTXA2 at 5 μM also blocked cell proliferation up to 50 % (Fig. 1c, d). Interestingly, similar efficacy of these chemicals to block cell proliferation was also found at 48 h and 72 h treatments (Fig. 1c, d). The optimal concentrations selected were applied for further experiments. Time-course experiments were subsequently conducted using MTS assays to confirm the above data. As shown in Fig. 1e–g, consistent with the data observed by BrdU cell proliferation assay, all of these chemicals showed the similar effects to block cell proliferation in a time-dependent manner. However, there was no statistical difference among the treatments with TXAS inhibitor furegrelate, TXA2R antagonist SQ29548, dual TXA2 modulators BM567 and PTXA2. Our study has demonstrated that dual TXA2 modulators, which dually block TXA2 synthesis and action, are not more efficacious to kill lung tumor cells than either single TXAS inhibitor or TXA2R antagonist.
Comparison of single TXAS inhibitor or TXA2R antagonist and dual TXA2 modulators. a–d BrdU cell proliferation assays were used to select the optimal concentration of chemicals. NCI-H23 cells were treated with graded concentrations of furegrelate, SQ29548, BM567, and PTXA2 for 24, 48, and 72 h, respectively. e–g The proliferation of NCI-H23 cells was further evaluated using MTS assays after treatment with furegrelate (1 mM), SQ29548 (5 μM), BM567 (10 μM) or PTXA2 (5 μM) for 24, 48 and 72 h. The results were presented as percentages of the control. Data are expressed as mean ± SD of three independent experiments done in quadruplicate. *p < 0.05, and **p < 0.01, ***p < 0.001 when compared to controls
Dual blockade of TXA2 synthase and action induced G2/M arrest
To our best knowledge, there is no information about the effect of TXA2 blockers on cell cycle progression in lung tumor. To examine whether the inhibitory effect of dual block of TXA2 synthesis and action on cell proliferation could be reflected by the arrest cells, the cell cycles after 24-h treatment were analyzed by flow cytometric analysis of propidium iodide (PI) stained cells. NNK has been established as a TXA2 stimulator in the growth of lung tumor cells (Huang and Chen 2011; Huang et al. 2011). Therefore, NNK treatment is employed in the subsequent experiments to act as the additional controls. In this study, we found that the proportion of cells in S phase was moderately increased in the presence of 10 μM NNK (p < 0.05, Fig. 2a). There was a significant increase in G2/M phase cells treated with either PTXA2 (5 μM) or BM567 (10 μM), compared with the non-treatment controls (p < 0.001). Results of three independent experiments showed that approximately 31 and 29 % of the cells were arrested in G2/M phase after treatment of PTXA2 and BM567, respectively. In the presence of NNK (10 μM), both PTXA2 and BM567 could arrest about 26 % of the cells at G2/M phase (p = 0.003 and <0.001, respectively, as compared to NNK treatment alone). The arrest in G2/M phase was accompanied with a concomitant decrease in G0/G1 and S phase cells.
Effects of dual TXA2 modulators on cell cycle progression. a The number of NCI-H23 cells in G0/G1, G2/M, and S phases was determined by flow cytometry after staining the cells with PI. Values are expressed as mean ± SD of three independent experiments. *p < 0.05 and **p < 0.01 when compared to control. b The protein levels of cyclinB1 (58 kDa) and CDK1 (34 kDa) were measured by Western blot analysis, and actin was used as a loading control. Figure is the representative result selected from three independent experiments, and the densitometry for relative protein expression was shown in right panel. *p < 0.05 and **p < 0.01, as compared to control. In these experiments, NNK (10 μM) treatment was used as the additional controls
The finding of cell cycle analysis was further confirmed by the experiments of Western blot detection analyzing the expression of cyclinB1 and cyclin-dependent kinase-1 (CDK1). The CDK1–cyclin B1 complex is known to be critical for entry into mitosis (Moussa et al. 2008b). In line with the data obtained by flow cytometry, the levels of cyclinB1 and CDK1 were consistently down-regulated by PTXA2 in a dose-dependent but NNK-independent manner. Together, these results suggest that blockade of TXA2 synthesis and action down-regulates cyclinB1 and CDK1 expression and causes the G2/M phase cell cycle arrest in lung tumor cells.
