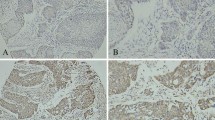
Abstract
Purpose
The purpose of this study was to comprehensively and quantitatively summarize available evidences for the use of VEGF protein to evaluate the clinicopathological and prognostic role of VEGF expression in Asian patients with gastric cancer.
Method
Searches were applied to MEDLINE, EMBASE, and the Cochrane Library databases until June 2010, without language restrictions. A meta-analysis was performed to clarify the impact of VEGF expression on clinicopathological parameters or over survival (OS) in gastric cancer.
Results
Our combined results showed that VEGF expression in Asian patients with gastric cancer was significantly higher in the case–control studies (1,194 patients and 1,618 controls) (odds ratio [OR] = 112.41, 95% confidence interval [CI] = 64.12–197.06). All the analyses estimated favored a stronger link between the high VEGF expression and the poor 5-year overall survival (1,236 patients) (risk ratio [RR] = 2.45, 95% CI = 2.11–2.83, P = 0.000). When stratifying the studies by the pathological variables, the depth of invasion (3,094 patients) (OR = 1.95, 95% CI = 1.40–2.71, P = 0.000), lymph node metastasis (3,240 patients) (OR = 1.82, 95% CI = 1.29–2.57, P = 0.001), distant metastasis (1,980 patients) (OR = 2.76, 95% CI = 1.22–6.25, P = 0.015), vascular invasion (1,803 patients) (OR = 2.61, 95% CI = 2.09–3.27, P = 0.000), and TNM stage (1,819 patients) (OR = 1.92, 95% CI = 1.57–2.36, P = 0.000) provided significant prognostic information.
Conclusion
Our results indicate that VEGF can potently predict the overall survival in Asian patients with gastric cancer. Importantly, VEGF may be converted from candidate to the routine clinical setting for clinicians to predict the outcome of single patient with gastric carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the second leading cause of death among all cancers worldwide. Despite the decline in gastric cancer rates in most of the Western world (Parkin et al. 2005), a 2005 analysis of the incidence and mortality showed that gastric cancer remains a serious fatal disease and the most common cancer in both sexes in Eastern Asia (Parkin et al. 2001). To improve clinical care, biological prognostic markers are highly desirable and must be identified in early stages of gastric cancer.
Angiogenesis is defined as the process of new capillary formation from preexisting vasculature (Folkman 1995). In regulating tumor angiogenesis, the vascular endothelial growth factor (VEGF) family plays a determinant role. VEGF induces cell proliferation, differentiation, and migration of vascular endothelial cells (Ferrara and Davis-Smyth 1997). VEGF is also required for the establishment of haematopoiesis in malignant tumors, which benefits primary tumor growth and metastasis (Kut et al. 2007). Recently, targeting constitutive VEGF and/or its receptors has been an attractive approach for cancer therapy (Jubb et al. 2006).
The prognostic potential of VEGF immunohistochemical expression has been reported by many literatures (Cheng et al. 2010; Wei et al. 2009; Li et al. 2008; Wang et al. 2008; Liu et al. 2007; Chen et al. 2007; Han et al. 2007; Ma et al. 2007; Gao et al. 2007; Lou et al. 2005; Du et al. 2003; Song et al. 2002; Jiao et al. 2005; Tang et al. 2008; Yu et al. 2003; Shi et al. 2003; Feng et al. 2002; Kolev et al. 2007; Mizokami et al. 2006; Urano et al. 2006; Aoyagi et al. 2005; Koga et al. 2004; Kaneko et al. 2003; Takahashi et al. 2003; Kawabe et al. 2002; Kimura et al. 2001; Ichikura et al. 2001; Kabashima et al. 2001; Maehara et al. 2000; Saito et al. 1999; Tomoda et al. 1999; Tanigawa et al. 1997; Baba et al. 1998; Maeda et al. 1998; Lee et al. 2009, 2010; Oh et al. 2008; Choi et al. 2006; Park et al. 2005; Joo et al. 2002, 2003). And, the most widely studied prognostic factors on VEGF refer to variables including tumor size, location, histo-differentiation, depth of invasion, lymph node status, distant metastasis, TNM stage, and vascular invasion. Although implicated in gastric cancer pathogenesis, the results on the correlation between VEGF and those factors are conflicting and inconclusive (Kyzas et al. 2005). It is unknown whether the differences in these investigations have been due to their limited sample size or genuine heterogeneity. It is estimated that almost two-thirds of gastric cancer occur in Asia (China and Japan) (Parkin et al. 2005). Therefore, in order to gain a full insight into the prognostic value of VEGF immunohistochemical expression in Asian patients with gastric cancer, we enrolled data only from cohorts of medical centers in Asia. The prognostic significance of our present analysis allows a better understanding of the natural history of gastric cancer; in addition, the use of VEGF may be converted from candidate to the routine clinical setting for clinicians to predict the outcome of single patient.
