Abstract
Purpose
Melanoma-associated antigens-A (MAGE-A) family is a group of well-characterized cancer/testis antigens (CTA), because they are strictly tumor specific and are shared by many kinds of tumors. However, the expression pattern of MAGE-A10 and MAGE-A11 in breast cancer patients is still unclear. The purpose of our study is to investigate the expression pattern and prognostic significance of MAGE-A10 and MAGE-A11 in breast cancer patients.
Methods
Formalin-fixed and paraffin-embedded tissues and the clinicopathological parameters from 75 primary breast cancer patients were collected. The expressions of MAGE-A10 and MAGE-A11 proteins were immunohistochemically detected, and the association of MAGE-A10 and MAGE-A11 expressions with the clinicopathological parameters and the survival of breast cancer patients were analyzed.
Results
The expression rates of MAGE-A10 and MAGE-A11 in breast cancer specimens were 73.3 and 52.0%, respectively. MAGE-A11 expression was more frequent in estrogen-receptor (ER)-positive breast carcinomas compared with ER-negative breast carcinomas (P = 0.004). MAGE-A11 expression was positively associated with HER-2 expression (P = 0.003). Overall survival of patients with MAGE-A11-negative expression was significantly longer than those patients with positive MAGE-A11 expression (P = 0.030), but no difference of overall survival was found between patients with MAGE-A10-negative and -positive expression (P = 0.881).
Conclusions
MAGE-A10 and MAGE-A11 are tumor-specific antigens, and MAGE-A11 expression probably is a potential poor prognostic factor for breast cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common malignancy in women (Garcia et al. 2007). Its clinical course may vary from indolent and slowly progressive to rapidly metastatic disease. Therapeutic options for this type of cancer range from primary surgery to adjuvant chemotherapy, radiotherapy, hormonal therapy or targeted therapy. Breast cancer is a heterogeneous disease, and therefore, no golden standard therapy exists suitable for all tumors of the mammary gland (Goldhirsch et al. 2005; Weigelt and Reis-Filho 2009; Polyak 2007). Identification of prognostic and predictive factors that reflect the biology of breast cancer is important for the assessment of prognosis and selection of patients who may benefit from adjuvant and/or systemic therapy. Therefore, there is a great need to identify molecular targets for developing novel therapeutic approaches for breast cancer patients.
Cancer/testis antigens (CTA) possess several features of ideal targets for cancer immunotherapy (Simpson et al. 2005). They are expressed in a wide variety of malignant tumors, but their expression in normal tissue is mostly restricted to germ cells, which are immunoprivileged because of their lack of human leukocyte antigen (HLA) molecules (Zendman et al. 2003; Scanlan et al. 2004). Melanoma-associated antigens (MAGE) are a group of well-characterized members of the CTA family that includes at least 55 closely related proteins (Van der Bruggen et al. 1991). The MAGE family has been divided into two big subfamilies: MAGE-I and MAGE-II (Sang et al. 2011a, b; Ohman et al. 2001). The MAGE-I family includes MAGE-A, MAGE-B and MAGE-C subfamilies. Most of them are relevant cancer/testis antigens and therefore are rarely expressed in normal adult tissues except for testis, but highly expressed in various forms of cancer. MAGE-II family that includes MAGE-D group differs from the MAGE-I family members in their expression pattern. It is almost universally expressed in all normal tissues and not related to cancer.
The MAGE-A antigens are of particular interest for cancer immunotherapy because they are strictly tumor specific and are shared by many kinds of tumors. The MAGE-A gene family that located on chromosome Xq28 is comprised of 12 family members called MAGE-A1-MAGE-A12 (De Plaen et al. 1994; Rogner et al. 1995). However, to identify which MAGE-A antigen should be the target of a breast cancer vaccine, the expression of each individual member of MAGE-A family still has to be defined. A few studies reported on the expression of specific MAGE-As in breast cancers. Otte et al. (2001) analyzed the expressions of MAGE-A1, -A2, -A3, -A4, -A6 and -A12 in primary breast cancers by multiplex semi-nested RT-PCR and found that their expressions were more frequently detected in patients at a high risk of tumor recurrence. Bandić et al. (2006) detected the MAGE-A4 expression in patients with invasive ductal breast cancer by immunohistochemistry and found that MAGE-A4-positive patients had a significantly longer survival than the MAGE-A4-negative patients.
