Abstract
Purpose
To assess the clinical outcomes of single or oligo-fractionated stereotactic radiotherapy (SRT) using dynamic conformal arcs (DCA) for head and neck tumors (HNTs).
Methods
Thirty-four consecutive patients with 35 lesions treated between 2005 and 2009 were retrospectively evaluated, of whom 85.7 % had recurrent or metastatic disease, and 45.7 and 34.3 % had previous radiotherapy and surgery, respectively. The median SRT dose was 22.3 Gy (11.2–32.8) in 2–4 fractions with a median interval of 7 days and 10.4 Gy (9.2–12.4) in one fraction. SRT was combined with upfront conventionally fractionated RT in 48.6 % of patients.
Results
The median follow-up periods were 18.4 months (2–84.1) for the entire cohort and 49.6 months for the survivors. The 1- and 2-year local control (LC) rates were 84.3 and 70.5 %, with the 1- and 2-year overall survival (OS) rates of 78.6 and 51.6 %. LC was significantly better for tumor volumes <25.6 cm3 (p = 0.001). OS was significantly longer in patients without any disease outside the SRT site (p < 0.001), whereas LC after the SRT did not affect the OS. Late adverse events occurred in 9 patients, including cranial nerve (CN) injury (grade 3/4) in 2, brain radionecrosis in 5 (grade 1), and fatal bleeding in 2 patients harboring uncontrolled lesions abutting the carotid artery.
Conclusions
DCA-based SRT can confer relatively long-term LC with acceptable toxicity in selected patients with HNTs. The patients with CN involvement or tumor volume ≥25.6 cm3 were deemed unsuitable for this treatment regimen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Treatment of patients presenting with recurrent or metastatic disease after previous surgery and/or radiotherapy (RT) for head and neck tumors (HNTs) poses a genuine challenge (Vermorken and Specenier 2010; Chen et al. 2011). Stereotactic radiotherapy (SRT), also referred to as stereotactic body radiotherapy (SBRT), has emerged as a promising strategy for these patients (Gardner et al. 2003; Heron 2009; Siddiqui et al. 2011; Yamazaki et al. 2011). Furthermore, SRT has been adopted as a boost for persistent or residual disease after conventionally fractionated RT (cRT) (Ahn et al. 2000; Uno et al. 2010; Al-Mamgani et al. 2012), or even as a definitive therapy for primary disease (Siddiqui et al. 2009; Kodani et al. 2011). Data, however, have been rather limited, particularly with regard to the nasopharynx as a treatment site (Yamazaki et al. 2011; Seo et al. 2009), cyberknife (CK) as a treatment modality (Roh et al. 2009; Heron et al. 2009; Truong et al. 2009; Unger et al. 2010; Kawaguchi et al. 2010; Cengiz et al. 2011; Rwigema et al. 2011; Vargo et al. 2012), squamous cell carcinoma (SCC) in histological specimens (Vargo et al. 2012), and the lack of long-term observations (Ryu et al. 2004; Unger et al. 2010; Cengiz et al. 2011). Despite encouraging results in local tumor control, severe late toxicities, such as carotid blowout (CB), have been reported (Yamazaki et al. 2011; Kodani et al. 2011; Cengiz et al. 2011; Milano et al. 2011; McDonald et al. 2012).
The prevailing treatment technique is intensity-modulated SRT (IMSRT) using a CK or the Trilogy linear accelerator (LINAC) using static conformal multi-beams (Siddiqui et al. 2009; Heron et al. 2009; Rwigema et al. 2010; Rwigema et al. 2011). Dynamic conformal arcs (DCA) using a micromultileaf collimator (mMLC) is one of the state-of-the-art techniques of LINAC-based SRT mainly used for intracranial disease (Jin et al. 2011; Kung et al. 2011; Ohtakara et al. 2012a). To our knowledge, there is only one report describing the preliminary results of DCA-based SRT for the treatment of limited cases with HNT (Ryu et al. 2004). The dose/fractionation schemes (DFS) for SRT varies substantially between institutions. Furthermore, SRT is generally delivered on consecutive days or within a 1- or 2-week period (Yamazaki et al. 2011). Thus, several issues regarding SRT for HNT have remained unresolved, including optimal patient selection, DFS, and treatment techniques (Siddiqui et al. 2011; Yamazaki et al. 2011).
Starting with 2005, we extended the use of DCA-based SRT to selected patients with HNT. The use of mMLC as an add-on device for non-dedicated linac and the machine capacity at our institution required us to use a very limited number of fractions (1–4) and to adopt a non-daily delivery scheme with ≥3 days of interval, resulting in 1 or 2 fraction(s) per week. In addition, upfront cRT was incorporated in selected patients. This DFS of SRT with or without cRT appeared to be quite unique compared with those described in other reports.