Materials and methods
Literature search
The meta-analysis was performed according to a predefined written protocol. Searches were applied to the following electronic databases: MEDLINE, EMBASE, and the Cochrane Library (last search update June 2010), without language restrictions. The search strategy was based on combinations [(VEGF or neovascularization) and “prognosis” and (“gastric” or “stomach”), “carcinoma” or “cancer” or “tumor”]. References of retrieved articles were cross-searched to identify any studies missed by the electronic search strategies. Our initial selection of all candidate articles was relied on careful screening of their abstracts by two independent reviewers. Primary studies that reported data required for meta-analysis were identified and categorized based on full-text review. Authors of eligible studies were contacted for the supplement of additional data relevant to meta-analysis.
Inclusion criteria for primary studies were as follows: (1) proven diagnosis of gastric cancer and normal gastric epithelial mucosa in humans; (2) VEGF evaluation with immunohistochemistry methods, and (3) data performed using cohorts from medical centers in Asian population.
Data extraction
Required information from all full publications was extracted carefully in duplicate by two of the authors (Chen and Li), using a prespecified data collection form with the following item: the first author, year of publication, nation, VEGF assessment method, cutoff value of VEGF positivity, number of readers, blinded reading, number of patients and controls included in the study, number of association between VEGF expression and overall 5-year survival, and number of events in each category of VEGF expression on clinicopathological factors including sex, age, tumor location, size, histo-differentiation, depth of invasion, lymph node status, distant metastasis, TNM stage, and vascular invasion of gastric cancer analyzed. Disagreement was resolved by consensus to all items.
Methodological assessment
There was no prespecified sample size or follow-up period for a study to be included in our meta-analysis. We did not weigh each study by a quality score, because no such score has received general agreement for use in a meta-analysis, especially of observational studies, making more difficult the evaluation of its quality (Altman 2001). Studies were not blinded to the readers, but exclusion was always decided without the knowledge of clinical outcome of each study. We tried carefully to avoid duplication of data, by examining each publication the names of all authors and the different medical centers involved. When studies has same author, however, individuals came from different cohorts, we regarded as two independent data analyzed. Whenever reports pertained to overlapping patients, we selected the study where the most individuals were investigated.
Statistical analysis
Actually, we analyzed three categories of stratified models: the first stratified multivariate model was performed to confirm whether VEGF was highly expressed in gastric cancer patients in comparison with the normal gastric mucosa. The second outcome of meta-analysis was to measure the impact of VEGF expression on survival by estimating the risk ratio (RR) between the positive or negative VEGF groups. And the third interest was to examine the prognostic value of VEGF expression that was corrected for the clinical variables including age, sex, tumor size, location and histo-differentiation, depth of invasion, vascular invasion, lymph node status, distant metastasis, and TNM stage.
According to clinical characteristics, well and moderate differentiation were combined and poor and undifferentiation were combined; T1 and T2 were combined and T3 and T4 were combined; Stage I and Stage II were combined and Stage III and Stage IV were combined; tumors larger than 5 cm in size were combined and tumors less than 5 cm were combined; also, patients who had greater than 60 years of age were combined and patients who had lesser than 60 years of age were combined.
All statistical analyses were performed using Statistical Analysis System software (STATA SE 9.0), and the P value for the summary effect <0.05 with two-tailed was considered statistically significant. The heterogeneity of all involved studies was assessed by a statistical value I 2. When I 2 was lower than 50%, the studies with an acceptable heterogeneity were considered, and then the fixed-effects model with Mantel–Haenszel method was used; otherwise, a random effect model with the DerSimonian and Laird (DL) method was adopted. The combined RRs or odd ratio (ORs) were initially estimated using Forrest plots graphically.