In the present study, we collected the formalin-fixed and paraffin-embedded tissues and the clinicopathological parameters from 75 primary breast cancer patients, immunohistochemically detected the expressions of MAGE-A10 and MAGE-A11 proteins, and analyzed their association with the clinicopathological parameters and the survival of breast cancer patients. The purpose of our study is to investigate the expression pattern and prognostic significance of MAGE-A10 and MAGE-A11 in breast cancer patients.
Materials and methods
Clinical specimens
Five human normal testis specimens were obtained from the prostate patients who underwent the surgical castration orchiectomy at the Department of Urinary Surgery, the Fourth Clinical Hospital of Hebei Medical University in 2006. All 75 primary breast cancer specimens and tumor-free breast specimens analyzed in our study were obtained from the same patients who had invasive breast cancer and underwent the surgical treatment at the Breast Center, the Fourth Clinical Hospital of Hebei Medical University in 2006. All patients did not undergo the preoperative adjuvant chemotherapy and radiotherapy. After surgery, all the specimens were sent to the pathology department of the hospital to be fixed and paraffin-embedded for routine immunohistochemistry analysis. All the patients provided written informed consent before enrollment. The study was approved by the Medical Ethics Committee of the Fourth Clinical Hospital of Hebei Medical University.
All of the patients were followed up by interview in clinic or phone call. The total period of follow-up was 16–60 months.
Collection of clinicopathological parameters
The clinicopathological parameters of the patients with breast cancer were collected from the case history of the patients at study entry, including age of patients, pathological types, histological grades, clinical stages, tumor size, metastatic state of lymph node, ER (estrogen receptor) and PR (progestogen receptor) status.
Immunohistochemistry (IHC) and evaluation
Five-micrometer sections from the formalin-fixed and paraffin-embedded tissue blocks were mounted on silanized slides and were dewaxed and rehydrated through toluene and an alcohol series. The sections were then washed with phosphate-buffered saline (PBS, pH 7.2) for 3 × 5 min. Then, the sections were heated in a microwave oven for 5 min in 10 mmol/l Na-citric buffer (pH 6.0) for antigen retrieval and washed with PBS. The sections were immersed in 0.3% hydrogen peroxide in methanol for 20 min to suppress endogenous peroxidase activity. After washed with PBS, the sections were incubated in 1:10 diluted normal goat serum at room temperature in a humidified chamber for 30 min to prevent nonspecific immunoglobulin binding. The sections were then treated with the 1: 200 diluted rabbit-anti-human MAGE-A10 or MAGE-A11 polyclonal antibodies (Epitomics, California, USA) or rabbit-anti-human HER-2 polyclonal antibody at 4°C overnight. Normal IgG replaced of the primary antibody served as the control. A streptoavidin-biotinylated horseradish peroxidase-based detection system was used to reveal specific binding. Sections were counterstained with hematoxylin for light microscopic review and evaluation.
MAGE-A10 and MAGE-A11 expressions were always positively detected in both the cytoplasm and the nucleus of cells. HER-2 was detected with strong complete membrane staining. Immunoreactivity was scored in the following ways: −, no positive cells (negative); +, <20% positive cells (“mild reaction”); ++, 21–50% positive cells (“moderate reaction”); and +++, >50% positive cells (“strong reaction”). The immunoreactivity scores for MAGE-A10 and MAGE-A11 were presented as either “negative” or “positive,” with positive including moderate and strong reactions. Percent of positive cells and staining intensity were scored by 2 independent observers.
