Abstract
Purpose
Sphingolipids are bioactive lipids implicated in apoptosis, cell survival and proliferation. We analyzed the prognostic value of enzymes from sphingolipid metabolism in breast cancer.
Methods
Differences in expression of ceramide galactosyl transferase (UGT8), ceramide kinase (CERK), and Ganglioside GD3-Synthase (ST8SIA1) in breast cancer cells were investigated by using microarray data of 1,581 tumor samples.
Results
UGT8, CERK, and ST8SIA1 were associated with poor pathohistological grading (P < 0.001). High CERK expression was correlated with ErbB2 status (P = 0.006). Among ER positive breast cancers a significant worse prognosis for patients with tumors showing low ST8SIA1 and UGT8 expression was observed. In the ER negative subgroup those samples with high CERK expression displayed a worse prognosis. In a multivariate analysis only ST8SIA1 and tumor size remained significant.
Conclusions
Our experiments reveal that expression of enzymes from the sphingolipid metabolism has prognostic implications in breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sphingolipids metabolites are important regulators of cell activation with a broad spectrum of activities controlling cell growth and death as well as signal transduction processes (Gomez-Munoz 2006; Gouaze-Andersson and Cabot 2006; Hannun and Obeid 2008). Links to various aspects of cancer, like tumor growth, neoangiogenesis, and response to therapy have been described for various sphingolipids (Gouaze-Andersson and Cabot 2006). One can distinguish ordinary and rather complex sphingolipids (Gomez-Munoz 2006). Ceramide, sphingosine, sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P) belong to the first group while glucosylceramide, α-galactosylceramide (a-GC) and GD3-ganglioside are typical examples of complex sphingolipids. Ceramide and S1P are counterplayer in the so called sphingolipid rheostat and determine the balance between life and death of the cell (Taha et al. 2006). Beside these major players C1P has gained interest in the last decade. C1P seems to inhibit cell death while promoting cell survival (Gomez-Munoz 2006) and functions as a mediator in inflammation (Hinkovska-Galcheva et al. 2005; Mitsutake et al. 2004). The complex sphingolipids GD3 ganglioside and α-galactosylceramide have been investigated to some extent regarding their role in cell physiology. They have been shown to act as antigens, as mediators of cell adhesion, binding agents for microbial toxins and growth factors, as well as modulators of signal transduction (Lahiri and Futerman 2007).
Recently, we could demonstrate that the S1P producing shingosine kinase 1 (SPHK1) is significantly higher expressed in ER negative tumors. High SPHK1 Expression was associated with poor prognosis in clinical breast cancer (Ruckhäberle et al. 2007). In contrast, we found that glucosyl ceramide synthase (GCS) displayed overexpression in ER positive samples but had no significant impact on patients’ prognosis neither in ER positive nor ER negative cancer (Ruckhäberle et al. 2008). In this report we present the impact of the gene expression of three further enzymes from the sphingolipid metabolism on the prognosis of breast cancer patients. All these three enzymes were higher expressed in ER negative tumors. Ceramide kinase (CERK) metabolizes ceramide to C1P (Fig. 1), while the ceramide galactosyltransferase is metabolizing ceramide to α-galactosylceramide. The ganglioside GD3-synthase (ST8SIA1) synthesizes GD3 from GM3 in the lumen of the golgi apparatus (Huwiler et al. 2000).