Dual blockade of TXA2 synthase and action induced cell apoptosis
To examine whether apoptosis was also induced by dual TXA2 modulators, flow cytometric analysis was employed to evaluate annexin V labeling to phosphatidylserine, a membrane phospholipid indicative for apoptosis (Moussa et al. 2008b). As shown in Fig. 3a, the percentage of annexin V-positive cells was significantly higher in cells treated with BM567 (10 μM) for 24 h, in the absence or presence of NNK (10 μM), compared to the controls (without treatments).
Effects of dual TXA2 modulators on cell apoptosis. a flow cytometric analysis of cells treated with BM567 (10 μM) for 24 h. The percentage of cells in early or late apoptosis is provided in the lower right and upper right quadrants, respectively. b Protein expression of PARP (full length: 116 kDa; cleavage: 89 kDa) and Survivin (17 kDa) was analyzed by Western blotting. Actin was used as loading control. Figure is the representative result selected from three independent experiments, and densitometry for blots was shown in lower panel. *p < 0.05 and **p < 0.01, as compared to control. NNK (10 μM) treatment was used as the additional control in these experiments
During apoptosis, the PARP protein is cleaved by caspases, and an 85-kDa fragment appearance is a hallmark of apoptosis. Also, Survivin has a prominent role at networks of intracellular signaling to exert anti-apoptotic effects (Cufer et al. 2013). Total protein was extracted and subjected to Western blot analysis. Figure 3b showed the appearance of a 116-kDa native form and an 85-kDa cleaved form in cells treated with graded concentrations of PTXA2 for 24 h. Moreover, anti-apoptotic protein Survivin was reduced in a dose-dependent manner. These data reveal that the dual blockade of TXA2 synthesis and action is able to suppress lung tumor cell proliferation via induction of apoptosis.
COX-2 was identified to be responsible for TXA2 production in lung tumor cells
Though the positive role of TXA2 in tumor progression has been established recently, the underlying mechanism remains largely unknown. Although TXA2 is produced upon the action of TXAS which is controlled by COX-2, a well-known oncogene (Cathcart et al. 2011; Huang and Chen 2011), the involvement of COX-2 in TXA2 production is likely but may not always be the case. We thus determined whether the elevation of TXA2 in lung tumor cells was COX-2 derived. To this end, a series of lung cells were screened for COX-1 and COX-2 expression using RT-PCR. CCL-202 and CCL-75.1 are normal lung fibroblast, while NCI-H23, A549, and NCI-H460 are NSCLC cells, as indicated in Materials and Methods. As illustrated in Fig. 4a, COX-1 was highly expressed in normal cells rather than tumor cells, whereas COX-2 had a higher expression in tumor cells than normal cells, supporting oncogenic role of COX-2 in lung tumors (Cathcart et al. 2011; Huang and Chen 2011). As an enzyme downstream of COXs, TXAS converts PGH2 to TXA2 (Huang and Chen 2011). To further confirm whether TXA2 synthesis is dependent on COX-2 activity, lung adenocarcinoma cells NCI-H23 and A549 were treated with 1 μM SC560, a selective COX-1 inhibitor (IC50 for COX-1 is 9nM, for COX-2, 6.3 μM) (Huang et al. 2013), or 10 μM NS398, a highly selective inhibitor for COX-2 with IC50 of 1 μM (Wang and Dubois 2006). As shown in Fig. 4b, SC560 did not significantly inhibit TXA2 production, while treatment of cells with NS398 decreased TXA2 production by about 42 % (p < 0.05, as compared to control), strongly confirming that biosynthesis and action of TXA2 are associated with COX-2, but not COX-1, in lung tumor cells. As expected, the level of TXA2 was significantly inhibited by the specific TXAS inhibitor furegrelate (1 mM) but not by TXA2R antagonist SQ29548 (10 μM). The similar results were observed in A549 cells.
