Abstract
Background
The prognosis of HCC remains poor mainly because of the lack of diagnosis biomarkers especially in patients with cirrhosis background.
Methods
To identify serum biomarkers for HCC, we use cleavable stable isotope labeling (cICAT) combined with LC–electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) to compare the serum proteome between liver cirrhosis and HCC patients. Sera from nine liver cirrhosis patients and nine HCC patients were selected for screening study. Then the alpha-1-acid glycoprotein (AGP) was chosen for validation by western blot. AGP was measured in the separate validation study including 52 HCC patients and 40 liver cirrhosis patients by rate nephelometry. Its value for HCC diagnosis from liver cirrhosis was also appreciated through receiver operating curve (ROC).
Results
We quantified and identified 31 different proteins which include AGP, complement C4, haptoglobin, alpha-1-antitrypsin precursor, alpha-2-macroglobulin precursor, prothrombin precursor and ubiquitin carboxyl-terminal hydrolase 44 (USP44), etc. The serum concentration of AGP in HCC patients (n = 52) was significantly higher than in liver cirrhosis (n = 40) (P < 0.005) in validation study. AGP was useful for discrimination of the HCC cases from LC patients when the AFP levels were below 500 ng/ml. The area under curve (AUC) of the AGP and the combination of AGP and AFP were 0.834 (P < 0.0005, 95% CI: 0.734–0.934) and 0.880 (P < 0.0005, 95% CI, 0.789-0.970) separately, which are higher than AFP alone (0.538, P = 0.604, 95% CI: 0.386–0.689).
Conclusion
cICAT combined with LC-MS/MS-based serum proteome analysis can be useful in the screening of serum biomarkers for HCC. Alpha-1-acid glycoprotein combining AFP could aid the diagnosis of HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a leading cause of cancer death worldwide and the overall 5-year survival rate remains less than 5% (Parkin et al. 2005). Chronic liver injury and cirrhosis, most often caused by viral hepatitis or alcoholism, are the major risk factors for development of HCC since most HCCs arise in patients with coexisting chronic liver disease and liver cirrhosis (LC). Worldwide, more than 52% of HCC cases are associated with chronic hepatitis B virus (HBV) infection and 25% with hepatitis C virus (HCV) infection (Liaw 2005). Surgical resection, liver transplantation are only applicable to those in whom the tumor is detected at an early stage, typically less than 3 cm in diameter without vascular involvement, but tumors rarely present with symptoms at this stage (Bruix et al. 2001). Current methods for the diagnosis of HCC rely on serological markers such as alpha fetoprotein (AFP) and certain liver enzymes, together with physical assessments and imaging technologies (e.g. ultrasound or computed tomography) (Caturelli et al. 2002). These serum biomarkers are much less useful in patients with earlier/small tumors where its sensitivity is low (Bolondi 2003; Ward and Robinson 2002). Therefore, new predictor has become a priority and would obviously improve the prognosis.
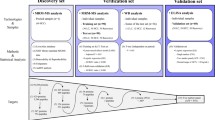
The discovery of novel candidate protein biomarkers from serum using high-throughput proteomic technologies has extracted enthusiasm in the biomedical research community. Two-dimensional gel electrophoresis (2 DE) is a widely used and robust method for studying differentially expressed proteins between cancer and non-cancer. Steel et al. (2003) has found apoA1 and complement C3 as the serum marker for HCC patient through 2DE strategy. Undoubtedly 2DE has the limit in narrow resolution magnitude (102) and labor consuming. SELDI TOF/MS is able to analyze the proteins with low molecular weight in serum, which is a complementation to 2 DE. This strategy has been used previously to identify peak patterns in the mass spectrum of serum that distinguish HCC from non-HCC/cirrhosis samples (Cui et al. 2007). A common pitfall of SELDI, however, is the inability to determine the actual proteins or peptides that may be contributing to these differential peak patterns. LC-MS/MS is an alternative approach that couples protein fractionation with mass spectrometry to generate a comprehensive catalog of the proteome of analyzed fluid samples. The majority of these relative quantification techniques employ the introduction of stable isotopes into the samples such as isotope-coded affinity tags (ICAT), amine-reactive isobaric tagging reagents (iTRAQ), in vivo stable isotope labeling with amino acids in cell culture (SILAC), and 18O labeling (Gygi et al. 1999; Ross et al. 2004; Kuster et al. 2005).