Materials and methods
Microarray expression data
A database 1581 Affymetrix microarray experiments from primary breast cancer patients was established. One hundred and twenty samples from our own institution were included (dataset Frankfurt) which have been described previously (Ahr et al. 2002; Rody et al. 2007; Ruckhäberle et al. 2007) as well as 1,461 samples from nine different publicly available datasets (Table 1): Uppsala (Miller et al. 2005); Stockholm (Pawitan et al. 2005); Rotterdam (Wang et al. 2005; Minn et al. 2007), Oxford-Untreated (Sotiriou et al. 2006), Oxford-Tamoxifen and London (Loi et al. 2007), New York (Minn et al. 2005), Villejuif (Desmedt et al. 2007), and ExpO (http://www.intgen.org/). Tissue samples of primary invasive breast cancer cases of the University of Frankfurt were obtained with informed consent and approval of the institutional review board of the University of Frankfurt. For comparability only data from Affymetrix HG-U133A microarrays were used. The clinical characteristics of the patients in the different datasets are given in Table 1. For 1,263 of the 1,581 patients follow up information was available (no follow up data has been reported for dataset expO). The median follow-up time was 79 months. 1,135 of the 1,581 samples (71.9%) were ER positive. Since methods of Affymetrix microarray normalization can have significant effects on the levels for individual probe sets, several uniform normalization methods (Li and Wong 2001; Irizarry et al. 2003) of CEL file data has been developed to allow the analysis of sets of multiple arrays. However, important discrepancies between different datasets depend on the dynamics of the measurements originating from different hybridization efficiencies and even uniform normalization methods are incapable in compensating those experimental differences. In addition, no CEL files are available for some studies (e.g. the Rotterdam dataset). Therefore, we used a conservative strategy for dataset stratification, which relies on a ranking of samples in each cohort. Each dataset of microarrays was normalized separately using the originally proposed method of the respective study (see Table 1). Log transformed expression values were median centered over each array. Subsequently median centering and normalization for genes were done separately in each dataset.
Since standard pathology for ER and ErbB2 was not available for all samples and to allow comparison of different datasets, receptor status was determined based on Affymetrix expression data as previously described (Alexe et al. 2007; Bonnefoi et al. 2007; Foekens et al. 2006; Gong et al. 2007) by fitting two normal distributions on normalized data. ER status was based on Affymetrix ProbeSet 205225_at, the ErbB2 status on ProbeSet 216836_s_at. A specificity of 86.1% and a sensitivity of 92.2% was observed when the chip based ER status was compared to the ER status obtained from biological assay (available for 1,233 samples), while the specificity and sensitivity of chip based ErbB2 status was 98.6 and 45.8%, respectively, compared to 3+ staining in immunohistochemistry with HER2 antibody (data available for 206 samples). To allow comparison of expression of the analyzed enzymes from sphingolipid metabolism from the Affymetrix HG-U133A array between different datasets we used a median or quartile split among each dataset. Samples were characterized as high or low expressing based on a split of the cohorts according to CERK (ProbeSet 218421_at), ST8SIA1 (ProbeSet 210073_at), and UGT8 (ProbeSet 208358_s_at).
Statistical analysis
All reported P values are two sided and P values of less than 0.05 were considered to indicate a significant result. Subjects with missing values were excluded from the analyses. Chi-square test was used for categorical parameters. Mann–Whitney U test was applied to test for differential expression of the investigated enzymes in ER positive and negative samples. Survival intervals were measured from the time of surgery to the time of death from disease or of the first clinical or radiographic evidence of disease recurrence. Data for women in whom the envisaged end point was not reached were censored as of the last follow-up date or at 120 months. We constructed Kaplan–Meier curves and used the log rank test to determine the univariate significance of the variables. A Cox proportional-hazards regression model was used to examine simultaneously the effects of multiple covariates on survival. The effect of each variable was assessed with the use of the Wald test and described by the hazard ratio, with a 95% confidence interval. The model included age, tumor size, lymph node status, ER-Status, and ErbB2 expression as well as all three analyzed enzymes from sphingolipid metabolism (UGT8, CERK, ST8SIA1). All analyses were performed using SPSS 15.0 (SPSS Inc., Chicago, IL).
Results
Correlation of gene expression of ST8SIA1, UGT8 and CERK with the ER status of the tumor
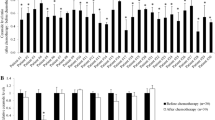
Initially, the mRNA expression of ceramide galactosyl transferase (UGT8), CERK and Ganglioside GD3-Synthase (ST8SIA1) that either synthesize or metabolize ceramide (Fig. 1) was analyzed in our microarray data base of 1,581 invasive breast cancer samples. Since estrogen receptor status is one of the most important predictive and prognostic factors the tumor samples were stratified into groups based on their estrogen receptor status and these groups were analyzed for differences in expression. As shown in Fig. 2a–c, UGT8 (P < 0.001), CERK (P < 0.001) and ST8SIA1 (P < 0.001) displayed significant higher expression among ER negative tumors.
