Abstract
This review explores the difficulties encountered in the management of head and neck cancer (HNC), with special attention to the challenges presented by locoregional recurrences, which impact a substantial number of patients. While maximal surgical resection remains the gold standard for treatment, surgery is often not feasible due to various factors. In such cases, reirradiation has emerged as a potential strategy, albeit with a heightened risk of severe toxicity. Stereotactic Body Radiation Therapy (SBRT) is introduced as a promising approach for unresectable recurrent HNC. SBRT offers precise radiation doses and shorter treatment durations, making it a potentially optimal treatment modality. Despite the growing interest in SBRT, there is a lack of consensus guidelines for its use in HNC, particularly in India. Nevertheless, recommendations are provided for the benefit of SBRT in reirradiation settings, considering factors like tumour size, dose, and treatment duration. The article highlights the safety and effectiveness of SBRT-based reirradiation with existing evidence. The literature review discusses various studies and their findings, emphasizing the importance of high-dose SBRT for improved overall survival. The article also explores the combination of SBRT with systemic therapy as a potential synergistic approach to enhance patient outcomes. In conclusion, SBRT shows promise as a valuable therapeutic tool for patients with inoperable recurrent HNC, offering acceptable safety. However, further research and well-designed trials are needed to optimize its use and identify the most suitable patient cohorts. Establishing comprehensive working guidelines and a nationwide prospective database will be crucial in advancing this treatment approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck cancer (HNC) poses a formidable challenge in the field of oncology, primarily owing to its high likelihood of locoregional recurrences, affecting nearly half of all patients, particularly those who initially present with advanced disease [1]. Additionally, approximately 15% of HNC patients may encounter a second primary tumour post-treatment during follow-up [2]. While the gold standard for treatment remains maximal surgical resection, various factors, including the extent of recurrence, the proximity of tumours to vital structures, and comorbidities can render surgery unfeasible [3, 4]. Regrettably, only a minority of HNC patients facing locoregional recurrence or the emergence of a second primary tumour are diagnosed with resectable disease. In cases of unresectable recurrent head and neck cancer (rHNC), reirradiation has emerged as a potential strategy to enhance local control [5]. However, reirradiation, typically administered in doses of 66–70 Gy divided into 2 Gy fractions, presents a multitude of challenges due to the heightened risk of severe toxicity [6]. Notably, Langer and colleagues reported that nearly 85% of patients subjected to irradiation on an RTOG trial experienced grade 3 or more severe toxicities within the initial two years of treatment, with treatment-related deaths accounting for 8% of cases [7].
SBRT in Recurrent Head and Neck Cancer
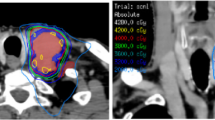
The need to improve results for patients dealing with inoperable rHNC, especially for those who have previously undergone radiation treatment, has led to a growing interest in the utilization of stereotactic body radiation therapy (SBRT). Originating in September 1991 at Sweden’s Karolinska Hospital and introduced by Lax and Blomgren, SBRT represents a radiotherapy technique that offers greater precision in controlling the distribution of radiation doses and shorter treatment durations, typically spanning just five fractions.
Furthermore, SBRT harnesses accelerated fractionation, enabling the delivery of elevated radiation doses in each fraction. This unique approach, despite resulting in a lower cumulative dose over the treatment regimen, can achieve a biologically equivalent dose advantageous for the target tissue. SBRT may be an optimal treatment modality for rHNC, as it offers logistical advantages for patients, with a comparatively lower incidence of increased toxicity when contrasted with traditional radiation techniques. It has garnered widespread international acceptance, proving especially valuable in the context of reirradiation. However, it’s important to note that the high radiation dose per fraction does entail a risk of severe toxicity [8].
Literature Review
Many investigations have been conducted to assess the safety and effectiveness of SBRT-based reirradiation. Nevertheless, these studies often featured small and diverse patient cohorts, encompassing various inclusion and exclusion criteria and treatment protocols. This diversity in study design represents a substantial hurdle in establishing definitive conclusions and hinders the widespread adoption of this approach in clinical reirradiation practices.
Currently, the most comprehensive body of evidence supporting SBRT-based reirradiation can be found in the meta-analysis conducted by Lee et al. [9]. This analysis scrutinized the effectiveness of this treatment in addressing local and regional recurrences, as well as second primary tumours. The meta-analysis incorporated ten studies published between 2006 and 2016, each encompassing different numbers of patients, with cohort sizes spanning from 22 to 107 individuals.