Statistical analysis
The associations between MAGE-A10 and MAGE-A11 expressions and clinicopathological parameters were evaluated using chi-square or continuity-corrected Pearson’s chi-square test, as appropriate. Overall survival of patients was estimated by the Kaplan–Meier method, and differences between groups were compared by the long-rank test. All the statistical analyses were performed with SPSS 13.0 software. P value less than or equal to 0.05 was considered statistically significant.
Results
Expressions of MAGE-A10 and MAGE-A11 in tumor-free breast specimens and breast cancer specimens
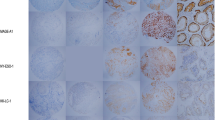
MAGE-A10 and MAGE-A11 antibodies were firstly used to stain sections of formalin-fixed and paraffin-embedded human normal testis specimens. Both MAGE-A10 and MAGE-A11 expressions were mainly observed on spermatogonia and primary spermatocytes, and both the cytoplasm and the nucleus were stained (Fig. 1), which was considered as the positive control, suggesting that MAGE-A9 expression was associated with early spermatogenesis.
Then, immunohistochemical staining with MAGE-A10 and MAGE-A11 antibodies was carried out on 75 paired tumor-free breast specimens and primary breast cancer specimens. Overall, no MAGE-A10 (Fig. 2) and MAGE-A11 (Fig. 3) expressions were found in 75 tumor-free breast specimens. 55 out of 75 (73.3%) and 39 out of 75 breast cancer specimens (52.0%) were found positive with MAGE-A10 and MAGE-A11 antibodies, respectively (Table 1). In most tumors, both MAGE-A10 (Fig. 2) and MAGE-A11 (Fig. 3) were seen in both the cytoplasm and the nucleus of breast cancer cells, suggesting that MAGE-A10 and MAGE-A11 are dynamic proteins that can shuttle in and out the nucleus as needed.
Immunohistochemical analysis of MAGE-A10 expression in human breast cancer tissues and tumor-free breast tissues. Magnification, a breast cancer tissues, ×200; b breast cancer tissues, ×400; c tumor-free breast tissues, ×200; d tumor-free breast tissues, ×400. In human breast cancer tissues, MAGE-A10 was expressed in both the cytoplasm and the nucleus
Immunohistochemical analysis of MAGE-A11 expression in human breast cancer tissues and tumor-free breast tissues. Magnification, a breast cancer tissues, ×200; b breast cancer tissues, ×400; c tumor-free breast tissues, ×200; d tumor-free breast tissues, ×400. In human breast cancer tissues, MAGE-A11 was expressed in both the cytoplasm and the nucleus
Association between MAGE-A10 and MAGE-A11 expressions and the clinicopathological features of breast cancer patients
The association between MAGE-A10 and MAGE-A11 expressions and the clinicopathological features were statistically evaluated. As shown in Table 2, no association at all was observed for MAGE-A10 expression and the clinicopathological features of breast cancer patients. MAGE-A11 expression was more frequent in estrogen-receptor (ER)-positive breast carcinomas (25/36, 69.4%) compared with ER-negative breast carcinomas (14/39, 35.9%) (P = 0.004). No correlation was found between MAGE-A11 expression and any other clinicopathological features of breast cancer patients.
Association between MAGE-A10 and MAGE-A11 expression and the prognosis of breast cancer patients
Because HER-2 has been reported to be overexpressed in 25–30% breast cancers and is correlated with the poor prognosis of breast cancer (Slamon et al. 1987, 1989; Ross and Fletcher 1998; Ross et al. 2003), we immunohistochemically examined the expression of HER-2 in breast cancer specimens and analyzed the association between MAGE-A10 and MAGE-A11 expressions and HER-2 expression, in order to explore the association between MAGE-A10 and MAGE-A11 expressions and the prognosis of breast cancer patients. As shown in Fig. 4, overexpression of HER-2 was detected in some of breast cancer specimens, with strong complete membrane staining. As shown in Table 3, no association at all was observed between MAGE-A10 expression and HER-2 expression. MAGE-A11 expression was positively associated with HER-2 expression (P = 0.003).