Assessment of publication bias was investigated for each of the pooled study groups mainly by the Egger’s linear regression test. As supplement approach, the Begg’s rank correlation also was applied to assess the potential publication bias, when P > 0.05 was considered that there was no publication bias in the study.
Result
Description of studies identified in meta-analysis
A total of 239 references were retrieved for initial review using search strategies as described. After exclusion of the articles that were out of the scope of our meta-analysis, we identified 99 potential studies for detail evaluation. Upon further review, 58 articles were eliminated due to the following reasons: 5 studies performed different cohorts outside Asian (1 report originated from Timisoara, 1 from Turkey, 1 from Italy, and 2 from Greece); 21 studies overlapped with others; 7 studies measured VEGF with other method rather than immunohistochemistry; and 25 studies lacked informative clinical data to create 2 × 2 tables for meta-analysis. Finally, 18 studies were performed on the association of VEGF expression between gastric cancer and normal gastric mucosa (Cheng et al. 2010; Li et al. 2008; Liu et al. 2007; Chen et al. 2007; Han et al. 2007; Du et al. 2003; Song et al. 2002; Jiao et al. 2005; Tang et al. 2008; Feng et al. 2002; Koga et al. 2004; Kawabe et al. 2002; Saito et al. 1999; Tomoda et al. 1999; Maeda et al. 1998; Lee et al. 2010; Joo et al. 2002, 2003); 11 studies dealt with the impact of VEGF expression on overall survival (Wei et al. 2009; Li et al. 2008; Ma et al. 2007; Kolev et al. 2007; Aoyagi et al. 2005; Kimura et al. 2001; Ichikura et al. 2001; Maehara et al. 2000; Saito et al. 1999; Maeda et al. 1998; Song et al. 2002); and 31 studies evaluated the prognostic value of VEGF expression and clinicopathological factors (Cheng et al. 2010; Li et al. 2008; Wang et al. 2008; Ma et al. 2007; Gao et al. 2007; Lou et al. 2005; Du et al. 2003; Song et al. 2002; Tang et al. 2008; Yu et al. 2003; Shi et al. 2003; Feng et al. 2002; Kolev et al. 2007; Mizokami et al. 2006; Urano et al. 2006; Kaneko et al. 2003; Takahashi et al. 2003; Kimura et al. 2001; Ichikura et al. 2001; Kabashima et al. 2001; Maehara et al. 2000; Saito et al. 1999; Tanigawa et al. 1997; Baba et al. 1998; Maeda et al. 1998; Lee et al. 2009, 2010; Oh et al. 2008; Choi et al. 2006; Park et al. 2005; Joo et al. 2002, 2003). After selection, a total of 41 literatures were finally enrolled in our meta-analysis including both English and non-English language articles, 5 for Chinese with English abstract (Wei et al. 2009; Wang et al. 2008; Chen et al. 2007; Du et al. 2003; Jiao et al. 2005), and 1 for Korean (Park et al. 2005). For all the patients, measurements had been taken in the primary tumor, and all specimens had been taken before chemotherapy or radiotherapy. The main features of eligible studies in our meta-analysis and the number of relation of VEGF expression with clinicopathological variables are summarized in Tables 1 and 2, respectively.
Main results
Correlation of VEGF expression between gastric cancer and normal gastric mucosa
The combined results from all studies showed that VEGF expression in patients with gastric cancer was extremely higher in comparison with the normal gastric mucosa in 18 studies (1,194 patients and 1,618 controls) (OR = 112.41; 95% CI = 64.12–197.06; P = 0.000). When stratifying for race, results were similar among China, Japan, and Korea (Table 3).
Correlation between VEGF expression and overall survival in 5 years
In total, there were 11 studies including 1,236 patients to evaluate the relation of VEGF expression and overall 5-year survival. All studies favored a stronger link between high VEGF expression and poor survival. Mortality was much higher in VEGF-positive patients than VEGF-negative patients among Asians (RR = 2.45; 95% CI = 2.11–2.83; P = 0.000). When stratifying for race, results were also consistent with China and Japan (Figs. 1, 2, 8; Table 3).