Several factors could have contributed to the overall survival (OS) rates. Certain studies have indicated that radiation dose and tumour size can affect OS following reirradiation with SBRT [10,11,12]. There is a broad consensus that high-dose SBRT is pivotal in achieving prolonged OS, especially in recurrent tumours that might harbour radioresistant tumour cells left unaddressed by previous chemoradiation [13]. Furthermore, Vargo and colleagues reported that smaller gross tumour volumes, less than 25 cm3, were associated with improved OS compared to larger tumours [14].
In the previously referenced meta-analysis [9], reirradiation with SBRT emerges as a safe option, characterized by a pooled event rate of grade ≥ 3 complications at 9.6%, with only three studies reporting rates exceeding 10%. Among the studies included in the analysis, Vargo and colleagues reported grade 3 toxicity in 6% of patients and no grade ≥ 4 toxicities after eight fractions of 5–5.5 Gy. Furthermore, Lartigau et al. found that 30% of patients experienced grade 3 toxicities following six fractions of 6 Gy [14, 15].
Additionally, organ-sparing SBRT demonstrated by Gogineni E. et al. achieved excellent tumour coverage while protecting the organs at the highest risk of re-irradiation-related complications, thus maintaining quality of life [16]. Patients with reirradiation to the skull base maintained stable dysphagia-related scores. In contrast, those treated in the aerodigestive tract initially saw a slight decrease in overall scores, which later returned to near baseline. The M. D. Anderson Symptom Inventory - Head and Neck Module (MDASI-HN) showed an early increase in symptoms for the skull base group. In contrast, the aerodigestive tract group had a delayed symptom onset.
Several studies have explored SBRT-based reirradiation with systemic therapy, hoping to achieve a synergistic effect and improve OS. However, it is important to note that the treatment schemes employed in these studies have been highly diverse [9]. For instance, Vargo et al. found that combining cetuximab with SBRT resulted in a 1-year OS of 40% [14]. Lartigau EF et al. demonstrated that SBRT-based reirradiation combined with cetuximab offers a valuable alternative to salvage surgery, yielding a 1-year OS of 48% [15]. More recently, immune checkpoint inhibitors such as pembrolizumab and nivolumab have shown enduring antitumor activity in recurrent and metastatic HNC where radiation therapy or surgery are not viable options, both in the first line (Keynote-048) and second line (Checkmate-141, Keynote-012, and Keynote-040) settings [17,18,19,20]. Therefore, there is a growing need to further investigate the combined therapeutic efficacy of systemic agents and local modalities like SBRT, even in cases of recurrent HNC.
Regrettably, there is currently a lack of Indian consensus guidelines for the use of SBRT in HNC. Despite this absence, many single-institute retrospective series have been published. Just recently, the American Radium Society issued an executive summary that provides guidance on the appropriate use of reirradiation, including the application of SBRT in HNC. It is worth noting that the committee could not reach a consensus regarding the use of SBRT. However, it is crucial to emphasize that higher dosages exceeding 35–40 Gy administered in 5 fractions, stringent adherence to organ-at-risk (OAR) constraints, and consideration of patients falling under RPA category III continue to be of utmost importance for further evaluation and investigation [21].
Conclusion
SBRT seems promising for patients with inoperable recurrent HNC or second primary HNC, providing acceptable safety and short overall treatment times. OS following SBRT reirradiation remains moderate, possibly due to insufficient doses used in published studies. There is a need for well-designed trials of SBRT-based reirradiation in terms of dose escalation and combined treatment strategies with systemic agents in well-defined patient groups. Based on the available literature, the following recommendations can be made for using SBRT in the reirradiation of HNC.
Only patients with small local or regional recurrences are good candidates; ideally, the gross tumour volume should be below 25 cm3. The dose should range from 35 to 40 Gy in five fractions. The patients may be treated with three fractions per week, with overall treatment time not exceeding 14 days.
SBRT in rHNC represents a valuable therapeutic tool, but its successful utilization necessitates comprehensive training and safe delivery methods to achieve the best possible outcomes. Establishing a nationwide prospective database and developing comprehensive working guidelines will be pivotal in identifying the most favourable patient cohorts for this treatment approach.