Relationship between COXs and TXA2 synthesis. a Differential expression of COX-1 and COX-2 in normal lung cell lines and lung tumor cells was detected by RT-PCR. Actin was used as a loading control. b Biosynthesis of TXA2 was determined by measuring the level of TXB2 in the culture medium. The results were presented as the percentage of the control. Data are expressed as mean ± SD of three independent experiments done in triplicate. *p < 0.05 and **p < 0.01, as compared to control
Dual TXA2 modulator BM567 and COX-2 inhibitor NS398 had the similar effects to suppress lung tumor proliferation
It is reported that inhibition of COX-2 can induce G2/M arrest in various types of cells, including A549 lung adenocarcinoma cells (Liu et al. 2003; Kim and Pyo 2013). To further explore whether TXA2 functioned as a mediator for tumor-promoting effects of COX-2, we compared effects between COX-2 inhibitor NS398 and dual TXA2 modulator BM567. As illustrated in Fig. 5a, MTT assays showed that 10 μM NNK increased cell proliferation by 134.79 ± 1.62 %. However, such obvious stimulation was significantly abolished by 25 μM NS398 and 25 μM BM567 (about 47.28 and 56.09 % decrease, respectively, p < 0.001). Moreover, both NS398 and BM567 could dramatically reduce expression of anti-apoptotic protein Survivin, though BM567 had more pronounced effects than NS398.
Relationship between the effects of COX-2 and TXA2. a The upper panel, the proliferation of NCI-H23 cells was determined by MTT assays. The results were presented as the percentages of the control. Data are expressed as mean ± SD of three independent experiments done in triplicate. *p < 0.05 and **p < 0.01. Lower panel, Survivin (17 kDa) was evaluated by Western blot detection. Actin was used as a loading control. Figure is the representative result selected from three independent experiments and densitometry was shown below the blots image. *p < 0.05 and **p < 0.01. NNK treatment was used as the additional control. b The inhibitory effect of siRNA on expression of COX-2 mRNA was examined by RT-PCR after 24 h transfection. c The protein level of Survivin was detected using Western blot analysis. Actin was used as a loading control. Figure is the representative result selected from three independent experiments, and densitometry was shown. *p < 0.05 and **p < 0.01. NNK treatment was used as the additional control. d cell proliferation was determined by MTS assays after treatment of 1 μM U46619 in NCI-H23 cells transfected with COX-2 siRNA. Data were presented as percentages of the control and expressed as mean ± SD of three independent experiments done in triplicate. *p < 0.05 and **p < 0.01
TXA2 mimetic U46619 recovered cell proliferation from COX-2 silencing
U46619 is a stable analog of PGH2 and exhibits properties similar to TXA2 (Abramovitz et al. 2000; de Leval et al. 2006). U46619 was therefore adopted to stimulate cells after transfection of COX-2 siRNA for 24 h. The inhibitory effect of COX-2 siRNA on COX-2 expression was shown in Fig. 5b. Consistent with the effects of chemical COX-2 inhibitor NS398, molecular inhibition of COX-2 using siRNA suppressed NNK-induced Survivin expression. Intriguingly, the administration of U46619 at 1 μM reconstituted the expression of Survivin, indicating that TXA2 plays a role in relaying the signal of COX-2. To further confirm this notion, MTS assay was carried out to detect the proliferation of cells transfected with COX-2 siRNA in the presence of 1 μM U46619. Similar results were observed in both NCI-H23 and A549 cells, and data from NCI-H23 cells were demonstrated below. As shown in Fig. 5d, consistent with pharmacological effect of NS398, COX-2 siRNA inhibited cell proliferation by about 53 %. Importantly, U46619 promoted cell proliferation by about 34 % in cells transfected with control siRNA, and COX-2 siRNA-mediated inhibition of cell proliferation was recovered by U46619 above the control level, which is basically in line with Survivin data. These findings support the intermediatory role of TXA2 for COX-2 signal in terms of the Survivin expression and cell proliferation in lung adenocarcinoma cells. In support of our results, U46619 has been demonstrated to stimulate cellular proliferation in several cell types (Moussa et al. 2008a; Belvisi et al. 1998; Huang et al. 2011).