We herein describe the clinical outcomes of DCA-based SRT with or without cRT for the treatment of HNT based on relatively long-term observations, addressing the feasibility, efficacy, and toxicity and also exploring the factors significantly associated with tumor control and survival.
Materials and methods
Study population
Thirty-five lesions in 34 consecutive patients with residual, persistent, recurrent, or metastatic HNT who were treated with DCA-based SRT between January 2005 and September 2009 at our institution were the subjects of this study. All the patients had a neoplasm proven by pathological examination. All the cases were discussed among the head and neck surgeons and radiation oncologists regarding the optimal treatment of choice, especially for further operability, based on a multidisciplinary approach. Written informed consent was obtained from the patients or guardians. The lesions that were deemed susceptible to intrafractional motion, such as that induced by swallowing, were excluded. Upfront cRT was prescribed to selected patients, with room for additional large-volume irradiation to compensate for the aforementioned limitations of SRT delivery. Concurrent chemotherapy was also administered in the selected cases to augment the radiosensitivity. This study was performed in accordance with Declaration of Helsinki in 1964.
Treatment procedures
SRT was performed by using the m3 mMLC (BrainLAB AG, Feldkirchen, Germany) as an add-on device on the Clinac 21EX (Varian, Palo Alto, CA, USA) with 6-MV photon energy (Ohtakara et al. 2012a). The DCA plans were generated by using the BrainSCAN version 5.3 (BrainLAB). The dose calculation was based on a pencil-beam (PB) algorithm with the radiological path length (RPL) for heterogeneity correction. The patients were immobilized with the BrainLAB thermoplastic mask. The clinical target volume (CTV) was defined based on simulation computed tomographic (CT) images fused with contrast-enhanced magnetic resonance imaging (MRI) and/or [18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET)-CT, coupled with the clinical examination. The contours were rather overdrawn for the tumors exhibiting poor demarcation. A dedicated image-guidance system (IGS) was not available; therefore, each planning target volume (PTV) was generated with the addition of ≥2-mm margin to the CTV. The PTV was basically covered with 80 % isodose surface (IDS) normalized to 100 % at the isocenter. A conformal blocking technique was used in the selected cases to reduce the dose to an organ at risk (OAR) such as the brainstem or optic apparatus, although it resulted in a substantial compromise of the PTV coverage for the intended %IDS. The DFS for each PTV was chosen on a case-to-case basis by considering various factors, such as PTV size, proximity to OARs, complexity in shape, dose and field of previous RT and/or combined upfront cRT, expected survival, and presumed radiosensitivity. Combined cRT was delivered using the Clinac 21EX with a 6-MV photon beam. The dose calculations were performed using the Eclipse version 7.3 (Varian) with the Batho power law method for heterogeneity correction. The CTV for cRT was generally defined with adequate margin (≥5 mm) to the PTV for SRT.
Review of the planning parameters
The dose-volume histogram data in all the cases were reviewed with the grid size set to 1.0 mm. The dose encompassing at least 95 % of the PTV (PTV D95) was defined as a prescription dose in this cohort (Ohtakara et al. 2012a). The conformity index (CI) available in the BrainSCAN (Ohtakara et al. 2012c) and the homogeneity index (HI) defined as maximum dose/PTV D95 were calculated, respectively.
A biologically effective dose (BED) was calculated according to the linear-quadratic (LQ) formula BEDn LQ (Gy) = total dose × [1 + (dose per fraction)/n], where n represents the α/β ratio, α/β = 10 for early-responding tissue, α/β = 3 for late-responding normal tissue. The LQ model is the de facto standard method to compare the effects of different DFSs and has been used in many literatures (Siddiqui et al. 2011; Roh et al. 2009; Unger et al. 2010; Brenner 2008). However, debate continues regarding the applicability of the LQ model for dose per fraction of >8–10 Gy that was given to the substantial cases in this study (Siddiqui et al. 2011; Yamazaki et al. 2011; Kirkpatrick et al. 2008; Shibamoto et al. 2012a). We therefore also calculated the BED using an alternative method: linear-quadratic-cubic (LQC) model (Joiner 2009; Wiggenraad et al. 2011). The BED based on the LQC model (BEDn LQC) was defined as total dose × [1 + (dose per fraction)/n − (dose per fraction)2/(α/γ)]. According to Joiner, the survival curve becomes straightened at dose Dl by choosing γ = β/(3Dl) and the LQC curve becomes a straight line at a dose of 18 Gy (Joiner 2009).