Correlation of clinical-pathological characteristics and the prognostic value of ceramide kinase (CERK) expression
For correlation with clinical parameters samples were categorized according to CERK expression by use of a median split. Univariate analysis of clinical parameters stratified according to high and low expression of CERK are given in Table 2. We observed an association of high CERK expression with ER negative samples (P < 0.001) and Her 2 neu positive samples (P = 0.006) as well as in Grade 3 tumors (P = 0.01). In contrast CERK expression showed no association with age, lymph node status and tumor size. Since CERK expression was associated with ER negative samples and patients with ER negative tumors have per se a worse prognosis than those with ER positive tumors we performed survival analyses separately in the ER positive and ER negative subgroups to avoid a confounding effect. When we used a median split of CERK expression a worse prognosis with lower 5-year (63.2 ± 3.3 vs. 75.5 ± 4.1%) and 10-year survival rates (59.5 ± 3.6 vs. 72.9 ± 4.4%) for patients with high CERK was observed in the ER negative subgroup (P = 0.035) while we found no significant difference among the ER positive samples (Fig. 3a, b).
Correlation of clinical-pathological characteristics and the prognostic value of UGT8
For correlation with clinical parameters a median split in UGT8 expression was performed. There was no significant difference in tumor size, lymph node status and Her 2 neu status between the two groups (Table 2). A significant correlation with higher expression was observed for older patients (P = 0.003) and tumors with poor pathohistological grading (P < 0.001). Survival analysis of UGT8 revealed a significant better prognosis for ER positive breast cancer patients with high expression of UGT8 (P = 0.011, Fig. 4). The 5- and 10-year disease free survival (DFS) was 79.8 ± 2.1 and 69.1 ± 2.6% in the UGT8 high group. In contrast 71.4 ± 2.0 and 61.6 ± 2.4 survival rates for 5 and 10 years were observed in the UGT8 low group. In contrast, there was no difference in survival among the ER negative tumors (log rank P = 0.67).
Correlation of clinical-pathological characteristics and the prognostic value of the ganglioside GD3-synthase (ST8SIA1)
Regarding the clinical parameters there were no significant differences between the groups with high and low ST8SIA expression for patient’s age, tumor size, lymph node status, and Her 2 neu overexpression. In contrast a larger number of tumors with higher histological grade was detected among the tumors with high expression of ST8SIA1 (P < 0.001, Table 2). When using the median split the survival analysis in our sample cohort failed to show a significant difference in the prognosis. In contrast, when only the highest quartile of ST8SIA1 expression was used to stratify the samples, we observed a significant better 5 year (84.1 ± 3.1 vs. 73.2 ± 1.6) as well as 10-year survival (72.3 ± 4.2 vs. 63.4 ± 2.0) for samples with high ST8IA1 expression among ER positive patients (P = 0.021; Fig. 5a, b). No significant difference was found for ER negative tumors.
Disease free survival in the breast cancer subgroups according to their expression of ST8SIA1 (blue line higher expression, green line lower expression; a significant better prognosis (P = 0.021) with higher ST8SIA1 expression in the ER positive subgroup; b no significant differences between the two groups in ER negative samples
Multivariate Cox regression analysis
A multivariate Cox regression analysis was performed in n = 605 patients for which data on all standard parameters (tumor size, lymph node status, grading, age, ER status and ErbB2) were available. The results are presented in Table 3. Analysis of these standard parameters and CERK, UGT8, and ST8SIA1 expression in relation to DFS revealed that only ST8SIA1 (HR 1.59, 95% CI 1.08–2.33, P = 0.017) remained a significant prognostic marker beside tumor size (HR 2.10, 95% CI 1.52–2.91, P < 0.001).
Discussion
Our results demonstrate that CERK, UGT8 and ST8SIA1 are higher expressed in ER negative tumor samples. In addition we observed a better prognosis for those ER positive samples with high expression of UGT8 and ST8SIA1. In contrast, ER negative patients with high CERK expression had a worse prognosis then those with low CERK expression. To our knowledge this is the first report of an ER status dependent expression of CERK, UGT8 and ST8SIA1 and a prognostic impact of these enzymes in clinical breast cancer. In concordance with our previous data these results strongly support that sphingolipid expression is estrogen dependent (Ruckhäberle et al. 2007, 2008).