Correlation between VEGF expression and clinicopathological characteristics
When stratifying for the different variables by the depth of invasion of gastric cancer, statistical significance was observed. Patients with T3 and T4 gastric cancer had a much higher VEGF expression in 25 studies (3,094 patients) (OR = 1.95; 95% CI = 1.40–2.71; P = 0.000) than those with T1 and T2 gastric cancer (Fig. 3; Table 3). When stratifying for lymph node status of gastric cancer, statistically significant results also appeared that VEGF expression was associated with lymph node metastasis in 28 studies (3,240 Asian patients) (OR = 1.82; 95% CI = 1.29–2.57, P = 0.001) (Fig. 4; Table 3), but not in Japanese in 11 studies (1,308 patients) (OR = 0.97; 95% CI = 0.61–1.57; P = 0.915). When stratifying for the distant metastasis of gastric cancer, there was a statistical significance that VEGF expression was associated with distant metastasis in 16 studies (1,980 patients) (OR = 2.76; 95% CI = 1.22–6.25; P = 0.015) (Fig. 5; Table 3), but negative in both Japanese in 6 studies (768 patients) (OR = 2.37; 95% CI = 0.85–6.57; P = 0.097) and Korean in 5 studies (813 patients) (OR = 0.89; 95% CI = 0.26–2.99; P = 0.848). When stratifying for vascular invasion, the overexpression of VEGF is significantly linked to the presence of vascular invasion in 14 studies (1,803 patients) (OR = 2.61; 95% CI = 2.09–3.27; P = 0.000) (Fig. 6; Table 3). When further stratifying for TNM stage, VEGF expression of patients with stages III and IV gastric cancer was much higher than those with stage I and II gastric cancer in 17 studies (1,819 patients) (OR = 1.92; 95% CI = 1.57–2.36; P = 0.000) (Figs. 7, 8; Table 3).
We also observed trends toward a correlation of VEGF positivity with age older than 60 years (P = 0.005), but not for sex (P = 0.331), size (P = 0.551), location (P = 0.837), and degree of differentiation (P = 0.396) in the whole Asians, except for those patients with poor differentiation in Chinese who had a significantly higher VEGF expression than those with well differentiation (1,028 patients) (OR = 1.82; 95% CI = 1.25–2.66; P = 0.002). Men tended to have a higher VEGF expression than women in Korea including 9 studies (1,213 patients), but the effect was modest (OR = 1.43; 95% CI = 1.04–1.99, P = 0.03) (Table 3).
Assessment of publication bias
Egger′s linear regression test and Begg’s test were used to examine publication bias. The potential for publication bias could not be ruled out except assessment of the relation between VEGF expression and histo-differentiation; however, the effect of bias was slight (P = 0.033) (Table 3).
Discussion
To our best knowledge, it is the first time that a comprehensive and detailed meta-analysis has assessed the prognostic role of VEGF for gastric cancer clinical outcome. High VEGF expression, as detected by immunohistochemistry, was confirmed in patients with gastric cancer according to the evidence-based medicine in our study. The pooled statistical data showed that VEGF protein, an independent marker of angiogenesis, can potently predict the 5-year survival. Further, when stratifying for baseline characteristics of patients, including sex, age, tumor size, location, histo-differentiation, depth of invasion, lymph node status, distant metastasis, vascular invasion, and TNM stage, our results showed that VEGF expression provided significant prognostic value, which increased the predictive accuracy of prognosis in patients with gastric cancer.
Our current finding was in agreement with the recent meta-analysis reports on VEGF expression in hepatocellular cancer (Schoenleber et al. 2009), colorectal cancer (Des Guetz et al. 2006), and head and neck squamous cell carcinoma (Kyzas et al. 2005). As a rule of the thumb, a prognostic factor with RR > 2 is considered as useful practical value (Hayes et al. 2001). In the present study, we found the global RR is 2.45, indicating that the statistical link between VEGF expression and survival in gastric cancer was rather strong. Although there was heterogeneity between studies, the effect was modest (I 2 = 51%), and all of the studies were in the same direction. During analysis, we strictly considered the most important variables that might confound the impact of high VEGF expression on survival. Our results showed that VEGF expression was significantly correlated with the depth of invasion, lymph node status, distant metastasis, vascular invasion, and poor TNM staging (Table 3), which collectively contribute to the survival of patients with gastric cancer. Although VEGF initially had no association with lymphangiogenesis (Jubb et al. 2006), recent experimental studies showed that VEGF overexpression could induce the formation of new lymph vessels (Nagy et al. 2002; Kunstfeld et al. 2004) by targeting VEGF receptor 2 (VEGFR2) signaling pathway. Furthermore, VEGF overexpression was correlated with larger metastatic deposits, which has been found in malignant lymphoma (Kadowaki et al. 2005), lung cancer (Niki et al. 2000), and breast cancer (Mohammed et al. 2007). All of these reports were consistent with our finding in gastric cancer. High VEGF expression induces the formation of a rich vascular network and nutritious environment (Breslin et al. 2003), which is an active process that requires the degradation of the extracellular matrix and the increase in vascular permeability both in blood and lymphatic vessels, favoring the progression of tumor cells into the vascular space and lymphatics. This may offer a possible explanation for the observed strong statistical association between VEGF overexpression and tumor invasion and metastasis. Our present study is the first to reinforce the relationship between tumor angiogenesis and the spread of metastases in gastric cancer.