References
Cooper JS, Zhang Q, Pajak TF, Forastiere AA, Jacobs J, Saxman SB et al (2012) Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 84:1198–1205
Cooper JS, Pajak TF, Rubin P, Tupchong L, Brady LW, Leibel SA et al (1989) Second malignancies in patients who have Head and Neck cancer: incidence, effect on survival and implications based on the RTOG experience. Int J Radiat Oncol Biol Phys 17:449–456
Jayaram SC, Muzaffar SJ, Ahmed I, Dhanda J, Paleri V, Mehanna H (2016) Efficacy, outcomes, and complication rates of different surgical and nonsurgical treatment modalities for recurrent/residual oropharyngeal carcinoma: a systematic review and meta-analysis. Head Neck 38(12):1855–1861
Wong SJ, Heron DE, Stenson K, Ling DC, Vargo JA (2016) Locoregional recurrent or second primary Head and Neck cancer: management strategies and challenges. Am Soc Clin Oncol Educ Book 35:e284–292
Kim YS (2017) Reirradiation of Head and Neck cancer in the era of intensity-modulated radiotherapy: patient selection, practical aspects, and current evidence. Radiat Oncol J 35:1–15
Dionisi F, Fiorica F, D’Angelo E, Maddalo M, Giacomelli I, Tornari E et al (2019) Organs at risk’s tolerance and dose limits for Head and Neck cancer re-irradiation: a literature review. Oral Oncol 98:35–47
Langer CJ, Harris J, Horwitz EM et al (2007) Phase II study oflow-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Protocol 9911. J Clin Oncol 25(30):4800–4805
Fowler JF (1983) Dose response curves for organ function or cell survival. Br J Radiol 56:497–500
Lee J, Kim W, Yoon W, Koom W, Rim C (2020) Reirradiation using stereotactic body radiotherapy in the management of recurrent or second primary Head and Neck cancer: a meta-analysis and systematic review. Oral Oncol 107:104757
Spencer SA, Harris J, Wheeler RH, Machtay M, Schultz C, Spanos W et al (2008) Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck 30:281–288
Rwigema JCM, Heron DE, Ferris RL, Andrade RS, Gibson MK, Yang Y et al (2011) The impact of Tumor volume and radiotherapy dose on outcome in previously irradiated recurrent squamous cell carcinoma of the head and neck treated with stereotactic body radiation therapy. Am J Clin Oncol 34:372–379
Yamazaki H, Ogita M, Himei K, Nakamura S, Suzuki G, Yoshida K et al (2016) Reirradiation using robotic image-guided stereotactic radiotherapy of recurrent Head and Neck cancer. J Rad Res 57:288–293
Weichselbaum RR, Beckett MA, Schwartz JL, Dritschilo A (1988) Radioresistant Tumor cells are present in head and neck carcinomas that recur after radiotherapy. Int J Radiat Oncol Biol Phys 15:575–579
Vargo JA, Ferris RL, Ohr J, Clump DA, Davis KS, Duvvuri U et al (2015) A prospective phase 2 trial of reirradiation with stereotactic body radiation therapy plus cetuximab in patients with previously irradiated recurrent squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 91(3):480–488
Lartigau EF, Tresch E, Thariat J, Graff P, Coche-Dequeant B, Benezery K et al (2013) Multi institutional phase II study of concomitant stereotactic reirradiation and cetuximab for recurrent Head and Neck cancer. Radiother Oncol 109:281–285
Gogineni E, Zhang I, Rana Z, Marrero M, Gill G, Sharma A, Riegel AC, Teckie S, Ghaly M (2019) Quality of life outcomes following organ-sparing SBRT in previously irradiated recurrent Head and Neck Cancer. Front Oncol 9:836. https://doi.org/10.3389/fonc.2019.00836
Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr et al (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomized, open-label, phase 3 study. Lancet 394:1915–1928
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L et al (2016) Nivolumab for recurrent squamous-cell carcinoma of the Head and Neck. N Engl J Med 375:1856–1867
Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP et al (2016) Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 17:956–965
Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ et al (2019) Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomized, open-label, phase 3 study. Lancet 393:156–167
Ward MC, Koyfman SA, Bakst RL, Margalit DN, Beadle BM, Beitler JJ, Chang SS, Cooper JS, Galloway TJ, Ridge JA, Robbins JR, Sacco AG, Tsai CJ, Yom SS, Siddiqui F (2022) Retreatment of recurrent or second primary Head and Neck Cancer after Prior Radiation: executive summary of the American Radium Society Appropriate Use Criteria. Int J Radiat Oncol Biol Phys 113(4):759–786
Funding
There is no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
NA.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nath, J., Neog, M., Bhattacharyya, M. et al. Stereotactic Body Radiation Therapy in Recurrent Head and Neck Cancer: Where Do We Stand?. Indian J Otolaryngol Head Neck Surg 76, 2212–2215 (2024). https://doi.org/10.1007/s12070-023-04450-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-023-04450-5