The prognostic benefit of higher expression of UGT8, the gene for α-galactosylceramide, might be explained by possible immune modulating effects of this glycolipid. α-Galactosylceramide (α-GC) is a commonly used ligand for the study of natural killer T (NKT) cell activation. Presented on CD1d molecules it leads to a cascade of immunologic reactions like the production of both T helper (Th) 1 and Th2 cytokines or activation of dendritic cell, B cells and NKT cells (Godfrey and Kronenberg 2004). Use of α-galactosylceramide significantly inhibited tumor growth in mice and tumor associated angiogenesis (Liu et al. 2005; Hayakawa et al. 2002; Teng et al. 2007). It has even been applied clinically in several phase I studies on small cell lung cancer, head neck cancer and other solid tumors (Ishikawa et al. 2005; Motohashi et al. 2006; Uchida et al. 2008; Giaccone et al. 2002). The analog α-C- galactosylceramide seems to be an even more potent inducer of the described immunological phenomena (Fujii et al. 2006). With the combination of whole body hyperthermia and α-galactosylceramide antitumor effects in colon cancers were achieved in mice (Hattori et al. 2007). Recently, it was also shown that CD1d-restricted α-galactosylceramide ligands for iNKT cells in combination with anti-DR5 and anti-4-1BB monoclonal antibodies (termed “NKTMab” therapy) can substantially reject breast and renal tumors in mice (Teng et al. 2007). Taking these results into consideration it gives future perspectives of immune modulating and antibody treatment for breast cancer. Possibly those patients with high expression of the enzyme UGT8 in the tumor samples might especially profit from these therapeutic approaches.
Another immune modulating agent is GD3 ganglioside that is synthesized by the ganglioside GD3-synthase (ST8SIA1). GD3 ganglioside was described to be highly expressed in neuroectoderm like melanomas and small cell lung cancer. GD3 ganglioside induces NKT-cell response in the mouse (Wu et al. 2003). Park et al. (2008) discovered a fine specificity of GD3 NKT cells by demonstrating that immunization with GD3 induced two populations of GD3-reactive NKT cells. We observed a higher expression of ganglioside GD3-synthase (ST8SIA1) in ER negative compared to ER positive tumors. Interestingly, however, those ER positive breast cancers with higher expression of ST8SIA1 were characterized by a better prognosis than those with lower expression. A possible explanation of this effect could be an immunologic advantage of patients with higher GD3 ganglioside levels.
The enzyme CERK is responsible for the balance between ceramide and C1P. Earlier studies suggested that C1P inhibits apoptosis (Gomez-Munoz et al. 2004) and acts as a mitogen (Gomez-Munoz et al. 1995). More recently, however, Mitra et al. (2007) demonstrated that low doses of natural C1P promote survival in lung adenocarcinoma cells while higher doses enhance apoptosis. In their studies they also show that high concentrations of exogenous C1P are converted to ceramide (Mitra et al. 2007). In our data we found significant higher expression of CERK in ER negative than in ER positive breast cancers. Moreover, when focusing on this ER negative subgroup we found that those ER negative tumors with highest expression of CERK had an even worse prognosis. These results would be in line with the rheostat model where CERK reduces the level of apoptotic ceramide in the cell. Exogenous addition of high levels of C1P might have an inverse effect, e.g. through secondary increase of ceramide levels as described by Mitra et al. (2007) above.
Generally, the sphingolipid metabolism represents a target with great therapeutical potential (Modrak et al. 2006; Huwiler and Pfeilschifter 2006). Especially the modulation of intracellular levels of ceramide and S1P holds interesting options since they are critically important for response to chemotherapy. Thus coadministration of ceramide and chemotherapeutic agents have been studied in mice (Devalapally et al. 2007). Ceramide levels have also been targeted by ceramide analogs (Struckhoff et al. 2004; Dahm et al. 2008), inhibition of CERK (Saxena et al. 2008; Lamour and Chalfant 2008) and glucosylceramide synthase (Gouaze et al. 2005; Gouaze-Andersson et al. 2007; Liu et al. 2008). S1P signaling has been targeted by monoclonal anti-S1P antibodies (Visentin et al. 2006) and inhibitors of sphingosine kinase as well as S1P receptor agonists (see Takabe et al. 2008; Shida et al. 2008). In addition small interfering RNAs against Gangliosid GD3 synthase were shown to reduce tumor growth in mice (Ko et al. 2006).
