Abstract
Purpose
CCAAT/enhancer binding protein alpha (C/EBPα) is a transcription factor and a tumor suppressor. We aimed to assess its protein expression and prognostic value in human hepatocellular carcinoma (HCC).
Methods
We conducted a retrospective cohort study on 50 HCC patients and performed immunohistochemistry against C/EBPα on tumors and adjacent nontumor specimens. Relationships of C/EBPα expression with clinical parameters and patient survival were analyzed.
Results
C/EBPα expression was not influenced by chronic alcohol exposure, viral hepatitis, or cirrhosis, but was reduced in 60% of HCC. Reduction of C/EBPα was associated with advanced tumor stage (P = 0.001). Patients with markedly reduced C/EBPα expression had a significantly shorter survival with a hazard ratio of 5.45 (95% confidence interval, 1.93–15.40; P = 0.001).
Conclusions
C/EBPα may be a potential prognostic marker or therapeutic target in HCC regardless of different etiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary liver cancer is the sixth most common cancer in the world, and the third most common cause of cancer-related death (Parkin et al. 2005). There are 564,000 new cases diagnosed yearly, and the incidence is still increasing (Bosch et al. 2004). Hepatocellular carcinoma (HCC) is complex in etiology and pathogenesis. Risk factors include aflatoxin, alcohol, hepatitis B virus (HBV), hepatitis C virus (HCV), and cirrhosis. Different risk factors lead to different molecular pathways, making pathogenesis of HCC complex to interpret (Farazi and DePinho 2006; Suriawinata and Xu 2004). Altered expression of genes such as p53, p16, p18, p27, nm-23, and survivin has been identified involving carcinogenesis and having prognostic value (Mann et al. 2007), but extensive validation is needed before they can be used as markers. Since HCC is diverse in pathogenic pathways, more potential markers should be discovered to aid in the prediction of prognosis or the selection of a therapeutic target.
The CCAAT/enhancer binding protein alpha (C/EBPα), a leucine zipper transcription factor, is expressed highly in the liver, lung, adipose and myeloid tissues, mediating the expression of terminally-differentiated genes (Ramji and Foka 2002). Through protein–protein interaction, it also functions to exit cell cycle with mechanisms not yet completely elucidated (Schuster and Porse 2006). It is strongly antiproliferative in various types of cultured cells (Hendricks-Taylor and Darlington 1995; Shim et al. 2005), and is also noted reduced in acute myeloid leukemia (AML) (Pabst et al. 2001), lung cancer (Costa et al. 2007; Halmos et al. 2002; Tada et al. 2006), breast cancer (Gery et al. 2005), head and neck squamous cell carcinoma (Bennett et al. 2007), evidencing its tumor-suppressive function in multiple tissues.
In the liver, C/EBPα deficiency increases hepatic proliferation rate in mice models (Flodby et al. 1996; Timchenko et al. 1997), C/EBPα overexpression inhibits proliferation of transformed rat hepatocytes (Diehl et al. 1996) and human hepatoma cells (Hendricks-Taylor and Darlington 1995), C/EBPα knock-in mice are more resistant to carcinogen-induced HCC (Tan et al. 2005). Thus C/EBPα also plays a tumor suppressor role in the liver; its alteration in expression may contribute to HCC development and progression.
A few studies have shown C/EBPα reduced in human HCC (Tomizawa et al. 2003; Xu et al. 2001; Xu et al. 1994). One of them reported that lower C/EBPα mRNA predicted shorter survival of patients, but the sample size was 11 which might not ensure sufficient power (Tomizawa et al. 2003). To date, little is known about C/EBPα expression in chronic liver diseases which precede HCC, and clinical significance and prognostic value of C/EBPα in HCC have not been assessed at the protein level.
To address these problems, we conducted a retrospective cohort study on 50 HCC patients, with immunohistochemistry performed on samples obtained from surgery, and evaluated the association of C/EBPα expression with clinicopathological parameters and patients’ survival.
Patients and methods
Patients
Fifty patients of primary HCC who underwent hepatic resection from 2000 to 2003 at the Department of Surgery, Changhua Christian Hospital were included for retrospective cohort study. The patients consisted of 39 men and 11 women, with a mean age of 54.5 years (SD, 14.9 years; range, 10–75 years). Data of clinicopathological parameters, dates of the last follow-up or death, were obtained from medical records. Survival was calculated from the time of surgery. The median follow-up time was 1,376 days (95% CI, 261–2,491 days). To date, 28 of the 50 patients have died, while 22 patients were censored at the last follow-up visit.
Tissue specimens
Hepatocellular carcinoma specimens and adjacent nontumor tissue specimens were collected during hepatic resection, and were formalin-fixed and paraffin-embedded until staining. All specimens were confirmed pathologically. Histologic grade of HCC was based on the WHO grading system (Hirohashi et al. 2000), while in this study the poorly differentiated and undifferentiated were combined and designated as poorly differentiated. Thus the numbers of patients with well, moderately, and poorly differentiated HCC were 6, 29, and 15, respectively. Tumor stage was based on pTNM staging system of the American Joint Committee on Cancer (American Joint Committee on Cancer 1997). The numbers of patients in stage I, II, IIIA, IIIB, and IV were 14, 17, 6, 3, and 10, respectively. The greatest diameter of each tumor was defined as tumor size (mean ± SD, 5.4 ± 3.3 cm; range, 1.6–15.5 cm). This study was approved by the Institutional Review Board of Changhua Christian Hospital.