Discussion
Lung cancer concerns a world-wide health problem, and treatment possibilities are still relative inefficient. Efforts to identify new and more effective therapeutic strategies are therefore urgently needed. Lung is a TXA2-abundant organ (McLemore et al. 1988), and lung tumor has been elucidated as a disease involving TXA2 disorders (Huang et al. 2011; Wei et al. 2010; McLemore et al. 1988). Recently, either TXAS or TXA2R has been documented to play a role in tumor development (Huang et al. 2011; Cathcart et al. 2011; Moussa et al. 2008a, b; Nie et al. 2008; Wei et al. 2010), suggesting that both of them may be the therapeutic targets. The oncogenic role of TXAS is thought to be realized via its product, TXA2. TXA2 is usually functional via its receptor TXA2R but TXA2R may not be a must for the oncogenic role of TXA2 (Nakahata 2008; Patscheke 1990). Considering these facts, it would be interesting to see comparison of the anti-lung cancer effects between single TXAS inhibitors and TXA2R antagonists and to determine whether it could be more effective to block tumor growth using dual blockers of TXA2 synthase and receptor. The present study examined this possibility for the first time in lung adenocarcinoma cells. Unexpectedly, the results turned out that dual TXA2 modulators BM567 and PTXA2 failed to show a stronger inhibition of cell proliferation than either the single TXAS inhibitor furegrelate or the single TXA2R antagonist SQ29548. This finding may suggest that the TXAS and TXA2R do not function in a paralleled fashion but work along the same route to promote the tumor growth. Nevertheless, the simultaneous inhibition of both may still be a better option as lung cancer frequently develops resistance to treatments that target only one specific onco-molecule (Cufer et al. 2013).
It is well known that cell cycle progression, as well as apoptosis inactivation, is required for tumor cell proliferation and growth. The information about the role of TXA2 in cell cycles progression is limited. We therefore analyzed cell cycle pattern in lung cancer cells treated with the dual TXA2 modulators BM567 and PTXA2. It was found that both BM567 and PTXA2 arrested cells at G2/M phase, which was further supported by the data showing that PTXA2 down-regulated the levels of CDK1 and cyclin B1 proteins, both of which are known to be critical for entry into mitosis (Moussa et al. 2008b). It was noted that the amount of cells arrested at G2/M phase is not more than 50 %, suggesting that G2/M arrest is not the only mechanism responsible for the suppression of cell proliferation by the dual TXA2 modulators. The increased cleaved-PARP companied with the decreased full length of PARP indicates that the dual TXA2 modulators can induce apoptosis, which is confirmed by the flow cytometric result and the reduction of anti-apoptotic protein Survivin. Therefore, in addition to the G2/M phase arrest, the induction of apoptosis contributes to the dual TXA2 modulator-mediated inhibition in lung cancer cell proliferation.
The positive role of TXA2 in lung tumor growth was confirmed by our findings that dual blockade of TXA2 synthesis and action using dual TXA2 modulators suppressed lung tumor cell proliferation via G2/M arrest and induction of apoptosis. However, whether the tumor-promoting function of TXA2 depends on COXs is virtually unknown in lung cancer. We found that COX-1 is highly expressed in normal lung cells but its level is limited in tumor cells. On the contrary, COX-2 is over-expressed in tumor cells, but its expression is minimal in normal lung cells. These findings are consistent with the other reports showing that COX-2, but not COX-1, is over-expressed in various tumors, such as lung, colon, pancreas, gastric, bladder, head and neck cancers (Strillacci et al. 2010). Furthermore, the increased expression of COX-2, but not COX-1, with a concomitant increase in TXAS was observed in lung cancer specimens, as compared to normal lung tissues (Ermert et al. 2003). These data suggest that biosynthesis of TXA2 in lung tumor correlates with COX-2, which is verified by our data showing that TXA2 production in lung tumor cells was significantly inhibited by COX-2 inhibitor NS398 but not COX-1 inhibitor SC-560.