In summary, our data demonstrate that expression of UGT8, CERK and ST8SIA has a prognostic impact in breast cancer and depends on ER status. These results are in line with in vitro and in vivo data demonstrating an important role of sphingolipids in various cancers. Investigation of a predictive value of markers from the sphingolipid metabolism for specific therapeutic approaches is still pending and should be a goal for future work. Moreover, development of new immune modulating therapies including a-galactosylceramide or GD3-ganglioside could offer additional treatment options in specific subgroups of breast cancer patients.
References
Ahr A, Karn T, Solbach C, Seiter T, Strebhardt K, Holtrich U, Kaufmann M (2002) Identification of high risk breast-cancer patients by gene expression profiling. Lancet 359:131–132. doi:10.1016/S0140-6736(02)07337-3
Alexe G, Dalgin GS, Scanfeld D, Tamayo P, Mesirov JP, DeLisi C, Harris L, Barnard N, Martel M, Levine AJ, Ganesan S, Bhanot G (2007) High expression of lymphocyte-associated genes in node-negative HER2 + breast cancers correlates with lower recurrence rates. Cancer Res 67:10669–10676. doi:10.1158/0008-5472.CAN-07-0539
Bonnefoi H, Potti A, Delorenzi M, Mauriac L, Campone M, Tubiana-Hulin M, Petit T, Rouanet P, Jassem J, Blot E, Becette V, Farmer P, André S, Acharya CR, Mukherjee S, Cameron D, Bergh J, Nevins JR, Iggo RD (2007) Validation of gene signatures that predict the response of breast cancer to neoadjuvant chemotherapy: a substudy of the EORTC 10994/BIG 00–01 clinical trial. Lancet Oncol 8:1071–1078. doi:10.1016/S1470-2045(07)70345-5
Dahm F, Bielawska A, Nocito A, Georgiev P, Szulc ZM, Bielawski J, Jochum W, Dindo D, Hannun YA, Clavien PA (2008) Mitochondrially targeted ceramide LCL-30 inhibits colorectal cancer in mice. Br J Cancer 98(1):98–105
Desmedt C, Piette F, Loi S, Wang Y, Lallemand F, Haibe-Kains B, Viale G, Delorenzi M, Zhang Y, d’Assignies MS, Bergh J, Lidereau R, Ellis P, Harris AL, Klijn JG, Foekens JA, Cardoso F, Piccart MJ, Buyse M, Sotiriou C (2007) TRANSBIG Consortium. (2007) Strong time dependence of the 76-gene prognostic signature for node-negative breast cancer patients in the TRANSBIG multicenter independent validation series. Clin Cancer Res 13:3207–3214
Devalapally H, Duan Z, Seiden MV, Amiji MM (2007) Paclitaxel and ceramide co-administration in biodegradable polymeric nanoparticulate delivery system to overcome drug resistance in ovarian cancer. Int J Cancer 121(8):1830–1838. doi:10.1002/ijc.22886
Foekens JA, Atkins D, Zhang Y, Sweep FC, Harbeck N, Paradiso A, Cufer T, Sieuwerts AM, Talantov D, Span PN, Tjan-Heijnen VC, Zito AF, Specht K, Hoefler H, Golouh R, Schittulli F, Schmitt M, Beex LV, Klijn JG, Wang Y (2006) Multicenter validation of a gene expression-based prognostic signature in lymph node-negative primary breast cancer. J Clin Oncol 24:1665–1671. doi:10.1200/JCO.2005.03.9115
Fujii S, Shimizu K, Hemmi H, Fukui M, Bonito AJ, Chen G, Franck RW, Tsuji M, Steinman RM (2006) Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci USA 103(30):11252–11257 (Epub 2006 Jul 14)
Giaccone G, Punt CJA, Ando Y et al (2002) A phase I study of the natural killer T-cell ligand a-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res 8:3702–3709
Godfrey DI, Kronenberg M (2004) Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest 114:1379–1388
Gomez-Munoz A (2006) Ceramide 1-phosphate/ceramide, a switch between life and death. Biochim Biophys Acta 1758:2049–2056. doi:10.1016/j.bbamem.2006.05.011