In this study, LC-MS approach combined with ICAT is presented to quantitatively compare the serum of HCC and cirrhosis patients. We quantified and identified 31 proteins differentially expressed between liver cirrhosis and HCC patients group. Alpha-1-acid glycoprotein (AGP) was further validated; area under ROC for AGP was calculated to evaluate the ability for diagnosis of HCC.
Materials and methods
Patients
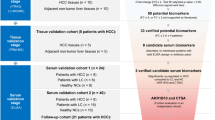
Serum samples were collected between March 2005 and December 2007 at the Affiliated Hospital of Guangxi Medical University, Nanning, China. Sample collection was officially registered and all patients gave informed consent. All patients were tested positive for hepatitis B on the day of sampling. Patients with other liver diseases, HCV co-infections, other melanomas or antiviral treatment were excluded. Hepatocellular carcinoma was diagnosed histologically according to the Barcelona criteria (Bruix et al. 2001). All cirrhosis patients were followed for at least 18 months to exclude individuals with asymptomatic HCC. Classification of the cirrhosis stage was done according to Child’s classification. Venous blood samples were taken in the morning’s fasting state. After at least 30 min, but within 2 h, the tubes were centrifuged at 20°C for 15 min at 1,200g, and the sera were stored frozen in plastic vials at −80°C until the time of consecutive analyses.
The information of patients is listed in Table 1. Totally 49 clinical pathologically proven liver cirrhosis and 61 hepatocellular carcinoma patients were enrolled. Equal volume from nine individuals of each group constitutes the pool of LC and HCC for screening study in ICAT analysis. The remaining 40 sera of LC and 52 sera of HCC were used for validation study in nephelometry assay.
ICAT labeling and MS spectrometry
Before labeling, serum albumin and immunoglobulin were depleted through Montage Albumin depletion kit (Millipore) and protein G Sepharose (Sigma) according to the manufacturer’s instructions. YM-3 centrifugal filter (Millipore) was used for desalting and concentration. The protein concentration of all concentrated elution was measured by 2D Quant protein assay (Amersham Biosciences, USA).
Protease inhibitor PMSF was added to depleted serum samples at a final concentration of 0.5 mM to prevent protein degradation. For each group, 0.1 mg protein was labeled with the acid cleavable ICAT reagents, either in an isotopically light C12 for pooled liver cirrhosis depleted serum or in heavy C13 isoform for HCC depleted serum. Reagents and detailed protocol for ICAT labeling were obtained from Applied Biosystems as a Commercial Kit (ABI, USA). The two labeled samples were then combined and digested into peptides by trypsin (Promega, USA) at 37°C overnight. ICAT-labeled peptides were subsequently separated from the excess ICAT reagents and detergents in the lysis buffer and were fractioned with strong cation exchange chromatography (SCX). The ICAT-labeled peptides were first acidified with mobile phase A (10 mM KH2PO4, 20% ACN at pH 2.7) to a final volume of 500 μL and injected. The peptides were first separated at 1.0 mL/min with 0–25% mobile phase B (350 mM KCl, 10 mM KH2PO4, and 20% ACN at pH 2.7) for 30 min, followed by 25–100% B over 20 min, and remained at 100% B for an additional 10 min. Peptide fractions were collected at 2 min intervals and dried. ICAT-labeled peptides were then purified by avidin-affinity cartridges (ABI) and dried. Biotin moieties were cleaved off with TFA at 37°C for 2 h. After the cleavage, the peptides were dried and reconstituted in 5% ACN and 0.1% TFA (mobile phase A for reversed phase LC, RPLC) and then separated with microcapillary reverse-phase column in line with Finnigan LCQ ion trap mass spectrometer. RPLC gradient consisted of first 5–30% mobile phase B (MPB, 95% ACN, and 0.1% TFA) over 120 min, then 30–90% MPB for 15 min, and maintained at 90% MPB for an additional 10 min at a flow rate of 400 nL/min.