Interestingly, in the meta-analysis of subgroups, we also observed the correlation of VEGF positivity with older patients (P = 0.005) and poor differentiation in Chinese (P = 0.002), which may explain its prognostic effect to some extent. Similar findings were also reported by other studies both on age (Lee et al. 2009; Oh et al. 2008; Park et al. 2005; Joo et al. 2002, 2003) and histo-differentiation (Kyzas et al. 2005). We did not detect significant heterogeneity for the above two independent factors; however, further studies are intended to assess the relation of VEGF on histo-differentiation and sex.
Several limitations of the current studies could not be ignored. First, although we did not detect significant publication bias between studies, it is uncertain whether the cases are comparably representative in Asia. All the patients and references enrolled in our meta-analysis came from main cancer centers, and they are observational studies, more prone to many biases than prospective randomized controlled studies. Obviously, we could not account for unpublished studies, and it is unavoidable that some data could still be missing. Missing information may reflect “negative” or more conservative association of VEGF with overall survival, which could reduce the significance of VEGF expression as a predictor of mortality (Uzzan et al. 2004). The discrepancies in the conclusion of various studies encouraged researchers to publish their data whatever the results mean, thus limiting the publication bias. Secondly, studies included in our meta-analysis used immunohistochemistry to assess VEGF expression status, which represented potential selection bias. The choice of the cutoff value for VEGF positivity varied from 5 to 50% among studies, whereas 19 studies used 10%. The most commonly used VEGF antibody was A20 (Santa Cruz Biotechnology, Santa Cruz, CA). And 7 studies had evaluated the correlation between VEGF and clinical outcome using reverse transcription-PCR, ELISA, or western blotting. Although results obtained from different methods are fixed, these findings are consistent with our meta-analysis. And finally, it should be noted that several potential sources of heterogeneity were identified to investigate the variables, including “the depth of invasion,” “lymph node status,” and “distant metastasis.” This may contribute to the variability in assessment of these variables between different studies. However, the DerSimonian and Laird method (random effect model) we used took them together into account.
In conclusion, our meta-analysis showed that VEGF overexpression has a detrimental effect on survival in Asian patients with gastric cancer. VEGF protein might be a powerful prognostic marker, which can help to identify high-risk patients earlier and guide clinical decision-making regarding therapy and outcome. However, this conclusion should be interpreted cautiously since this analysis would ideally be performed on individual patient data. Further investigation into this subset of patients from other cohorts should assess the generalization of results before VEGF is implemented in the routine clinical management of gastric cancer.