Outcome assessment
To determine the efficacy of SRT, the actuarial overall survival (OS), local control (LC), and disease-failure-free (DFF) rates from the commencement of SRT were estimated. LC was defined as no increase in tumor size or no appearance of a new lesion within or in the periphery of the SRT site at the last follow-up and was evaluated by clinical examinations and CT/MRI scans, augmented with PET when available. Disease failure was defined as local and/or distant. Acute and late toxicity was scored using Common terminology Criteria for Adverse Events v3.0 (CTCAE) (Trotti et al. 2003), where late toxicities were defined as symptoms or imaging changes that developed more than 3 months after the completion of SRT.
Statistical analyses
Comparison of paired numerical variables was performed using Wilcoxon signed-rank test for the median value and Levene’s test for the equality of variances, respectively. Spearman’s rank correlation coefficient was applied to evaluate any correlations between the variables. LC, and DFF probability, and OS from the commencement of SRT were estimated using Kaplan–Meier method, and the differences between the subgroups were compared using the log-rank test. Univariate Cox regression analyses were performed in order to identify factors associated with LC and OS. The significant (p < 0.05) and marginally significant (p < 0.10) variables were included in multivariate analyses using a forward stepwise selection with the likelihood-ratio criterion. The receiver operating characteristic (ROC) curves were determined to assess the area under the curve (Az) and the optimal cutoff value for numerical variables. Statistical significance was considered to be p < 0.05. Statistical analyses were performed using the PASW Statistics 18.0 (SPSS Inc, Chicago, IL Illinois, USA).
Results
Patients and treatment characteristics
The demographic and clinical characteristics of the patients and the treatment parameters are summarized in Table 1, of whom 45.7 and 34.3 % had received prior radiotherapy and surgery, respectively. Most lesions (85.7 %) were recurrent or metastatic disease, and most cases (94.3 %) were deemed further inoperable. Most of the primary (88.2 %) and treated sites (94.3 %) corresponded to non-nasopharyngeal lesions, and the predominant site of treatment was the retropharyngeal lymph node (RPLN) (20 %). Approximately half (48.6 %) of the lesions showed non-SCC histologic features. Upfront cRT and concurrent chemotherapy was administered in 48.6 and 37.1 % of the patients, respectively. All the patients completed the planned cRT and SRT without toxicity-related deferral. The median number of fractions was 2, and the median interfractional interval for SRT was 7 days. The BED10 or 3 values for SRT based on the LQC formula were lower than those for the LQ model (p < 0.001). The variance of the BED3 LQC values for SRT was significantly smaller than that of the BED3 LQ (Levene’s test, p = 0.017), whereas these values for BED10 showed a similar variance (p = 0.911). The cumulative BED10 LQ or LQC values of cRT and SRT were inversely correlated with previous RT dose (Spearman’s ρ = −0.53, p = 0.001), but not with the PTV (ρ = −0.04, p = 0.831). The BED10 LQ values of SRT were inversely correlated with the HI values (ρ = −0.59, p < 0.001).
Clinical outcomes
The median follow-up period was 18.4 months (range, 2.0–84.1), and 15 patients (44.1 %) were alive at the last follow-up visit. The median follow-up duration for the survivors was 49.6 months (range, 2.9–84.1). Fifteen patients (42.9 %) had symptoms related to the treated site, and the symptoms of 80 % of them abated at least temporarily.
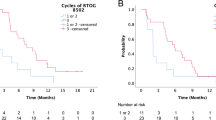
The 1- and 2-year LC rates were 84.3 and 70.5 %, respectively. The ROC analyses failed to determine any cutoff value of either nominal or BED-based doses for better LC. Previous reports suggested that SRT doses ≥30 or 35 Gy administered in 5 fractions was significantly associated with better LC (Unger et al. 2010; Rwigema et al. 2011; Vargo et al. 2012). These doses correspond to 48/59.5 and 46/56.3 Gy for the BED10 LQ and BED10 LQC, respectively. These cutoff values were therefore applied to each dose parameter as a dichotomous variable for Cox regression analyses.
The multivariate analyses revealed that a PTV <25.6 cm3 was the only significant factor associated with better LC (Table 2, Fig. 1c). The cumulative BED10 LQ of cRT and SRT (≥59.5 vs. <59.5 Gy) and HI (<1.24 vs. ≥1.24) were included but were not significant in the final multivariate model. The patients receiving a cumulative BED10 LQ ≥59.5 Gy showed better LC (log-rank test, p = 0.020, Fig. 1d). The DFF probability (Fig. 1e) was significantly lower than the LC probability (log-rank test, p = 0.001).