Peptide identification by collision induced dissociation (CID) was carried out in an automated fashion followed by automated data processing for protein identification.
MS data analysis
Tandem mass spectrometry data was analyzed by using the database search software SEQUEST (Bioworks 3.2, ThermoFinnigan, USA) to interpret MS/MS spectra. The DTA files were generated from production scan data with the threshold intensity set at 10,000 and these were used to search against the IPI human protein database (version 3.35). Peptides that showed SEQUEST scores >2, and delta correlation scores (DelCN, ΔCn) of ≥0.1 were further analyzed by detailed spectral analysis. SEQUEST search parameters were set as follows: static modification was set to +330.1362 for ICAT-labeled cysteine, differential modification of +9.0302 for heavy ICAT-labeled cysteine residues, +16 for oxidized methionine. The XPRESS software 3.2 automates protein expression calculations by accurately quantifying the relative abundance of ICAT-labeled peptides from their chromatographic coelution profiles. Starting with the peptide identification, XPRESS isolates the C12 and C13 peptide elution profiles, determines the area of each peptide peak, and calculates the abundance ratio based on these areas.
Western blot assay
We selected alpha1-acid glycoprotein for validation through western blot in 18 sera of screening group. A 0.5 μL aliquot of serum was electrophoresed on a 10% polyacrylamide gel and transferred onto a PVDF membrane (Millipore). The membranes were incubated with 5% skim milk in phosphate buffered saline (PBS) overnight and then incubated with 1/2,000 diluted anti human AGP antibody (AbFrontier, Korea) for 2 h. After washing three times with tris-buffered saline-T (TBST) (150 mM NaCl, 50 mM Tris, 0.1% Tween 20, pH 7.4) for 10 min each, the membrane was incubated with peroxidase-conjugated rabbit IgG for 1 h. After washing the membrane three times with TBST for 10 min each, development was performed using a SuperSignal West Chemiluminescent Substrate (Pierce, USA) according to standard protocols. The band intensity was analyzed by scanning densitometry (Quantity One, Bio-Rad, USA).
Nephelometry assay
Serum AGP concentrations were measured in total 52 HCC patients and 40 LC patients through nephelometry using commercial kit (Beckman Coulter), 20 normal cohorts was also assayed as control.
Statistical analysis
Student’s t test was used to compare the variables between the study groups, P < 0.05 was thought as significant difference. Receiver operating characteristics (ROC) curves were constructed by calculating the sensitivities and specificities of a biomarker or the diagnostic score of a logistic regression model at different cutoff points for differentiating HCC cases from liver cirrhosis.
Results
ICAT Identification and quantification result
Serum proteins from HCC group had heavy tag, and proteins from LC group had light tag. The protein quantification and identification were accomplished using XPRESS and SEQUEST software tools. 329 peptides which contain at least one ICAT modified cysteine were identified with a high level of confidence (>99%) on the basis of matching of tandem mass spectra of peptides to peptide sequences in the NCBI database. To obtain a cutoff value of the ICAT ratio that represents a significant difference, we performed a preliminary ICAT experiment of bovine serum albumin (BSA) standards in four predefined heavy-to-light ICAT ratios to assess the degree of random analytical variations. In all four samples, the CV values were less than 10%. Therefore, 20% (double CV) was set as cutoff value for the ICAT ratio reflected the genuine quantitative differences. ICAT ion pairs with at least 20% change were determined to have significant expression differences. Following review of all peptide match spectra and exclusion of redundant matches, we identified and quantified 31 differential expressed proteins with 20% change (Table 2) including 22 up-regulated and 19 down-regulated. Of them, 19 proteins (61%) were identified based on two or more peptides matches; 12 proteins (39%) were identified based on a single peptide match. The proteins were assigned into 13 functional categories based mainly on protein function as shown in Table 2. The MS/MS spectrum of a peptide KEQLGEFYEALDC*LR from alpha1-acid glycoprotein was presented with a complete series of y-ions and b-ions for confident identification (Fig. 1).The observation of a mass difference of 339 Da between y2 and y3 ions matched the mass of a heavy ICAT reagent modified cysteine.