Gomez-Munoz A, Duffy PA, Martin A, O’Brien L, Byun H-S, Bittman R, Brindley DN (1995) Short-chain ceramide-1-phosphates are novel stimulators of DNA synthesis and cell division: antagonism by cell-permeable ceramides. Mol Pharmacol 47:883–889
Gomez-Munoz A, Kong JY, Salh B, Steinbrecher UP (2004) Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages. J Lipid Res 45:99–105. doi:10.1194/jlr.M300158-JLR200
Gong Y, Yan K, Lin F, Anderson K, Sotiriou C, Andre F, Holmes FA, Valero V, Booser D, Pippen JE Jr, Vukelja S, Gomez H, Mejia J, Barajas LJ, Hess KR, Sneige N, Hortobagyi GN, Pusztai L, Symmans WF (2007) Determination of oestrogen-receptor status and ERBB2 status of breast carcinoma: a gene-expression profiling study. Lancet Oncol 8:203–211. doi:10.1016/S1470-2045(07)70042-6
Gouaze V, Liu YY, Prickett CS, Yu JY, Giuliano AE, Cabot MC (2005) Glucosylceramide synthase blockade down-regulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Res 65(9):3861–3867. doi:10.1158/0008-5472.CAN-04-2329
Gouaze-Andersson V, Cabot MC (2006) Glycosphingolipids and drug resistance. Biochim Biophys Acta 1758:2096–2103. doi:10.1016/j.bbamem.2006.08.012
Gouaze-Andersson V, Yu JY, Kreitenberg AJ, Bielawska A, Giuliano AE, Cabot MC (2007) Ceramide and glucosylceramide upregulate expression of the multidrug resistance gene MDR1 in cancer cells. Biochim Biophys Acta 1771(12):1407–1417
Hannun YA, Obeid LM (2008) Principles of bioactive lipid signaling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9:139–150. doi:10.1038/nrm2329
Hattori T, Kokura S, Okuda T, Okayama T, Takagi T, Handa O, Naito Y, Yoshida N, Yoshikawa T (2007) Antitumor effect of whole body hyperthermia with alpha-galactosylceramide in a subcutaneous tumor model of colon cancer. Int J Hyperthermia 23(7):591–598. doi:10.1080/02656730701708328
Hayakawa Y, Takeda K, Yagita H, Smyth MJ, Van Kaer L, Okumura K, Saiki I (2002) IFN-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood 100(5):1728–1733
Hinkovska-Galcheva V, Boxer LA, Kindzelskii A, Hiraoka M, Abe A, Goparju S, Spiegel S, Petty HR, Shayman JA (2005) Ceramide 1-phosphate, a mediator of phagocytosis. J Biol Chem 280:26612–26621. doi:10.1074/jbc.M501359200
Huwiler A, Kolter T, Pfeilschifter J, Sandhoff K (2000) Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim Biophys Acta 1485(2–3):63–99
Huwiler A, Pfeilschifter J (2006) Altering the sphingosine-1-phosphate/ceramide balance: a promising approach for tumor therapy. Curr Pharm Des 12(35):4625–4635. doi:10.2174/138161206779010422
Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP (2003) Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31:e15. doi:10.1093/nar/gng015
Ishikawa A, Motohashi S, Ishikawa E et al (2005) A phase I study of a-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res 11:1910–1917. doi:10.1158/1078-0432.CCR-04-1453
Ko K, Furukawa K, Takahashi T, Urano T, Sanai Y, Nagino M, Nimura Y, Furukawa K (2006) Fundamental study of small interfering RNAs for ganglioside GD3 synthase gene as a therapeutic target of lung cancers. Oncogene 25(52):6924–6935. doi:10.1038/sj.onc.1209683
Lahiri S, Futerman AH (2007) The metabolism and function of sphingolipids and glycosphingolipids. Cell Mol Life Sci 64(17):2270–2284. doi:10.1007/s00018-007-7076-0
Lamour NF, Chalfant CE (2008) Ceramide kinase and the ceramide-1-phosphate/cPLA2alpha interaction as a therapeutic target. Curr Drug Targets 9(8):674–682. doi:10.2174/138945008785132349