Kaplan–Meier estimates of overall survival (OS), local control (LC), disease-failure-free (DFF) probability, and the incidence of late adverse events from the commencement of SRT. a OS for the entire cohort, b comparison of OS with or without any disease outside the SRT site, c comparison of LC for PTV< versus ≥25.6 cm3, d comparison of LC between BED10 LQ< versus ≥59.5 Gy, e DFF probability, f the incidence of late adverse events
The actuarial OS rate is shown in Fig. 1a. The median survival time (MST) was 28.8 months, and the 1- and 2-year OS rates were 78.6 and 51.6 %, respectively. The multivariate analyses revealed that the absence of any disease outside the SRT site was the only significant factor for better OS (Table 2, Fig. 1b). Notably, the LC status after SRT did not affect the OS. The main cause of death was attributable to failed LC after SRT in only 4 patients (21.1 %).
On the other hand, any differences in histology and treatment sites or the use of chemotherapy were not prognostic for LC or OS (data not shown).
Additional salvage SRT for recurrence after SRT
Two patients received salvage SRT for in-field or marginal recurrence after SRT. The first case was that of a 56-year-old woman who received salvage SRT with a dose of 20.4 Gy in 3 fractions for marginal recurrence of middle ear carcinoma that was initially treated with cRT followed by SRT (Table 3-2). The lesion was controlled at the last follow-up at 59.6 months after the salvage SRT. The second case was that of a 47-year-old woman who received salvage SRT with a dose of 17.6 Gy in 2 fractions for the in-field recurrence of the RPLN metastasis from the nasopharyngeal cancer (NPC), resulting in local and out-of-field failure 7 months after the salvage SRT (Table 3-8). Despite the administration of oral chemotherapy, she exhibited abducens nerve palsy caused by extensive skull base invasion of the recurrent tumor 9 months after the recurrence and died of fatal bleeding 12.3 months after the recurrence. If the outcomes after the second SRT were included, the 1- and 2-year LC rates were 88.2 and 74.5 %, respectively.
Toxicity
With regard to acute toxicity, only 1 patient developed grade 3 neutropenia related to concurrent chemotherapy. Other complications included grade 1 or 2 mucositis and dermatitis, which subsided favorably.
The cumulative incidence of late adverse events is shown in Fig. 1f, and the details of the events are listed in Table 3. Apart from the aforementioned patient, fatal bleeding also occurred in one other patient (Table 3-9). This case was that of a 63-year-old man who received SRT with a dose of 28 Gy in 3 fractions for postoperative recurrence of RPLN metastasis. Subsequently, metastases along with the ipsilateral upper jugular lymph nodes developed 1.5 months after the SRT. He received additional cRT of 40 Gy in 16 fractions, resulting in inadequate control of the LN metastases. He died of fatal bleeding 6.5 months after the SRT (2 months after the additional cRT). Both cases had uncontrolled tumors near the ICA. In the first patient, fatal bleeding occurred >1 year after the tumor recurrence. The second case received additional cRT. However, the tumors did not encase but rather abutted the ICA with ≥180º of the wall surrounded by the tumor (Cengiz et al. 2011), and the PTV D95 dose for SRT was irradiated to the entire wall of the ICA. Furthermore, the cumulative dose (BED3 LQ) to the ICA was relatively high (277 and 224.7 Gy).
Other severe late toxicities were cranial nerve (CN) injury in 2 cases presenting with CN paresis that deteriorated after SRT. Brain radionecrosis (RN) was the most commonly observed complications (5 cases), and the MRI findings were scored as grade 3 in 1, grade 2 in 3, and grade 1 in 1, according to the late effects of normal tissue-subjective, objective, management, analytic (LENT SOMA) scoring system (LENT SOMA tables 1995), and, fortunately, all these patients had been asymptomatic. The median Karnofsky performance status (KPS) of these survived patients was 80 % (60–90) at the last follow-up visit. The BED3 LQ or LQC values of SRT alone, cRT + SRT, or previous RT were not associated with the incidence of late adverse events.
Discussion
Efficacy
The 1- and 2-year LC rates in this study were comparable with those for IMSRT (Siddiqui et al. 2009; Kodani et al. 2011; Roh et al. 2009; Unger et al. 2010; Rwigema et al. 2010). LC at more than 3- and 4-years was attained in 28.6 and 22.9 % of the patients, respectively. The hypofractionated regimens using a rather abrasive dose per fraction may conquer the potential intrinsic radioresistance of recurrent or persistent tumor cells after cRT (Unger et al. 2010) and originally radioresistant tumors, such as melanoma (Ozyigit et al. 2012).