Identification of the peptide KEQLGEFYEALDC*LR from Alpha-1-acid glycoprotein by MS/MS. a The relative abundance and the calculated C12:C13 ratio obtained using XPRESS software. b Subjected to alternating MS/MS scans of the peptide, the identity of the parent protein determined by sequence database searching using SEQUEST. A mass difference of 339 Da between y2 and y3 ions matched the mass of a heavy ICAT reagent modified cysteine. c Bolds showed the matched peptides (C* designates cysteine labeled with the heavy form of ICAT reagent
It should be noted that eight of the identified proteins had not been reported previously as differentially expressed in the HCC serum proteome, such as isoform 1 of lymphoid-specific helicase, isoform 1 of serine beta-lactamase-like protein LACTB mitochondrial precursor, isoform 1 of probable ATP- dependent RNA helicase DHX40, isoform 5 of calcium-activated potassium channel subunit alpha 1.
As expected, many of the proteins identified in serum were acute phase protein, including alpha-1-acid glycoprotein (AGP), alpha-1-antitrypsin precursor, alpha-2-macroglobulin precursor, prothrombin precursor (fragment), which supports that cirrhosis and HCC patients all have a background of chronic HBV infection.
Western blot validation and rate nephelometry of AGP
AGP is secreted mainly from the liver cell, its expression is related with the function of liver cell, but here is no report about if its elevation could aid the diagnosis of HCC, so we focus on its diagnosis value. Firstly, AGP was examined by western blotting in these 18 patients of screening group. The mean expression of AGP from HCC patients was 1.7-fold more than that from the patients with cirrhosis (P < 0.05) (Fig. 2a), which demonstrates the agreement between protein expression differences determined by ICAT and western blot. Meanwhile, we also found that western blot analysis of individual serum specimens showed variation in AGP expression among both LC group and HCC group (Fig. 2a). So we further assayed the AGP serum level in the separate control (n = 20), LC (n = 40) and HCC (n = 52) patients with rate nephelometry (Beckman). The serum levels of AGP in control and patients with LC and HCC are 654.45 ± 162.40 ng/ml, 369.09 ± 230.81 ng/ml and 895.73 ± 322.08 ng/ml separately (Fig. 2b). The difference of serum AGP levels between LC patients and HCC patients was statistically significant (P < 0.001).