All 75 patients were followed up for 16–60 months, of those, 26 patients were lost. Figure 5 showed the Kaplan–Meier plots of MAGE-A10 and MAGE-A11 expression levels in relation to overall survival. Overall survival of patients with MAGE-A11-negative expression was significantly longer than those patients with positive MAGE-A11 expression (χ2 = 4.697, P = 0.030), but no difference of overall survival was found between patients with MAGE-A10-negative expression and positive expression (χ2 = 0.022, P = 0.881), as shown in Fig. 5.
Discussion
The best studied CTAs are those of type I MAGEs, especially the MAGE-A subfamily, because the MAGE-A antigens are strictly tumor specific and are shared by many kinds of tumors (Sang et al. 2011a, b). Thus, MAGE-As are appealing targets for cancer immunotherapy. However, considering the limited specificity of the available anti-MAGE antibodies to distinguish different MAGE proteins, most investigators used microarray analysis, RT-PCR and RNA in situ hybridization to characterize MAGE gene expression (Sugita et al. 2002; Kufer et al. 2002). By using multiplex semi-nested RT-PCR analysis, Otte et al. (2001) detected the expressions of MAGE-A1, -A2, -A3, -A4, -A6 and -A12 in primary breast cancers and found that their expressions were more frequently detected in patients at a high risk of tumor recurrence. Similar results were obtained in triple-negative breast cancers (Karn et al. 2011), indicating that high expression of MAGE-A genes may be correlated with the worse survival of breast cancers.
In contrast to the mRNA expression, the expression of MAGE-As proteins in tumors has only been analyzed for a few MAGE antigens by using immunohistochemistry and Western blot analysis because of the high degree of homology of many MAGE family members and the lack of antibodies recognizing specific MAGE family members. The anti-MAGE-A1 antibody 6C1 that cross-reacts with MAGE-A1, -A2, -A3, -A4, -A6, -A10, and -A12 and the anti-MAGE-A3 antibody 57B which cross-reacts with MAGE-1, -4, -6, and -12 (Busam et al. 2000; Rimoldi et al. 2000) were better regarded as multi-MAGE antibodies. By using immunohistochemistry, Bandić et al. (2006) reported the expression of MAGE-A4 in invasive ductal breast cancer patients and found that MAGE-A4-positive patients had a significantly longer survival than the MAGE-A4-negative patients. In other tumors except for breast cancer, the higher frequency of MAGE-As expression was often associated with poor outcome except for some reports concerning MAGE-A4 (Bergeron et al. 2009; Bandić et al. 2006). Higher grade and metastatic tumors have also been found to have more frequent MAGEs expression than the primary tumors (Brasseur et al. 1995).
In the present study, to examine the expression patterns of MAGE-A10 and MAGE-A11 proteins in breast cancer and tumor-free breast specimens, we firstly detected their expressions in human normal testis specimens, which were considered as the positive control. Then, the expression patterns of them in breast tissues were analyzed. Our results showed that there were no MAGE-A10 and MAGE-A11 expressions in all tumor-free breast specimens, while 73.3 and 52.0% breast cancer specimens showed positive MAGE-A10 and MAGE-A11 expressions, respectively. Subsequently, we analyzed the association of MAGE-A10 and MAGE-A11 expressions with the clinicopathological parameters of breast cancer patients. No association at all was observed for MAGE-A10 expression and the clinicopathological features of breast cancer patients. MAGE-A11 expression was only associated with ER expression, but not with other clinicopathological features of breast cancer patients, suggesting that MAGE-A11 may be involved in the estrogen dependent cell proliferation.