Non-daily delivery with at least 3 days of interval inevitably leads to prolongation of overall treatment time (OTT) but may be beneficial in terms of tumor reoxygenation and repair from sublethal damage to normal tissues (Shibamoto et al. 2012a, b). Furthermore, if tumor shrinkage occurs immediately after the commencement of SRT, the PTV coverage with the selected IDS and the actual dose to the tumor boundary can be increased. Higuchi et al. (2009) adopted a unique SRT regimen for large brain metastases: 3 fractions with 2-week intervals, in which the CTV was redefined for each second- and third session according to the MRI findings in a response-adaptive manner. In the present study, the OTT of SRT administered either alone or combined with cRT did not affect LC significantly.
The combined use of upfront cRT may be beneficial in terms of providing more expeditious treatment for symptomatic patients, the potential reduction in tumor volumes (PTV for SRT) before SRT, the relative reduction in SRT dose, and the potential reduction in the risk for marginal failure after SRT for the lesions with ambiguous tumor boundaries on images or with predilection for local invasion. Unfortunately, the merit of combined cRT for LC or OS was not validated in this study.
A PTV <25.6 cm3 was the only significant factor associated with improved LC in our study. Other reported cutoff values of PTV resulting in better LC include 25 (Rwigema et al. 2011) and 15 cm3 (Kawaguchi et al. 2010), in which the PTVs were defined without any setup margin. In general, higher doses are required to control larger tumors, given that the numbers of clonogenic, quiescent, and hypoxic cells increase as the tumor volume increases. However, volume-based dose selection was not carried out in the study populations. The remarkable heterogeneity of the patients’ background also discouraged us from adopting a consistent DFS or commencing a dose-escalation trial (Heron et al. 2009). Several studies suggested that higher doses are associated with improved LC (Unger et al. 2010; Rwigema et al. 2011), and certainly, in the present study, the patients who received a BED10 LQ ≥59.5 Gy had better LC. Although stringent comparison of our SRT doses with those of other studies was essentially complicated by the differences in DFSs and OTT, this BED10 LQ value equivalent to 35 Gy/5 fractions may be a threshold dose to attain considerable LC. Nonetheless, the results suggest that the presented SRT regimen has considerable limitations in large tumors, especially to attain long-term LC. To attain better LC for large tumors without increasing toxicity to normal tissues, different strategies using more fractionated SRT regimens with increased BED should be considered.
The OS in this study was comparable with or better than that of other reports (Yamazaki et al. 2011; Siddiqui et al. 2009; Kodani et al. 2011; Roh et al. 2009; Unger et al. 2010; Rwigema et al. 2010; Vargo et al. 2012), given the patients’ backgrounds. Various factors affecting OS have been suggested in the literature: younger age (Unger et al. 2010), KPS >80 % (Heron et al. 2011), no prior RT (Kodani et al. 2011), the interval of >24 months from the previous RT (Kodani et al. 2011), SRT dose of >40 Gy (Heron et al. 2011), tumor volume <15 cm3 (Kodani et al. 2011), NPC (Heron et al. 2011), completely macroscopic resection (Unger et al. 2010), non-SCC histologic features (Unger et al. 2010), and the use of cetuximab (Heron et al. 2011), although none of these had a significant effect on OS in the present study. Absence of any disease outside the SRT site was the only significant factor for better OS, and a 3-year OS rate >70 % was attained in these patients (Fig. 1b). On the other hand, the LC status after the SRT did not affect the OS. The prognosis of the study populations appeared to hinge on rather systemic disease control. Further aggressive local treatment, including SBRT, for a limited burden of disease, such as oligometastases, along with systemic therapy, may lead to better OS (Lo et al. 2009). Nonetheless, better survival is expected in patients undergoing SRT for a solitary lesion; thus, the DFS of SRT should be optimized to attain long-term LC and minimum toxicities.
Toxicity
The incidence of late toxicities increased with the longer follow-up duration (Fig. 1f), suggesting that previous studies with rather short-term follow-up durations might have underestimated the possibility of late toxicities. One of the severe late toxicities was CN injury in this study. The precise localization of the involved CN on the images was difficult to determine in most cases, leading to inaccurate dose estimation. A more fractionated regimen with a reduced dose per fraction is preferable in these cases for functional preservation (Buatti et al. 1995; Mori et al. 2010). The most common toxicity was brain RN, with a median interval of 33 months, which was likely attributable to the dose spillage into these non-eloquent brain tissues as a trade-off for dose reduction to the OARs, such as the brainstem or optic apparatus. Further optimization with the appropriate %IDS selection and arc arrangement and by considering the balance between the target dose homogeneity and dose gradient outside the target is required (Leavitt 1998; Ohtakara et al. 2011).