The concentration of AGP in individual patient. a Western blot analysis of independent serum sample from screening group. The relative difference in alpha-1-acid glycoprotein expression in the pooled serum samples from liver cirrhosis (LC) versus serum from HCC patients cancer was 1.7 (P < 0.05). Individual serum specimens showed variation in alpha-1-acid glycoprotein expression among both LC (white bar) and HCC patients (grey bar); b Scatter plots of AGP concentration in validation group through nephelometry assay were presented. The serum levels of AGP in control and patients with LC and HCC are 654.45 ± 162.40 ng/ml, 369.09 ± 230.81 ng/ml and 895.73 ± 322.08 ng/ml separately. The difference of serum AGP levels between LC patients and control and between HCC and LC were statistically significant (LC vs. control: P < 0.001; HCC vs LC: P < 0.001)
Diagnostic usefulness of alpha-1-acid glycoprotein
To evaluate the diagnostic usefulness of AGP,HCC (n = 28) and LC (n = 38) cases with serum AFP levels below 500 ng/ml from the validation group were enrolled in the analysis. ROC curves for serum AGP and AFP level were constructed through SPSS. The area under curve (AUC) of the AFP level and the AGP were 0.538 (P = 0.604, 95% CI: 0.386–0.689) and 0.834 (P < 0.0005, 95% CI: 0.734–0.934), respectively. The sensitivity and accuracy of AGP were higher than that AFP at 90% specificity (Fig. 3), which means the diagnostic accuracy of serum AGP was higher than AFP in differentiating HCC from LC cases with AFP levels below 500 ng/ml. The cutoff value of AGP corresponding to the 90% specificity was 716.7 ng/ml. To evaluate the diagnostic value of AGP in combination of the conventional marker AFP, a multivariate logistic regression analysis was performed using the HCC and LC cases. The AUC of the ROC curve of the diagnostic score was 0.880 (P < 0.0005, 95% CI, 0.789–0.970, Fig. 3), which is better than AGP alone.
AGP level between patients with different tumor sizes
Further, we are interested with the effect of tumor size on the sensitivity of the serum AGP level for HCC diagnosis. 52 HCC patients were divided into two subgroups according to the tumor size (d < 3 cm and d > 3 cm). According to the diagnostic criteria with either AFP > 500 ng/ml or AGP > 716.7 ng/ml, 70% (14/20) of d < 3 cm HCC cases and 75% (24/32) of d > 3 cm HCC were accurately diagnosed. The sensitivities for tumors of different sizes were similar. This result suggests that AGP may be applicable to the diagnosis of early (small) HCC.
Discussion
The ideal biomarker should have low false-positive and negative rates and be sensitive, specific, quantifiable, and capable of detecting tumors at an early stage to allow appropriate therapy (Yoon 2008). AFP is routinely monitored as a marker for HCC, but it lacks sensitivity and specificity. Application of proteomic high-throughput approaches holds the promise of identifying new biomarkers (Kulasingam and Diamandis 2008).
In present study, we have used ICAT combining LC-MS/MS to quantify and identify the different expressed serum proteins between LC and HCC patients. Considering that the dynamic range of a mass spectrometer is on the order of two orders of magnitude, it is thought that a straight forward LC-MS/MS analysis will only result in the characterization of the highest abundance, and probably least interesting proteins. We use strong cation exchange (SCX) pre-fractionation prior to a reverse-phase LC-MS/MS analysis which has shown the ability of increasing low-abundant proteins in many proteomic studies. Protein concentration of serum ranged from 60 to 80 mg/ml, of which albumin with 40–55 mg/ml and globulin with 20–30 mg/ml take up the most abundant part. We adopt the affinity depletion approaches to delete albumin and globulin. With the depletion, some intracellular components or membrane protein such as guanine deaminase, ATP-dependent RNA helicase, calcium-activated potassium channel subunit alpha 1, lymphoid-specific helicase and ubiquitin carboxyl-terminal hydrolase 44, etc. could be identified through this strategy.
RNA helicase DHX40 belongs to RNA helicase family, which is highly conserved enzymes that utilize the energy derived from NTP hydrolysis to modulate the structure of RNA. The members of RNA helicase family often over expressed in tumor, such as lung cancer, colon cancer (Wei et al. 2004). Ubiquitin carboxyl-terminal hydrolase 44 (ubiquitin-specific protease 44, USP44) is a member of the ubiquitin proteasome pathway controlling intracellular protein degradation, functioning to maintain ubiquitin balance by associating with ubiquitin and by releasing ubiquitin from tandemly conjugated ubiquitin monomers and small adducts or unfolded proteins. Deubiquitinating enzyme USP44 is a critical regulator of the spindle checkpoint. Dysregulation of the spindle checkpoint can contribute to birth defects and tumorigenesis (Stegmeier et al. 2007).