Abbreviations
- VEGF:
-
Vascular endothelial growth factor
- RR:
-
Risk ratio
- OR:
-
Odds ratio
- OS:
-
Overall survival
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
References
Altman DG (2001) Systematic reviews of evaluations of prognostic variables. BMJ 323:224–228
Aoyagi K, Kouhuji K, Yano S, Miyagi M, Imaizumi T, Takeda J, Shirouzu K (2005) VEGF significance in peritoneal recurrence from gastric cancer. Gastric Cancer 8:155–163
Baba M, Konno H, Maruo Y, Tanaka T, Kanai T, Matsumoto K, Matsuura M, Nishino N, Maruyama K, Nakamura S, Baba S (1998) Relationship of p53 and vascular endothelial growth factor expression of clinicopathological factors in human scirrhous gastric cancer. Eur Surg Res 30:130–137
Breslin JW, Pappas PJ, Cerveira JJ, Hobson RW 2nd, Duran WN (2003) VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am J Physiol Heart Circ Physiol 284:H92–H100
Chen W, Ouyang XN, Lin QC (2007) Study on the relationship between vascular endothelial growth factor and syndrome type of traditional Chinese medicine in patients with gastric carcinoma. Zhongguo Zhong Xi Yi Jie He Za Zhi 27:127–130
Cheng P, Jiang FH, Zhao LM, Dai Q, Yang WY, Zhu LM, Wang BJ, Xu C, Bao YJ, Zhang YJ (2010) Human macrophage metalloelastase correlates with angiogenesis and prognosis of gastric carcinoma. Dig Dis Sci
Choi JH, Ahn MJ, Park CK, Han HX, Kwon SJ, Lee YY, Kim IS (2006) Phospho-Stat3 expression and correlation with VEGF, p53, and Bcl-2 in gastric carcinoma using tissue microarray. APMIS 114:619–625
Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, Breau JL, Perret GY (2006) Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer 94:1823–1832
Du JR, Jiang Y, Zhang YM, Fu H (2003) Vascular endothelial growth factor and microvascular density in esophageal and gastric carcinomas. World J Gastroenterol 9:1604–1606
Feng CW, Wang LD, Jiao LH, Liu B, Zheng S, Xie XJ (2002) Expression of p53, inducible nitric oxide synthase and vascular endothelial growth factor in gastric precancerous and cancerous lesions: correlation with clinical features. BMC Cancer 2:8
Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18:4–25
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1:27–31
Gao ZL, Zhang C, Du GY, Lu ZJ (2007) Clinical significance of changes in tumor markers, extracellular matrix, MMP-9 and VEGF in patients with gastric carcinoma. Hepatogastroenterology 54:1591–1595
Han JC, Zhang KL, Chen XY, Jiang HF, Kong QY, Sun Y, Wu ML, Huang L, Li H, Liu J (2007) Expression of seven gastric cancer-associated genes and its relevance for Wnt, NF-kappaB and Stat3 signaling. APMIS 115:1331–1343
Hayes DF, Isaacs C, Stearns V (2001) Prognostic factors in breast cancer: current and new predictors of metastasis. J Mammary Gland Biol Neoplasia 6:375–392
Ichikura T, Tomimatsu S, Ohkura E, Mochizuki H (2001) Prognostic significance of the expression of vascular endothelial growth factor (VEGF) and VEGF-C in gastric carcinoma. J Surg Oncol 78:132–137
Jiao ZY, Gou CZ, Cao N, Li YM (2005) Correlation of tissue factor expression to angiogenesis of gastric carcinoma and its clinical significance. Ai Zheng 24:880–884
Joo YE, Sohn YH, Joo SY, Lee WS, Min SW, Park CH, Rew JS, Choi SK, Park CS, Kim YJ, Kim SJ (2002) The role of vascular endothelial growth factor (VEGF) and p53 status for angiogenesis in gastric cancer. Korean J Intern Med 17:211–219
Joo YE, Rew JS, Seo YH, Choi SK, Kim YJ, Park CS, Kim SJ (2003) Cyclooxygenase-2 overexpression correlates with vascular endothelial growth factor expression and tumor angiogenesis in gastric cancer. J Clin Gastroenterol 37:28–33
Jubb AM, Oates AJ, Holden S, Koeppen H (2006) Predicting benefit from anti-angiogenic agents in malignancy. Nat Rev Cancer 6:626–635
Kabashima A, Maehara Y, Kakeji Y, Sugimachi K (2001) Overexpression of vascular endothelial growth factor C is related to lymphogenous metastasis in early gastric carcinoma. Oncology 60:146–150
Kadowaki I, Ichinohasama R, Harigae H, Ishizawa K, Okitsu Y, Kameoka J, Sasaki T (2005) Accelerated lymphangiogenesis in malignant lymphoma: possible role of VEGF-A and VEGF-C. Br J Haematol 130:869–877