Although we excluded the tumor locations susceptible to organ motion from the indications, such as the oropharynx or larynx, the treated sites (48.6 %) were located in or close to the mucosal tissue. None of the cases, however, exhibited severe mucosal injury. The dose calculation algorithm (PB with RPL) of the DCA was rather simple. The actual dose of the PTV near the air cavity may be overestimated at the border owing to the inadequate estimation of the buildup/builddown effect (Linthout et al. 2002). Compared with the CK technique, which uses non-isocentric conformal planning with multiple narrow beams to fully cover the entire PTV, the buildup/builddown effect of the DCA may render the mucosal tissue substantially protected from radiation damage. Furthermore, the use of non-coplanar arcs may be beneficial for steep dose falloff into the mucosal tissue, especially compared with modalities with basically coplanar delivery such as helical tomotherapy (Ohtakara et al. 2011).
CB has been one of the major late toxicity concerns (Siddiqui et al. 2011; Yamazaki et al. 2011; Kodani et al. 2011; Cengiz et al. 2011; Milano et al. 2011; McDonald et al. 2012). In 48.6 % of the cases, the tumors abutted or engulfed the ICA, and 2 of these sustained fatal bleeding, although there was no concrete evidence that the hemorrhage per se was from the ICA near the treated site in either case. On the other hand, none of the patients with controlled tumors developed CB. In the cases with fatal bleeding, the tumor progression near the ICA might have rendered the arterial wall vulnerable, leading to the disastrous results. Nonetheless, hypofractionated SRT for the lesions abutting the ICA is fraught with a substantial risk for CB. The scrupulous effort to reduce the dose to the ICA is thus imperative in treatment planning.
This study bore several intrinsic limitations, including its retrospective nature, potential selection bias, and small cohort size and the heterogeneities of the patient characteristics and treatment parameters. In addition, the treatment of the study populations without the use of a dedicated IGS had room for improvement regarding treatment accuracy of SRT and cRT. Since December 2009, at our institution, the presented SRT method has been supplanted by the Novalis Tx platform equipped with dedicated IGSs (Ohtakara et al. 2012b), and since then, a DFS using more fractionations (≥5–10) and daily consecutive delivery has been adopted. Whether this DFS provides better LC and less toxicity compared with the presented regimens deserves further investigation.
In conclusion, single or oligo-fractionated SRT with non-consecutive (nondaily) delivery using DCA combined with or without upfront cRT can confer relatively long-term tumor control with acceptable late toxicities in selected patients with HNT. The patients presenting with CN involvement or large tumor volume (PTV ≥ 25.6 cm3) were deemed unsuitable for this SRT regimen with respect to tumor control and functional preservation. The results indicate that better survival should be expected for patients who do not show any disease outside the SRT treated site.
References
Ahn YC, Lee KC, Kim DY, Huh SJ, Yeo IH, Lim DH, Kim MK, Shin KH, Park S, Chang SH (2000) Fractionated stereotactic radiation therapy for extracranial head and neck tumors. Int J Radiat Oncol Biol Phys 48:501–505
Al-Mamgani A, Tans L, Teguh DN, van Rooij P, Zwijnenburg EM, Levendag PC (2012) Stereotactic body radiotherapy: a promising treatment option for the boost of oropharyngeal cancers not suitable for brachytherapy: a single-institutional experience. Int J Radiat Oncol Biol Phys 82:1494–1500
Brenner DJ (2008) The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin Radiat Oncol 18:234–239
Buatti JM, Friedman WA, Bova FJ, Mendenhall WM (1995) Linac radiosurgery for locally recurrent nasopharyngeal carcinoma: rationale and technique. Head Neck 17:14–19
Cengiz M, Özyiğit G, Yazici G, Doğan A, Yildiz F, Zorlu F, Gürkaynak M, Gullu IH, Hosal S, Akyol F (2011) Salvage reirradiaton with stereotactic body radiotherapy for locally recurrent head-and-neck tumors. Int J Radiat Oncol Biol Phys 81:104–109
Chen AM, Phillips TL, Lee NY (2011) Practical considerations in the re-irradiation of recurrent and second primary head-and-neck cancer: who, why, how, and how much? Int J Radiat Oncol Biol Phys 81:1211–1219