Some acute phase proteins (APPs) were included in our result. Although acute-phase response is an early and immediate reaction to tissue injury, infection and inflammation, they may also occur in some chronic inflammatory reactions and other stressful stimuli, such as physical trauma, or neoplasia (Kushner 1982) Alpha-1-acid glycoprotein (AGP), also called orosomucoid (ORM), is a small acute-phase glycoprotein (about 40 kDa), synthesized mostly by hepatocytes. Some groups (Kim et al. 2006; Song et al. 2003) have reported that serum asialo AGP was suggested as a potential marker for cirrhosis and hepatocellular carcinoma (HCC). Mooney et al. (2006) presented that glycosylation of AGP is influenced by the degree of fibrosis and as such may prove a valuable prognostic indicator of the development of cirrhosis. But there is no exact report about the value of AGP for differentiating HCC from LC patients.
In present study, pooled sample was used to screen the difference between different groups, which strategy may cover up the individual variation of target protein. So we validate the expression of AGP in each patient through western and nephelometry. From normal control, LC to HCC patients, the AGP level in serum was down-regulated in LC and increased reversely in HCC patients. The presence of cirrhosis would result in a reduction of the synthetic ability of the liver and thus a concordant decrease in AGP levels would be expected as well. The hepatoma cell could produce and secrete abnormally much more AGP into serum that may be used as an indicator of HCC.
About 20% of HCC patients have serum AFP levels within the normal range (<10 ng/ml), and another 20% of patients present a similar levels of AFP to the cases with liver cirrhosis (10–500 ng/ml) (Johnson 2001), while patients with AFP level greater than 500 ng/ml almost always had HCC. So the ability to identify the HCC subgroup without significant elevation in serum AFP levels is particularly important. Through ROC analysis, we found that AGP could differentiate HCC from LC cases with AFP levels below 500 ng/ml. It was possible to achieve better differentiation between HCC and LC cases by combining the AFP and AGP together.
Besides the sensitivity and specificity of a diagnostic test, its applicability to the early diagnosis of liver cancer is also important. The present study has shown that with the conventional AFP test and AGP, the sensitivity for the detection of small HCC (<3 cm) is similar to that for the detection of large HCC (>5 cm). This strongly suggests that combined use of the conventional AFP test and AGP may enable an early diagnosis of HCC arising in patients with liver cirrhosis.
Concluding remarks
In conclusion, we have successfully identified distinctive serological marker for the diagnosis of HCC among patients with liver cirrhosis by ICAT-LC-MS/MS. 31 proteins were identified differentially expressed. Among them, alpha-1-acid glycoprotein was further invalidated through western blot and nephelometry analysis. The serum concentration of AGP in HCC patients (n = 52) was significantly higher than in liver cirrhosis (n = 40) (P < 0.005). AGP was useful for discrimination of the HCC cases from LC patients when the AFP levels were below 500 ng/ml. Thus, cICAT combined with LC-MS/MS-based serum proteome analysis can be useful in the screening of serum biomarkers for HCC. Alpha-1-acid glycoprotein could aid the diagnosis of HCC; further validation is also needed in enlarged population.