Theoretically, lowest TXB2 level should be expected in cells treating with NS-398 when compared with TXAS inhibitor furegrelate, as TXAS is controlled by COX-2 and the inhibition of upstream genes in the same pathway yields bigger effect in general. However, in our experimental model, furegrelate was more effective in suppressing TXA2 production than NS-398. As stated previously, there are many products in the downstream of COX-2 pathway, and COX-2-derived products can constitute a positive auto-amplifying pathway (Huang and Chen 2011). For instance, in NSCLC cells, TXA2 mimetics can induce COX-2 expression through the activation of TXA2R, which is able to activate the COX-2 and TXAS gene regulators, nuclear factor kappa B (NF-κB), and cyclic adenosine monophosphate response element-binding (CREB), through extracellular signal-regulated kinases (ERK), p38 mitogen-activated protein kinase (MAPK), and cyclic adenosine monophosphate (cAMP)-dependent pathways (Wei et al. 2007; Huang et al. 2013). Through the similar mechanisms, PGE2 and its receptors induce AA release and COX-2 expression (Wang and Dubois 2006; Hinz et al. 2000; Minghetti et al. 1997; Rosch et al. 2005). Therefore, it is possible that although TXA2 was inhibited by COX-2 inhibitor NS-398, TXA2 still could be synthesized by the action of itself and the other prostanoids, such as PGE2 and PGI2, via these feedback loops. However, TXAS is a specific and terminal synthase for TXA2 biosynthesis, which means that the inhibition of TXAS can almost completely suppress TXA2 biosynthesis.
COX-2 is a well-known oncogene. For example, human urothelial cell line SV-HUC cells were stably transfected with a plasmid containing COX-2 under a CMV promoter, resulting in the enhancement of cellular growth ability (Gee et al. 2008). In addition, tumorigenicity in nude mice was induced by subcutaneous injection of SV-HUC cells overexpressing COX-2 (Gee et al. 2008). In human lung adenocarcinoma CL1.0 cells, lung tumor metastasis could be elevated by overexpressing COX-2 using an isopropyl-beta-D-thiogalactopyranoside-inducible COX-2 gene expression system (Su et al. 2004). Meta-analyses showed that high COX-2 expression is associated with poor survival in NSCLC patients (Hinz et al. 2000; Minghetti et al. 1997), and COX-2 expression has been established as a biomarker for patients with advanced non-small cell lung cancer (Yucel-Lindberg et al. 1999). The inhibition of this enzyme has been identified as a useful method for cancer prevention and even treatment (Cathcart et al. 2011). For instance, COX-2 inhibitors coxib and etoricoxib can against 9,10-dimethylbenz(a)anthracene (DMBA)-induced lung carcinogenesis in rats (Setia and Sanyal 2012).
Among the downstream of COX-2, the role of PGE2 is controversial because of the paradox in its effects, and PGES may be unrelated to COX-2 in NSCLC (Wu et al. 2010), while TXA2 has been established as a survival factor recently (Cathcart et al. 2010, 2011). Furthermore, our experiments showed that inhibition of COX-2 and TXAS by NS398 and BM567, respectively, generated a similar decrease in tumor cell proliferation, suggesting that there may be a close relationship between COX-2 and TXA2 in the growth of lung cancer. Considering the fact that TXA2 is downstream of COX-2, we thus asked whether TXA2 acted as a key mediator for the oncogenic function of COX-2 in lung cancer growth. To test this hypothesis, we employed TXA2 mimetic U46619 to exhibit the function of TXA2 and COX-2 siRNA to silence of the COX-2 function. It was found that U46619 reconstituted Survivin expression suppressed by COX-2 siRNA. Moreover, U46619 was able to fully recover the dramatic reduction of cell proliferation caused by COX-2 siRNA, suggesting that the COX-2-mediated proliferation is largely, if not 100 %, relayed by TXA2 in lung cancer cells tested.