Li C, Wong WH (2001) Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA 98(1):31–36. doi:10.1073/pnas.011404098
Liu YY, Yu JY, Yin D, Patwardhan GA, Gupta V, Hirabayashi Y, Holleran WM, Giuliano AE, Jazwinski SM, Gouaze-Andersson V, Consoli DP, Cabot MC (2008) A role for ceramide in driving cancer cell resistance to doxorubicin. FASEB J 22(7):2541–2551. doi:10.1096/fj.07-092981
Liu K, Idoyaga J, Charalambous A, Fujii S, Bonito A, Mordoh J, Wainstok R, Bai XF, Liu Y, Steinman RMJ (2005) Innate NKT lymphocytes confer superior adaptive immunity via tumor-capturing dendritic cells. Exp Med 202:1507–1516. doi:10.1084/jem.20050956
Loi S, Haibe-Kains B, Desmedt C, Lallemand F, Tutt AM, Gillet C, Ellis P, Harris A, Bergh J, Foekens JA, Klijn JG, Larsimont D, Buyse M, Bontempi G, Delorenzi M, Piccart MJ, Sotiriou C (2007) Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J Clin Oncol 25:1239–1246. doi:10.1200/JCO.2006.07.1522
Miller LD, Smeds J, George J, Vega VB, Vergara L, Ploner A, Pawitan Y, Hall P, Klaar S, Liu ET, Bergh J (2005) An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci USA 102:13550–13555. doi:10.1073/pnas.0506230102
Minn AJ, Gupta GP, Padua D, Bos P, Nguyen DX, Nuyten D, Kreike B, Zhang Y, Wang Y, Ishwaran H, Foekens JA, van de Vijver M, Massagué J (2007) Lung metastasis genes couple breast tumor size and metastatic spread. Proc Natl Acad Sci USA 104:6740–6745. doi:10.1073/pnas.0701138104
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J (2005) Genes that mediate breast cancer metastasis to lung. Nature 436:518–524. doi:10.1038/nature03799
Mitra P, Maceyka M, Payne SG, Lamour N, Milstien S, Chalfant CE, Spiegel S (2007) Ceramide kinase regulates growth and survival of A549 human lung adenocarcinoma cells. FEBS Lett 581(4):735–740 (Epub 2007 Jan 25)
Mitsutake S, Kim TJ, Inagaki Y, Kato M, Yamashita T, Igarashi Y (2004) Ceramide kinase is a mediator of calcium-dependent degranulation in mast cells. J Biol Chem 279:17570–17577. doi:10.1074/jbc.M312885200
Modrak DE, Gold DV, Goldenberg DM (2006) Sphingolipid targets in cancer therapy. Mol Cancer Ther 5(2):200–208. doi:10.1158/1535-7163.MCT-05-0420
Motohashi S, Ishikawa A, Ishikawa E et al (2006) A phase I study of in vitro expanded natural killer T cells inpatients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res 12:6079–6086. doi:10.1158/1078-0432.CCR-06-0114
Park JE, Wu DY, Prendes M, Lu SX, Ragupathi G, Schrantz N, Chapman PB (2008) Fine specificity of natural killer T cells against GD3 ganglioside and identification of GM3 as an inhibitory natural killer T-cell ligand. Immunology 123(1):145–155. doi:10.1111/j.1365-2567.2007.02760.x
Pawitan Y, Bjohle J, Amler L, Borg AL, Egyhazi S, Hall P, Han X, Holmberg L, Huang F, Klaar S, Liu ET, Miller L, Nordgren H, Ploner A, Sandelin K, Shaw PM, Smeds J, Skoog L, Wedren S, Bergh J (2005) Gene expression profiling spares early breast cancer patients from adjuvant therapy: derived and validated in two population-based cohorts. Breast Cancer Res 7:R953–R964. doi:10.1186/bcr1325
Rody A, Holtrich U, Gaetje R, Gehrmann M, Engels K, von Minckwitz G, Loibl S, Diallo-Danebrock R, Ruckhäberle E, Metzler D, Ahr A, Solbach C, Karn T, Kaufmann M (2007) Poor outcome in estrogen receptor-positive breast cancers predicted by loss of plexin B1. Clin Cancer Res 13:1115–1122. doi:10.1158/1078-0432.CCR-06-2433