Immunohistochemical analysis of C/EBPα
Formalin-fixed, paraffin-embedded tissue sections (4 μm thick) were deparaffinized. The slides were treated with 3% hydrogen peroxide to block endogenous peroxidase activity, and heated in 10 mM citrate buffer at 100°C for 20 min to retrieve antigen. Slides were then incubated with a 1:120 diluted goat polyclonal anti-C/EBPα antibody (Santa Cruz, CA, USA) at room temperature for 30 min, followed by incubation with a secondary antibody conjugated with horseradish peroxidase polymer (Zymed, CA, USA) for 10 min. Diaminobenzadine (DakoCytomation, Denmark) was used as chromogen and hematoxylin as counterstain. Positive staining was defined as C/EBPα staining detected in more than 5% of cancer cells (Tada et al. 2006), thus staining intensity was scored as 0 (<5% of positive cells), 1 (5–50% positive cells), or 2 (>50% positive cells) (Morrison et al. 2002).
Statistical analysis
Wilcoxon signed rank test was used to compare C/EBPα protein scores between tumor and nontumor specimens. The associations between C/EBP expression and clinical parameters were determined by Fisher’s exact test except when tumor size was used as a continuous variable, to which Spearman correlation analysis was applied. Survival functions were estimated by Kaplan–Meier method, with Log-rank test for the differences among groups. Cox proportional hazards model was used for analysis of prognostic factors, with fitness of proportionality assumptions checked before analysis. Multivariate Cox model was obtained by a backward elimination procedure with P > 0.10 as criterion to remove variables. All statistical analyses were performed with SPSS 13.0 for Windows and P < 0.05 (two-sided) was considered statistically significant.
Results
C/EBPα expression was downregulated in HCC
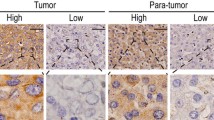
Immunohistochemistry showed tumor and nontumor specimens differed in C/EBPα staining intensities for protein (Fig. 1a). Most of the nontumor specimens had a score of 1 or 2, while most of the tumor specimens had a score of 0 or 1 (Fig. 1b). The score difference between tumor and the paired nontumor specimens (T − N) ranged from −2 to 2. In total, 60% of HCCs had reduced expression of C/EBPα (T − N < 0) (Fig. 1c). Wilcoxon signed rank test further confirmed that tumor specimens had a significantly lower C/EBPα expression than nontumor specimens (P < 0.001).
C/EBPα expression was not altered in chronic liver diseases preceding HCC
To clarify whether C/EBPα expression was already decreased in preceding liver diseases or influenced by exposure factors, we analyzed nontumor tissues. Fisher’s exact test showed C/EBPα expression was not associated with age, gender, alcohol drinking, smoking, HBV, HCV, or cirrhosis; there was also no difference of C/EBPα expression between HBV and HCV infection (Table 1). Thus among chronic liver diseases we studied, it was only HCC that presented a reduction in C/EBPα expression.
C/EBP expression in HCC was negatively correlated with pTNM tumor stage
We ranked C/EBP expression into three levels: I, T − N = −2; II, T − N = −1; and III, T − N ≥ 0, as T − N represented C/EBPα expression score in tumor normalized to that in the nontumor. Fisher’s exact test showed C/EBP expression in HCC was not associated with age, gender, HBV or HCV infection, cirrhosis, tumor size (cut off at 5 cm) and histologic grade, but was negatively associated with tumor stage (Table 2). To avoid bias from arbitrary cutoff of tumor size, we also checked Spearman correlation between C/EBPα expression (T − N, from −2 to 2, in original five ranks) and tumor size (as a continuous variable), and found a nearly significant inverse correlation between them (r = −0.277, P = 0.052). Therefore among the parameters we studied, C/EBPα downregulation was strongly associated with pTNM stage but weakly associated with tumor size.
Reduced C/EBPα expression in HCC was associated with shortened patient survival
Kaplan–Meier survival analysis showed patients with class I C/EBPα expression had a shortest median survival of 254 days [95% confidence interval (CI), 140–368 days], patients with class II C/EBPα expression had an intermediate median survival of 1,579 days (95% CI, 435–2,723 days), while the median survival time of patients with class III C/EBPα expression was not reached. Log-rank test further confirmed patients with class I C/EBPα expression had a significant poorer outcome than the other two groups (Fig. 2). To perform Cox proportional hazards analysis, we classified all the categorical variables into dichotomous ones, and used tumor size as a continuous variable. Univariate analysis identified that C/EBPα, tumor size and tumor stage were significant predictors for survival after surgery, while age, gender, HBV, HCV, cirrhosis, and histologic grade were not (Table 3). Being collinear with C/EBPα, both stage and tumor size were not included in the following multivariate model. The final model showed patients with class I C/EBPα expression had an increased risk for death, with a hazard ratio of 5.44 (95% CI, 1.93–15.40; P = 0.001) after adjusting for histologic grade, which also showed a nearly significant influence on survival (Table 4).