In summary, in addition to the confirmation of the positive role of TXA2 in lung tumor growth, this study has further demonstrated that the inhibition of both TAXS and TXA2R may not achieve a better efficiency in terms of tumor cell proliferation reduction and apoptosis induction, compared with the inhibition of either. More importantly, we have shown that the oncogenic function of COX-2 is likely dependent on TXA2, as TXA2 is able to relay the COX-2 signal for the cell proliferation and survival in lung cancer cells tested. Therefore, TXA2 should be considered to be a critical mediator for tumor-promoting effects of COX-2 in lung tumor cells (Fig. 6). The results reported here may have implications in the employment of drugs affecting TXA2 synthesis or action as the anti-cancer agents in lung carcinoma.
References
Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, Lamontagne S, Rochette C, Sawyer N, Tremblay NM, Belley M, Gallant M, Dufresne C, Gareau Y, Ruel R, Juteau H, Labelle M, Ouimet N, Metters KM (2000) The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta 1483(2):285–293
Belvisi MG, Saunders M, Yacoub M, Mitchell JA (1998) Expression of cyclo-oxygenase-2 in human airway smooth muscle is associated with profound reductions in cell growth. Br J Pharmacol 125(5):1102–1108
Brodskii V, Vasil’ev AV, Terskikh VV, Zvezdina ND, Fateeva VI, Mal’chenko LA, Kiseleva EV, Bueverova EI (2012) Mesenchymal stromal cells synchronize the rhythm of protein synthesis under the effect of an exogenous signal. Ontogenez 43(3):229–232
Cathcart MC, Reynolds JV, O’Byrne KJ, Pidgeon GP (2010) The role of prostacyclin synthase and thromboxane synthase signaling in the development and progression of cancer. Biochim Biophys Acta 1805(2):153–166. doi:10.1016/j.bbcan.2010.01.006
Cathcart MC, Gately K, Cummins R, Kay E, O’Byrne KJ, Pidgeon GP (2011) Examination of thromboxane synthase as a prognostic factor and therapeutic target in non-small cell lung cancer. Mol Cancer 10:25. doi:10.1186/1476-4598-10-25
Cheng L, Alexander RE, Maclennan GT, Cummings OW, Montironi R, Lopez-Beltran A, Cramer HM, Davidson DD, Zhang S (2012) Molecular pathology of lung cancer: key to personalized medicine. Mod Pathol 25(3):347–369. doi:10.1038/modpathol.2011.215
Cufer T, Ovcaricek T, O’Brien ME (2013) Systemic therapy of advanced non-small cell lung cancer: major-developments of the last 5-years. Eur J Cancer 49(6):1216–1225. doi:10.1016/j.ejca.2012.11.021
de Leval X, Dassesse T, Dogne JM, Waltregny D, Bellahcene A, Benoit V, Pirotte B, Castronovo V (2006) Evaluation of original dual thromboxane A2 modulators as antiangiogenic agents. J Pharmacol Exp Ther 318(3):1057–1067. doi:10.1124/jpet.106.101188
Ekambaram P, Lambiv W, Cazzolli R, Ashton AW, Honn KV (2011) The thromboxane synthase and receptor signaling pathway in cancer: an emerging paradigm in cancer progression and metastasis. Cancer Metastasis Rev 30(3–4):397–408. doi:10.1007/s10555-011-9297-9
Ermert L, Dierkes C, Ermert M (2003) Immunohistochemical expression of cyclooxygenase isoenzymes and downstream enzymes in human lung tumors. Clin Cancer Res 9(5):1604–1610
Gee J, Lee IL, Grossman HB, Sabichi AL (2008) Forced COX-2 expression induces PGE(2) and invasion in immortalized urothelial cells. Urol Oncol 26(6):641–645. doi:10.1016/j.urolonc.2007.05.015