Kaneko T, Konno H, Baba M, Tanaka T, Nakamura S (2003) Urokinase-type plasminogen activator expression correlates with tumor angiogenesis and poor outcome in gastric cancer. Cancer Sci 94:43–49
Kawabe A, Shimada Y, Uchida S, Maeda M, Yamasaki S, Kato M, Hashimoto Y, Ohshio G, Matsumoto M, Imamura M (2002) Expression of cyclooxygenase-2 in primary and remnant gastric carcinoma: comparing it with p53 accumulation, Helicobacter pylori infection, and vascular endothelial growth factor expression. J Surg Oncol 80:79–88
Kimura H, Konishi K, Nukui T, Kaji M, Maeda K, Yabushita K, Tsuji M, Miwa A (2001) Prognostic significance of expression of thymidine phosphorylase and vascular endothelial growth factor in human gastric carcinoma. J Surg Oncol 76:31–36
Koga T, Shibahara K, Kabashima A, Sumiyoshi Y, Kimura Y, Takahashi I, Kakeji Y, Maehara Y (2004) Overexpression of cyclooxygenase-2 and tumor angiogenesis in human gastric cancer. Hepatogastroenterology 51:1626–1630
Kolev Y, Uetake H, Iida S, Ishikawa T, Kawano T, Sugihara K (2007) Prognostic significance of VEGF expression in correlation with COX-2, microvessel density, and clinicopathological characteristics in human gastric carcinoma. Ann Surg Oncol 14:2738–2747
Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, Lin C, Fiebiger E, Wei X, Wu Y, Hicklin D, Bohlen P, Detmar M (2004) Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood 104:1048–1057
Kut C, Mac Gabhann F, Popel AS (2007) Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br J Cancer 97:978–985
Kyzas PA, Cunha IW, Ioannidis JP (2005) Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res 11:1434–1440
Lee SJ, Kim JG, Sohn SK, Chae YS, Moon JH, Kim SN, Bae HI, Chung HY, Yu W (2009) No association of vascular endothelial growth factor-A (VEGF-A) and VEGF-C expression with survival in patients with gastric cancer. Cancer Res Treat 41:218–223
Lee SA, Choi SR, Jang JS, Lee JH, Roh MH, Kim SO, Kim MC, Kim SJ, Jeong JS (2010) Expression of VEGF, EGFR, and IL-6 in gastric adenomas and adenocarcinomas by endoscopic submucosal dissection. Dig Dis Sci 55:1955–1963
Li SG, Ye ZY, Zhao ZS, Tao HQ, Wang YY, Niu CY (2008) Correlation of integrin beta3 mRNA and vascular endothelial growth factor protein expression profiles with the clinicopathological features and prognosis of gastric carcinoma. World J Gastroenterol 14:421–427
Liu L, Li Z, Feng G, You W, Li J (2007) Expression of connective tissue growth factor is in agreement with the expression of VEGF, VEGF-C, -D and associated with shorter survival in gastric cancer. Pathol Int 57:712–718
Lou G, Gao Y, Ning XM, Zhang QF (2005) Expression and correlation of CD44v6, vascular endothelial growth factor, matrix metalloproteinase-2, and matrix metalloproteinase-9 in Krukenberg tumor. World J Gastroenterol 11:5032–5036
Ma J, Zhang L, Ru GQ, Zhao ZS, Xu WJ (2007) Upregulation of hypoxia inducible factor 1alpha mRNA is associated with elevated vascular endothelial growth factor expression and excessive angiogenesis and predicts a poor prognosis in gastric carcinoma. World J Gastroenterol 13:1680–1686
Maeda K, Kang SM, Onoda N, Ogawa M, Sawada T, Nakata B, Kato Y, Chung YS, Sowa M (1998) Expression of p53 and vascular endothelial growth factor associated with tumor angiogenesis and prognosis in gastric cancer. Oncology 55:594–599
Maehara Y, Kabashima A, Koga T, Tokunaga E, Takeuchi H, Kakeji Y, Sugimachi K (2000) Vascular invasion and potential for tumor angiogenesis and metastasis in gastric carcinoma. Surgery 128:408–416
Mizokami K, Kakeji Y, Oda S, Irie K, Yonemura T, Konishi F, Maehara Y (2006) Clinicopathologic significance of hypoxia-inducible factor 1alpha overexpression in gastric carcinomas. J Surg Oncol 94:149–154
Mohammed RA, Green A, El-Shikh S, Paish EC, Ellis IO, Martin SG (2007) Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis. Br J Cancer 96:1092–1100
Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, Lawitts JA, Benjamin L, Tan X, Manseau EJ, Dvorak AM, Dvorak HF (2002) Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med 196:1497–1506