Abbreviations
- cICAT:
-
Cleavable isotope-coded affinity tag
- LC:
-
Liver cirrhosis
- HCC:
-
Hepatocellular carcinoma
- 2 DE:
-
2 Dimentional electrophoresis
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel
- MS:
-
Mass spectrometry
- MS/MS:
-
Tandem mass spectrometry
- SELDI-TOF:
-
Surface-enhanced laser desorption ionization time-of-flight
- AGP:
-
Alpha-1-acid glycoprotein
- AFP:
-
Alpha fetoprotein
- ROC:
-
Receiver operating curve
References
Bolondi L (2003) Screening for hepatocellular carcinoma in cirrhosis. J Hepatol 39:1076–1084
Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J, EASL Panel of Experts on HCC (2001) Clinical management of hepatocellular carcinoma.Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35:421–430
Caturelli E, Bartolucci F, Biasini E, Vigliotti ML, Andriulli A, Siena DA, Attino V, Bisceglia M (2002) Diagnosis of liver nodules observed in chronic liver disease patients during ultrasound screening for early detection of hepatocellular carcinoma. Am J Gastroenterol 97:397–405
Cui J, Kang X, Dai Z, Huang C, Zhou H, Guo K, Li Y, Zhang Y, Sun R, Chen J, Li Y, Tang Z, Uemura T, Liu Y (2007) Prediction of chronic hepatitis B, liver cirrhosis and hepatocellular carcinoma by SELDI-based serum decision tree classification. J Cancer Res Clin Oncol 133:825–834
Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R (1999) Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotechnol 17:994–999
Johnson PJ (2001) The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis 5:145–159
Kim KA, Lee EY, Kang JH, Lee HG, Kim JW, Kwon DH, Jang YJ, Yeom YI, Chung TW, Kim YD, Yoon do Y, Song EY (2006) Diagnostic accuracy of serum asialo-alpha1-acid glycoprotein concentration for the differential diagnosis of liver cirrhosis and hepatocellular carcinoma. Clin Chim Acta. 369:46–51
Kulasingam V, Diamandis EP (2008) Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nat Clin Pract Oncol 5:588–599
Kushner I (1982) The phenomenon of the acute phase response. Ann NY Acad Sci 389:39–48
Kuster B, Schirle M, Mallick P, Aebersold R (2005) Scoring proteomes with proteotypic peptide probes. Nat Rev Mol Cell Biol 6:577–583
Liaw YF (2005) Prevention and surveillance of hepatitis B virus-related hepatocellular carcinoma. Semin Liver Dis 25(Suppl 1):40–47
Mooney P, Hayes P, Smith K (2006) The putative use of α-1-acid glycoprotein as a non-invasive marker of fibrosis. Biomed Chromatogr 20:1351–1358
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJl (2004) Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics 3:1154–1169
Sherman M (2005) Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis 25:143–154
Song EY, Kim KA, Kim YD, Lee EY, Lee HS, Kim HJ, Ahn BM, Choe YK, Kim CH, Chung TW (2003) Elevation of serum asialo-alpha(1) acid glycoprotein concentration in patients with hepatic cirrhosis and hepatocellular carcinoma as measured by antibody-lectin sandwich assay. Hepatol Res 26:311–317
Steel LF, Shumpert D, Trotter M, Seeholzer SH, Evans AA, London WT, Dwek R, Block TM (2003) A strategy for the copmararative analysis of serum proteomes for the discovery of biomarkers for hepatocellular carcinoma. Proteomics 3:601–609
Stegmeier F, Rape M, Draviam VM, Nalepa G, Sowa ME, Ang XL, McDonald ER 3rd, Li MZ, Hannon GJ, Sorger PK, Kirschner MW, Harper JW, Elledge SJ (2007) Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature 446:876–881
Ward J, Robinson PJ (2002) How to detect hepatocellular carcinoma in cirrhosis. Eur Radiol 12:2258–2272
Wei X, Pacyna-Gengelbach M, Schluns K, An Q, Gao Y, Cheng S, Petersen I (2004) Analysis of the RNA helicase A gene in human lung cancer. Oncol Rep 11:253–258
Yoon SK (2008) Recent advances in tumor markers of human hepatocellular carcinoma. Intervirology 51(Suppl 1):34–41
Acknowledgments
This research was supported by Chinese National high tech Developing Programme (2006AA02A308) and National Major Program of Science-technology for Infectious Diseases (2008ZX10207, 2008ZX10208).
Conflict of interest statement
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled. There is no any conflict of interest with others.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, X., Sun, L., Guo, K. et al. Serum protein biomarkers screening in HCC patients with liver cirrhosis by ICAT-LC-MS/MS. J Cancer Res Clin Oncol 136, 1151–1159 (2010). https://doi.org/10.1007/s00432-010-0762-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-010-0762-6