Gardner E, Linskey ME, Peñagarícano JA, Hanna EY (2003) Stereotactic radiosurgery for patients with cancer of the head and neck. Curr Oncol Rep 5:164–169
Heron DE (2009) Stereotactic body radiation therapy for recurrent head & neck cancers: rethinking nonoperative salvage strategies. Future Oncol 5:1321–1325
Heron DE, Ferris RL, Karamouzis M, Andrade RS, Deeb EL, Burton S, Gooding WE, Branstetter BF, Mountz JM, Johnson JT, Argiris A, Grandis JR, Lai SY (2009) Stereotactic body radiotherapy for recurrent squamous cell carcinoma of the head and neck: results of a phase I dose-escalation trial. Int J Radiat Oncol Biol Phys 75:1493–1500
Heron DE, Rwigema JC, Gibson MK, Burton SA, Quinn AE, Ferris RL (2011) Concurrent cetuximab with stereotactic body radiotherapy for recurrent squamous cell carcinoma of the head and neck: a single institution matched case-control study. Am J Clin Oncol 34:165–172
Higuchi Y, Serizawa T, Nagano O, Matsuda S, Ono J, Sato M, Iwadate Y, Saeki N (2009) Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys 74:1543–1548
Jin JY, Wen N, Ren L, Glide-Hurst C, Chetty IJ (2011) Advances in treatment techniques: arc-based and other intensity modulated therapies. Cancer J 17:166–176
Joiner M (2009) Quantifying cell kill and survival. In: Joiner M, van der Kogel A (eds) Basic clinical radiobiology, 4th edn. Hodder Arnold, London, pp 41–55
Kawaguchi K, Sato K, Horie A, Iketani S, Yamada H, Nakatani Y, Sato J, Hamada Y (2010) Stereotactic radiosurgery may contribute to overall survival for patients with recurrent head and neck carcinoma. Radiat Oncol 5:51
Kirkpatrick JP, Meyer JJ, Marks LB (2008) The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol 18:240–243
Kodani N, Yamazaki H, Tsubokura T, Shiomi H, Kobayashi K, Nishimura T, Aibe N, Ikeno H, Nishimura T (2011) Stereotactic body radiation therapy for head and neck tumor: disease control and morbidity outcomes. J Radiat Res 52:24–31
Kung SW, Wu VW, Kam MK, Leung SF, Yu BK, Ngai DY, Wong SC, Chan AT (2011) Dosimetric comparison of intensity-modulated stereotactic radiotherapy with other stereotactic techniques for locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 79:71–79
Leavitt DD (1998) Beam shaping for SRT/SRS. Med Dosim 23:229–236
Linthout N, Verellen D, Van Acker S, Voordeckers M, Bretz A, Storme G (2002) Evaluation of dose calculation algorithms for dynamic arc treatments of head and neck tumors. Radiother Oncol 64:85–95
Lo SS, Fakiris AJ, Teh BS, Cardenes HR, Henderson MA, Forquer JA, Papiez L, McGarry RC, Wang JZ, Li K, Mayr NA (2009) Stereotactic body radiation therapy for oligometastases. Expert Rev Anticancer Ther 9:621–635
McDonald MW, Moore MG, Johnstone PA (2012) Risk of carotid blowout after reirradiation of the head and neck: a systematic review. Int J Radiat Oncol Biol Phys 82:1083–1089
Milano MT, Usuki KY, Walter KA, Clark D, Schell MC (2011) Stereotactic radiosurgery and hypofractionated stereotactic radiotherapy: normal tissue dose constraints of the central nervous system. Cancer Treat Rev 37:567–578
Mori Y, Hashizume C, Kobayashi T, Shibamoto Y, Kosaki K, Nagai A (2010) Stereotactic radiotherapy using Novalis for skull base metastases developing with cranial nerve symptoms. J Neurooncol 98:213–219
Ohtakara K, Hayashi S, Hoshi H (2011) Dose gradient analyses in Linac-based intracranial stereotactic radiosurgery using Paddick’s gradient index: consideration of the optimal method for plan evaluation. J Radiat Res 52:592–599
Ohtakara K, Hayashi S, Hoshi H (2012a) Characterisation of dose distribution in linear accelerator-based intracranial stereotactic radiosurgery with the dynamic conformal arc technique: consideration of the optimal method for dose prescription and evaluation. Br J Radiol 85:69–76
Ohtakara K, Hayashi S, Tanaka H, Hoshi H, Kitahara M, Matsuyama K, Okada H (2012b) Clinical comparison of positional accuracy and stability between dedicated versus conventional masks for immobilization in cranial stereotactic radiotherapy using 6-degree-of-freedom image guidance system-integrated platform. Radiother Oncol 102:198–205
Ohtakara K, Hayashi S, Hoshi H (2012c) The relation between various conformity indices and the influence of the target coverage difference in prescription isodose surface on these values in intracranial stereotactic radiosurgery. Br J Radiol. doi:10.1259/bjr/36606138