Ruckhäberle E, Karn T, Hanker L, Gätje R, Metzler D, Holtrich U, Kaufmann M, Rody A (2008) Prognostic relevance of glucosylceramide synthase (GCS) expression in breast cancer. J Cancer Res Clin Oncol 135(1):81–90
Ruckhäberle E, Rody A, Engels K, Gaetje R, von Minckwitz G, Schiffmann S, Grosch S, Geisslinger G, Holtrich U, Karn T, Kaufmann M (2007) Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat 112(1):41–52
Saxena S, Banerjee M, Shirumalla RK, Ray A (2008) Ceramide kinase: a potential anti-inflammatory target? Curr Opin Investig Drugs 9(5):455–462
Shida D, Takabe K, Kapitonov D, Milstien S, Spiegel S (2008) Targeting SphK1 as a new strategy against cancer. Curr Drug Targets 9(8):662–673. doi:10.2174/138945008785132402
Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B, Desmedt C, Larsimont D, Cardoso F, Peterse H, Nuyten D, Buyse M, Van de Vijver MJ, Bergh J, Piccart M, Delorenzi M (2006) Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 98:262–272
Struckhoff AP, Bittman R, Burow ME, Clejan S, Elliott S, Hammond T, Tang Y, Beckman BS (2004) Novel ceramide analogs as potential chemotherapeutic agents in breast cancer. J Pharmacol Exp Ther 309(2):523–532
Taha TA, Mullen TD, Obeid LM (2006) A house divided: ceramidesphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim Biophys Acta 1758:2027–2036. doi:10.1016/j.bbamem.2006.10.018
Takabe K, Paugh SW, Milstien S, Spiegel S (2008) “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev 60(2):181–195. doi:10.1124/pr.107.07113
Teng MW, Westwood JA, Darcy PK, Sharkey J, Tsuji M, Franck RW, Porcelli SA, Besra GS, Takeda K, Yagita H, Kershaw MH, Smyth MJ (2007) Combined natural killer T-cell based immunotherapy eradicates established tumors in mice. Cancer Res 67(15):7495–7504. doi:10.1158/0008-5472.CAN-07-0941
The International Genomics Consortium (IGC) The expO project (Expression Project For Oncology) http://www.intgen.org/
Uchida T, Horiguchi S, Tanaka Y, Yamamoto H, Kunii N, Motohashi S, Taniguchi M, Nakayama T, Okamoto Y (2008) Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother 57(3):337–345. doi:10.1007/s00262-007-0373-5
Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA (2006) Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell 9(3):225–238. doi:10.1016/j.ccr.2006.02.023
Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, Atkins D, Foekens JA (2005) Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 365:671–679
Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB (2003) Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med 198:173–181. doi:10.1084/jem.20030446
Acknowledgments
We thank Samira Adel and Katherina Kourtis for expert technical assistance. This work was supported by grants from the Deutsche Krebshilfe, the Margarete Bonifer-Stiftung, Bad Soden, the Dr. Robert Pfleger-Stiftung, Bamberg, and the BANSS-Stiftung, Biedenkopf. Tissue samples of breast cancer cases of the University of Frankfurt were obtained with informed consent and approval of the institutional review board of the University of Frankfurt.
Conflict of interest statement
There are no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Ruckhäberle and T. Karn contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Ruckhäberle, E., Karn, T., Rody, A. et al. Gene expression of ceramide kinase, galactosyl ceramide synthase and ganglioside GD3 synthase is associated with prognosis in breast cancer. J Cancer Res Clin Oncol 135, 1005–1013 (2009). https://doi.org/10.1007/s00432-008-0536-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-008-0536-6