Total HER-2 expression is an independent predictor of poor prognosis of breast cancer (Slamon et al. 1987, 1989; Ross and Fletcher 1998; Ross et al. 2003) and is also a clinical target for treatment (Rabindran 2005; Yeon and Pegram 2005). In order to explore the association of MAGE-A10 and MAGE-A11 with the prognosis of breast cancer patients, we firstly detected the expression of HER-2 in breast cancer specimens and analyzed the association of MAGE-A10 and MAGE-A11 expressions with HER-2 expression. 28% (21/75) of the patients had HER-2-positive (+++) breast tumors, and the expression of MAGE-A11 was positively associated with HER-2 expression. Further survival analysis also revealed that overall survival of patients with MAGE-A11-negative expression was significantly longer than those patients with positive MAGE-A11 expression, but no difference of overall survival was found between patients with MAGE-A10-negative expression and -positive expression.
However, why MAGE-A11, but not MAGE-A10 was associated with the poor prognosis of breast cancer is still unclear. From our consideration, it may be due to the following three reasons. Firstly, the limited numbers of the enrolled patients may affect the results of the statistical analysis. Secondly, although MAGE-A10 and MAGE-A11 have similar structural domains, substantial difference was also found in NH2-terminal region, COOH-terminal region and MAGE homology domains (Sang et al. 2011a, b). The different MAGE functional domains probably play different roles in the carcinogenesis of breast cancer. Thirdly, MAGE-A11 is likely to be more specific in breast cancer than MAGE-A10.
Since the study was a preliminary investigation, the number of patients was relatively small and 26 patients were lost during the follow-up, which may represent a limitation. In addition, the longest follow-up time is 60 months. Accordingly, the observation that the MAGE-A11 has a prognostic role is only an initial hypothesis that should be tested on a much greater number of patients with breast cancer.
References
Bandić D, Juretić A, Sarcević B, Separović V, Kujundzić-Tiljak M, Hudolin T, Spagnoli GC, Cović D, Samija M (2006) Expression and possible prognostic role of MAGE-A4, NY-ESO-1, and HER-2 antigens in women with relapsing invasive ductal breast cancer: retrospective immunohistochemical study. Croat Med J 47(1):32–41
Bergeron A, Picard V, LaRue H, Harel F, Hovington H, Lacombe L, Fradet Y (2009) High frequency of MAGE-A4 and MAGE-A9 expression in high-risk bladder cancer. Int J Cancer 125(6):1365–1371
Brasseur F, Rimoldi D, Liénard D, Lethé B, Carrel S, Arienti F, Suter L, Vanwijck R, Bourlond A, Humblet Y (1995) Expression of MAGE genes in primary and metastatic cutaneous melanoma. Int J Cancer 63(3):375–380
Busam KJ, Iversen K, Berwick M, Spagnoli GC, Old LJ, Jungbluth AA (2000) Immunoreactivity with the anti-MAGE antibody 57B in malignant melanoma: frequency of expression and correlation with prognostic parameters. Mod Pathol 13(4):459–465
De Plaen E, Arden K, Traversari C, Gaforio JJ, Szikora JP, De Smet C, Brasseur F, van der Bruggen P, Lethé B, Lurquin C (1994) Structure, chromosomal localization, and expression of 12 genes of the MAGE family. Immunogenetics 40(5):360–369
Garcia M, Jemal A, Ward EM, Center MM, Hao Y, Siegel RL, Thun MJ (2007) Global cancer facts & figures 2007. American Cancer Society, Atlanta
Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thürlimann B, Senn HJ, Panel members (2005) Meeting highlights: international expert consensus on the primary therapy of early breast cancer. Ann Oncol 16(10): 1569–1583
Karn T, Pusztai L, Ruckhäberle E, Liedtke C, Müller V, Schmidt M, Metzler D, Wang J, Coombes KR, Gätje R, Hanker L, Solbach C, Ahr A, Holtrich U, Rody A, Kaufmann M (2011) Melanoma antigen family A identified by the bimodality index defines a subset of triple negative breast cancers as candidates for immune response augmentation. Eur J Cancer 48(1):12–23