Ozyigit G, Cengiz M, Yazici G, Yildiz F, Sezen D, Yildiz D, Gurkaynak M, Zorlu F, Akyol F (2012) Robotic stereotactic body radiotherapy in the treatment of sinonasal mucosal melanoma: report of four cases. Head Neck. doi:10.1002/hed.21895
Roh KW, Jang JS, Kim MS, Sun DI, Kim BS, Jung SL, Kang JH, Yoo EJ, Yoon SC, Jang HS, Chung SM, Kim YS (2009) Fractionated stereotactic radiotherapy as reirradiation for locally recurrent head and neck cancer. Int J Radiat Oncol Biol Phys 74:1348–1355
Rwigema JC, Heron DE, Ferris RL, Gibson M, Quinn A, Yang Y, Ozhasoglu C, Burton S (2010) Fractionated stereotactic body radiation therapy in the treatment of previously-irradiated recurrent head and neck carcinoma: updated report of the University of Pittsburgh experience. Am J Clin Oncol 33:286–293
Rwigema JC, Heron DE, Ferris RL, Andrade RS, Gibson MK, Yang Y, Ozhasoglu C, Argiris AE, Grandis JR, Burton SA (2011) The impact of tumor volume and radiotherapy dose on outcome in previously irradiated recurrent squamous cell carcinoma of the head and neck treated with stereotactic body radiation therapy. Am J Clin Oncol 34:372–379
Ryu S, Khan M, Yin FF, Concus A, Ajlouni M, Benninger MS, Kim JH (2004) Image-guided radiosurgery of head and neck cancers. Otolaryngol Head Neck Surg 130:690–697
Seo Y, Yoo H, Yoo S, Cho C, Yang K, Kim MS, Choi C, Shin Y, Lee D, Lee G (2009) Robotic system-based fractionated stereotactic radiotherapy in locally recurrent nasopharyngeal carcinoma. Radiother Oncol 93:570–574
Shibamoto Y, Otsuka S, Iwata H, Sugie C, Ogino H, Tomita N (2012a) Radiobiological evaluation of the radiation dose as used in high-precision radiotherapy: effect of prolonged delivery time and applicability of the linear-quadratic model. J Radiat Res 53:1–9
Shibamoto Y, Hashizume C, Baba F, Ayakawa S, Manabe Y, Nagai A, Miyakawa A, Murai T, Iwata H, Mori Y, Mimura M, Ishikura S (2012b) Stereotactic body radiotherapy using a radiobiology-based regimen for stage I nonsmall cell lung cancer: a multicenter study. Cancer. doi:10.1002/cncr.26470
Siddiqui F, Patel M, Khan M, McLean S, Dragovic J, Jin JY, Movsas B, Ryu S (2009) Stereotactic body radiation therapy for primary, recurrent, and metastatic tumors in the head-and-neck region. Int J Radiat Oncol Biol Phys 74:1047–1053
Siddiqui F, Raben D, Lu JJ, Grecula JC, Lo SS, Huang Z, Mayr NA, Teh BS, Yao M (2011) Emerging applications of stereotactic body radiation therapy for head and neck cancer. Expert Rev Anticancer Ther 11:1429–1436
LENT SOMA tables (1995) Radiother Oncol 35:17–16
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176–181
Truong MT, Grillone G, Tschoe C, Chin L, Kachnic LA, Jalisi S (2009) Emerging applications of stereotactic radiotherapy in head and neck cancer. Neurosurg Focus 27:E11
Unger KR, Lominska CE, Deeken JF, Davidson BJ, Newkirk KA, Gagnon GJ, Hwang J, Slack RS, Noone AM, Harter KW (2010) Fractionated stereotactic radiosurgery for reirradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys 77:1411–1419
Uno T, Isobe K, Ueno N, Fukuda A, Sudo S, Shirotori H, Kitahara I, Fukushima T, Ito H (2010) Fractionated stereotactic radiotherapy as a boost treatment for tumors in the head and neck region. J Radiat Res 51:449–454
Vargo JA, Wegner RE, Heron DE, Ferris RL, Rwigema JC, Quinn A, Gigliotti P, Ohr J, Kubicek GJ, Burton S (2012) Stereotactic body radiation therapy for locally recurrent, previously irradiated nonsquamous cell cancers of the head and neck. Head Neck. doi:10.1002/hed.21889
Vermorken JB, Specenier P (2010) Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol 21(Suppl 7):252–261
Wiggenraad R, Verbeek-de Kanter A, Kal HB, Taphoorn M, Vissers T, Struikmans H (2011) Dose-effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother Oncol 98:292–297
Yamazaki H, Kodani N, Ogita M, Sato K, Himei K (2011) Reirradiation of head and neck cancer focusing on hypofractionated stereotactic body radiation therapy. Radiat Oncol 6:98
Conflict of interest
None of the authors has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohtakara, K., Hayashi, S., Mizuta, K. et al. Clinical outcomes of single or oligo-fractionated stereotactic radiotherapy for head and neck tumors using micromultileaf collimator-based dynamic conformal arcs. J Cancer Res Clin Oncol 138, 1511–1522 (2012). https://doi.org/10.1007/s00432-012-1225-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-012-1225-z