Hinz B, Brune K, Pahl A (2000) Prostaglandin E(2) upregulates cyclooxygenase-2 expression in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biochem Biophys Res Commun 272(3):744–748. doi:10.1006/bbrc.2000.2859
Huang RY, Chen GG (2011) Cigarette smoking, cyclooxygenase-2 pathway and cancer. Biochim Biophys Acta 1815(2):158–169. doi:10.1016/j.bbcan.2010.11.005
Huang RY, Li MY, Hsin MK, Underwood MJ, Ma LT, Mok TS, Warner TD, Chen GG (2011) 4-Methylnitrosamino-1-3-pyridyl-1-butanone (NNK) promotes lung cancer cell survival by stimulating thromboxane A2 and its receptor. Oncogene 30(1):106–116. doi:10.1038/onc.2010.390
Huang RY, Li MY, Ng CS, Wan IY, Kong AW, Du J, Long X, Underwood MJ, Mok TS, Chen GG (2013) Thromboxane A2 receptor alpha promotes tumor growth through an autoregulatory feedback pathway. J Mol Cell Biol 5(6):380–390. doi:10.1093/jmcb/mjt038
Kim Y-M, Pyo H (2013) Different cell cycle modulation by celecoxib at different concentrations. Cancer Biother Radiopharm 28(2):138–145
Larzabal L, Nguewa PA, Pio R, Blanco D, Sanchez B, Rodriguez MJ, Pajares MJ, Catena R, Montuenga LM, Calvo A (2011) Overexpression of TMPRSS4 in non-small cell lung cancer is associated with poor prognosis in patients with squamous histology. Br J Cancer 105(10):1608–1614. doi:10.1038/bjc.2011.432
Leung KC, Hsin MK, Chan JS, Yip JH, Li M, Leung BC, Mok TS, Warner TD, Underwood MJ, Chen GG (2009) Inhibition of thromboxane synthase induces lung cancer cell death via increasing the nuclear p27. Exp Cell Res 315(17):2974–2981. doi:10.1016/j.yexcr.2009.06.025
Li X, Tai HH (2012) Increased expression of matrix metalloproteinases mediates thromboxane A2-induced invasion in lung cancer cells. Curr Cancer Drug Targets 12(6):703–715
Li X, Tai HH (2013) Thromboxane A receptor-mediated release of matrix metalloproteinase-1 (MMP-1) induces expression of monocyte chemoattractant protein-1 (MCP-1) by activation of protease-activated receptor 2 (PAR2) in A549 human lung adenocarcinoma cells. Mol Carcinog. doi:10.1002/mc.22020
Liu W, Chen Y, Wang W, Keng P, Finkelstein J, Hu D, Liang L, Guo M, Fenton B, Okunieff P, Ding I (2003) Combination of radiation and celebrex (celecoxib) reduce mammary and lung tumor growth. Am J Clin Oncol 26(4):S103–S109. doi:10.1097/01.COC.0000074147.22064.67
McLemore TL, Hubbard WC, Litterst CL, Liu MC, Miller S, McMahon NA, Eggleston JC, Boyd MR (1988) Profiles of prostaglandin biosynthesis in normal lung and tumor tissue from lung cancer patients. Cancer Res 48(11):3140–3147
Minghetti L, Polazzi E, Nicolini A, Creminon C, Levi G (1997) Up-regulation of cyclooxygenase-2 expression in cultured microglia by prostaglandin E2, cyclic AMP and non-steroidal anti-inflammatory drugs. Eur J Neurosci 9(5):934–940
Moussa O, Ashton AW, Fraig M, Garrett-Mayer E, Ghoneim MA, Halushka PV, Watson DK (2008a) Novel role of thromboxane receptors beta isoform in bladder cancer pathogenesis. Cancer Res 68(11):4097–4104. doi:10.1158/0008-5472.CAN-07-6560
Moussa O, Riker JM, Klein J, Fraig M, Halushka PV, Watson DK (2008b) Inhibition of thromboxane synthase activity modulates bladder cancer cell responses to chemotherapeutic agents. Oncogene 27(1):55–62. doi:10.1038/sj.onc.1210629
Moussa O, Ciupek A, Watson DK, Halushka PV (2011) Urinary thromboxane B2 and thromboxane receptors in bladder cancer: opportunity for detection and monitoring. Prostaglandins Other Lipid Mediat 96(1–4):41–44. doi:10.1016/j.prostaglandins.2011.09.002