Kufer P, Zippelius A, Lutterbüse R, Mecklenburg I, Enzmann T, Montag A, Weckermann D, Passlick B, Prang N, Reichardt P, Dugas M, Köllermann MW, Pantel K, Riethmüller G (2002) Heterogeneous expression of MAGE-A genes in occult disseminated tumor cells: a novel multimarker reverse transcription-polymerase chain reaction for diagnosis of micrometastatic disease. Cancer Res 62(1):251–261
Ohman Forslund K, Nordqvist K (2001) The melanoma antigen genes–any clues to their functions in normal tissues? Exp Cell Res 2001 265(2): 185–194
Otte M, Zafrakas M, Riethdorf L, Pichlmeier U, Loning T, Jänicke F, Pantel K (2001) MAGE-A gene expression pattern in primary breast cancer. Cancer Res 61(18):6682–6687
Polyak K (2007) Breast cancer: origins and evolution. J Clin Invest 117(11):3155–3163
Rabindran SK (2005) Antitumor activity of HER-2 inhibitors. Cancer Lett 227(1):9–23
Rimoldi D, Salvi S, Schultz-Thater E, Spagnoli GC, Cerottini JC (2000) Anti-MAGE-3antibody 57B and anti-MAGE-1 antibody 6C1 can be used to study different proteins of the MAGE-A family. Int J Cancer 86(5):749–751
Rogner UC, Wilke K, Steck E, Korn B, Poustka A (1995) The melanoma antigen gene (MAGE) family is clustered in the chromosomal band Xq28. Genomics 29(3):725–731
Ross JS, Fletcher JA (1998) The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem cells 16(6):413–428
Ross JS, Fletcher JA, Linette GP, Stec J, Clark E, Ayers M, Symmans WF, Pusztai L, Bloom KJ (2003) The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist 8(4):307–325
Sang M, Wang L, Ding C, Zhou X, Wang L, Lian Y, Shan B (2011a) Melanoma-associated antigen genes-an update. Cancer Lett 302(2):85–90
Sang M, Lian Y, Zhou X, Shan B (2011b) MAGE-A family: attractive targets for cancer immunotherapy. Vaccine 29(47):8496–8500
Scanlan MJ, Simpson AJ, Old LJ (2004) The cancer/testis genes: review, standardization, and commentary. Cancer Immun 23:1
Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ (2005) Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 5(8):615–625
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science, 1989, 244(4905): 707–712
Sugita M, Geraci M, Gao B, Powell RL, Hirsch FR, Johnson G, Lapadat R, Gabrielson E, Bremnes R, Bunn PA, Franklin WA (2002) Combined use of oligonucleotide and tissue microarrays identifies cancer/testis antigens as biomarkers in lung carcinoma. Cancer Res 62(14):3971–3979
Van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T (1991) A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254(5038):1643–1647
Weigelt B, Reis-Filho JS (2009) Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat Rev Clin Oncol 6(12):718–730
Yeon CH, Pegram MD (2005) Anti-erbB-2 antibody trastuzumab in the treatment of HER2-amplified breast cancer. Invest New Drugs 23(5):391–409
Zendman AJ, Ruiter DJ, Van Muijen GN (2003) Cancer/testis-associated genes: identification, expression profile, and putative function. J Cell Physiol 194(3):272–288
Acknowledgments
This work was supported by National Nature Science Foundation of China (No. 81001178). The authors would like to greatly appreciate Dr. Qianglin Duan, a skilled English proofreader from Tongji University for paper revision.
Conflict of interest
The authors had no conflicts of interest to declare in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yishui Lian and Meixiang Sang contribute equally to this work.
Rights and permissions
About this article
Cite this article
Lian, Y., Sang, M., Ding, C. et al. Expressions of MAGE-A10 and MAGE-A11 in breast cancers and their prognostic significance: a retrospective clinical study. J Cancer Res Clin Oncol 138, 519–527 (2012). https://doi.org/10.1007/s00432-011-1122-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-011-1122-x