Niki T, Iba S, Tokunou M, Yamada T, Matsuno Y, Hirohashi S (2000) Expression of vascular endothelial growth factors A, B, C, and D and their relationships to lymph node status in lung adenocarcinoma. Clin Cancer Res 6:2431–2439
Oh SY, Kwon HC, Kim SH, Jang JS, Kim MC, Kim KH, Han JY, Kim CO, Kim SJ, Jeong JS, Kim HJ (2008) Clinicopathologic significance of HIF-1alpha, p53, and VEGF expression and preoperative serum VEGF level in gastric cancer. BMC Cancer 8:123
Park GS, Joo YE, Kim HS, Choi SK, Rew JS, Park CS, Kim SJ (2005) Expression of PTEN and its correlation with angiogenesis in gastric carcinoma. Korean J Gastroenterol 46:196–203
Parkin DM, Bray F, Ferlay J, Pisani P (2001) Estimating the world cancer burden: Globocan 2000. Int J Cancer 94:153–156
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Saito H, Tujitani S, Ikeguchi M, Maeta M, Kaibara N (1999) Neoangiogenesis and relationship to nuclear p53 accumulation and vascular endothelial growth factor expression in advanced gastric carcinoma. Oncology 57:164–172
Schoenleber SJ, Kurtz DM, Talwalkar JA, Roberts LR, Gores GJ (2009) Prognostic role of vascular endothelial growth factor in hepatocellular carcinoma: systematic review and meta-analysis. Br J Cancer 100:1385–1392
Shi H, Xu JM, Hu NZ, Xie HJ (2003) Prognostic significance of expression of cyclooxygenase-2 and vascular endothelial growth factor in human gastric carcinoma. World J Gastroenterol 9:1421–1426
Song ZJ, Gong P, Wu YE (2002) Relationship between the expression of iNOS, VEGF, tumor angiogenesis and gastric cancer. World J Gastroenterol 8:591–595
Takahashi R, Tanaka S, Kitadai Y, Sumii M, Yoshihara M, Haruma K, Chayama K (2003) Expression of vascular endothelial growth factor and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncology 64:266–274
Tang H, Wang J, Bai F, Zhai H, Gao J, Hong L, Xie H, Zhang F, Lan M, Yao W, Liu J, Wu K, Fan D (2008) Positive correlation of osteopontin, cyclooxygenase-2 and vascular endothelial growth factor in gastric cancer. Cancer Invest 26:60–67
Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T (1997) Correlation between expression of vascular endothelial growth factor and tumor vascularity, and patient outcome in human gastric carcinoma. J Clin Oncol 15:826–832
Tomoda M, Maehara Y, Kakeji Y, Ohno S, Ichiyoshi Y, Sugimachi K (1999) Intratumoral neovascularization and growth pattern in early gastric carcinoma. Cancer 85:2340–2346
Urano N, Fujiwara Y, Doki Y, Tsujie M, Yamamoto H, Miyata H, Takiguchi S, Yasuda T, Yano M, Monden M (2006) Overexpression of hypoxia-inducible factor-1 alpha in gastric adenocarcinoma. Gastric Cancer 9:44–49
Uzzan B, Nicolas P, Cucherat M, Perret GY (2004) Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res 64:2941–2955
Wang XL, Ai ZS, Fang JP, Tang RY, Chen XM (2008) Expression of vascular endothelial growth factors (VEGF)-A, -C and -D and their prognostic significance and relationship with angio- and lymphangiogenesis in gastric cancer. Zhonghua Zhong Liu Za Zhi 30:837–843
Wei YZ, Li CF, Xue YW (2009) Expression of transcription factor SP1, vascular endothelial growth factor and CD34 in serosa-infiltrating gastric cancer and their relationship with biological behavior and prognosis. Zhonghua Wei Chang Wai Ke Za Zhi 12:145–149
Yu HG, Li JY, Yang YN, Luo HS, Yu JP, Meier JJ, Schrader H, Bastian A, Schmidt WE, Schmitz F (2003) Increased abundance of cyclooxygenase-2 correlates with vascular endothelial growth factor-A abundance and tumor angiogenesis in gastric cancer. Cancer Lett 195:43–51
Acknowledgments
This work is supported by the Fundamental Research Funds for the Central Universities (78210021), the Chenguang Program from Shanghai Municipal Education Commission (10CG25), and the National Natural Science Foundation of China (81101683).
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., Li, T., Wu, Y. et al. Prognostic significance of vascular endothelial growth factor expression in gastric carcinoma: a meta-analysis. J Cancer Res Clin Oncol 137, 1799–1812 (2011). https://doi.org/10.1007/s00432-011-1057-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-011-1057-2