Nakahata N (2008) Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol Ther 118(1):18–35. doi:10.1016/j.pharmthera.2008.01.001
Nie D, Guo Y, Yang D, Tang Y, Chen Y, Wang MT, Zacharek A, Qiao Y, Che M, Honn KV (2008) Thromboxane A2 receptors in prostate carcinoma: expression and its role in regulating cell motility via small GTPase Rho. Cancer Res 68(1):115–121. doi:10.1158/0008-5472.CAN-07-1018
Patscheke H (1990) Current concepts for a drug-induced inhibition of formation and action of thromboxane A2. Blut 60(5):261–268
Rosch S, Ramer R, Brune K, Hinz B (2005) Prostaglandin E2 induces cyclooxygenase-2 expression in human non-pigmented ciliary epithelial cells through activation of p38 and p42/44 mitogen-activated protein kinases. Biochem Biophys Res Commun 338(2):1171–1178. doi:10.1016/j.bbrc.2005.10.051
Strillacci A, Griffoni C, Valerii MC, Lazzarini G, Tomasi V, Spisni E (2010) RNAi-based strategies for cyclooxygenase-2 inhibition in cancer. J Biomed Biotechnol 2010:828045. doi:10.1155/2010/828045
Setia S, Sanyal SN (2012) Downregulation of NF-κB and PCNA in the regulatory pathways of apoptosis by cyclooxygenase-2 inhibitors in experimental lung cancer. Mol Cell Biochem 369(1–2):75–86. doi:10.1007/s11010-012-1370-3
Su JL, Shih JY, Yen ML, Jeng YM, Chang CC, Hsieh CY, Wei LH, Yang PC, Kuo ML (2004) Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: a novel mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res 64(2):554–564
Wang D, Dubois RN (2006) Prostaglandins and cancer. Gut 55(1):115–122. doi:10.1136/gut.2004.047100
Wei J, Yan W, Li X, Chang WC, Tai HH (2007) Activation of thromboxane receptor alpha induces expression of cyclooxygenase-2 through multiple signaling pathways in A549 human lung adenocarcinoma cells. Biochem Pharmacol 74(5):787–800. doi:10.1016/j.bcp.2007.06.008
Wei J, Yan W, Li X, Ding Y, Tai HH (2010) Thromboxane receptor alpha mediates tumor growth and angiogenesis via induction of vascular endothelial growth factor expression in human lung cancer cells. Lung Cancer 69(1):26–32. doi:10.1016/j.lungcan.2009.09.009
Wu YC, Su LJ, Wang HW, Jeff Lin CF, Hsu WH, Chou TY, Huang CY, Lu CL, Hsueh CT (2010) Co-overexpression of cyclooxygenase-2 and microsomal prostaglandin E synthase-1 adversely affects the postoperative survival in non-small cell lung cancer. J Thorac Oncol 5(8):1167–1174. doi:10.1097/JTO.0b013e3181e2f4f5
Yucel-Lindberg T, Ahola H, Carlstedt-Duke J, Modeer T (1999) Involvement of tyrosine kinases on cyclooxygenase expression and prostaglandin E2 production in human gingival fibroblasts stimulated with interleukin-1beta and epidermal growth factor. Biochem Biophys Res Commun 257(2):528–532. doi:10.1006/bbrc
Acknowledgments
We thank for Ze-Bo Jiang’s technical assistance. This study was supported by National Natural Science Foundation of China (No. 81302799), China Postdoctoral Science Foundation (No. 2013M531838) and 2012 Excellent Young Scientist Foundation from Guangzhou University of Chinese Medicine (No. KAB111133K08).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Huang, RY., Li, SS., Guo, HZ. et al. Thromboxane A2 exerts promoting effects on cell proliferation through mediating cyclooxygenase-2 signal in lung adenocarcinoma cells. J Cancer Res Clin Oncol 140, 375–386 (2014). https://doi.org/10.1007/s00432-013-1573-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-013-1573-3