Discussion
Our findings demonstrated that C/EBPα was downregulated in HCC, but not in preceding liver diseases such as HBV, HCV hepatitis, and cirrhosis. Its decrease was associated with tumor stage and poor prognosis, compatible with its role as a tumor suppressor in HCC.
Apart from being a transcription factor of many tissue-specific genes, C/EBPα is a strongly antiproliferative molecule, which mediates exit from cell cycle by protein–protein interactions. Several mechanisms have been proposed, including stabilization of p21 (Timchenko et al. 1997), direct inhibition of Cdk2 and Cdk4 (Wang et al. 2001), repression of E2F (D’Alo et al. 2003), and interaction with chromatin remodeling complex (Muller et al. 2004). C/EBPα also regulates metallothionein expression to resist oxidative stress and malignant transformation of hepatocytes (Datta et al. 2007). Therefore by multiple mechanisms, C/EBPα acts as a suppressor of carcinogenesis.
In human cancers, C/EBPα has been shown reduced in AML (Pabst et al. 2001), lung (Costa et al. 2007; Halmos et al. 2002) and breast cancers (Gery et al. 2005), head and neck squamous cell carcinoma (Bennett et al. 2007), and HCC (Tomizawa et al. 2003; Xu et al. 2001; Xu et al. 1994). Consistent with these studies, our results showed C/EBPα protein decreased in 60% of HCCs, thus the reduction of C/EBPα might contribute to hepatocarcinogenesis.
From the nontumor samples, we demonstrated that C/EBPα expression was not influenced by chronic alcohol and smoking exposure, HBV, HCV, and cirrhosis. C/EBPα mRNA has been observed downregulated in acute liver injury of alcohol-fed rats (Bridle et al. 2006), and in regenerating rat liver after partial hepatectomy (Flodby et al. 1993). However, among chronic liver injuries, as was noted in our study, it was only HCC that had a reduction in C/EBPα expression. Our findings clarified that C/EBPα was not reduced in liver diseases preceding HCC, and persistent C/EBPα reduction was associated with HCC development and progression.
We observed that C/EBPα expression in HCC was negatively associated with pTNM stage, a result consistent with the findings in previous studies of lung cancer (Halmos et al. 2002) and HCC (Tomizawa et al. 2003), though the latter used clinical staging system instead. We also found a weak inverse correlation between C/EBPα expression and tumor size, similar to the finding in previous study of HCC, though it used a cutoff tumor size of 3 cm (Tomizawa et al. 2003). The agreement of these reports clearly evidenced C/EBPα functions to inhibit carcinogenesis, and its reduction facilitates tumor progression. To our surprise, though C/EBPα also functions to regulate expression of terminally-differentiated genes, we did not find an association between C/EBPα expression and histologic grade. The negative result was also seen in the study of breast cancer (Gery et al. 2005), but not in the lung cancer study where a trend toward loss of C/EBPα in less differentiated tumor samples was noted (Costa et al. 2007). Other clinical parameters such as age, gender, cirrhosis, and virus hepatitis, did not affect C/EBPα expression in tumor. HCC is diverse in etiology and subsequent molecular pathways, for example, HBV DNA integrates into human genome, resulting in chromosome instability, while HCV might use core protein to divert intracellular pathways (Suriawinata and Xu 2004). The finding that C/EBPα expression in HCC was not affected by HBV or HCV infection and other exposure agents suggests C/EBPα may serve as a therapeutic target regardless of different etiology.
The prognostic value of C/EBPα has been shown in studies of AML, where AML patients with C/EBPα mutations had a paradoxically favorable prognosis with reasons yet unknown (Frohling et al. 2004; Preudhomme et al. 2002). Prognosis in solid tumor was less studied. Previous study of HCC patients showed low C/EBPα mRNA was associated with unfavorable outcome (Tomizawa et al. 2003), but this association was not found in lung cancer patients with low C/EBPα protein (Costa et al. 2007). Both studies analyzed C/EBPα dichotomously. With C/EBPα categorized into three groups, our model clearly showed the patients with markedly reduced C/EBPα had a poorer prognosis, suggesting there was a threshold on which the amount of C/EBPα was no longer able to inhibit cell proliferation and hence tumor progression, thus leading to a shortened patient survival.
Other significant prognostic factors found in our study include tumor stage and tumor size, consistent with Nonami et al.’s observation that advanced pTNM stage and larger tumor size predict poor prognosis in HCC patients (Nonami et al. 1997). Low C/EBPα, advanced tumor stage and larger tumor size correlated with each other in our study, all reflecting rapid progression of tumor, and affecting patient survival in a similar mode. On the other hand, the influence of histologic grade on HCC patient survival is controversial (Haratake et al. 1993; Lai et al. 1979). Our Cox multivariate model showed histologic grade had a weak influence on patient survival. On the whole, the relationship among C/EBPα expression, histologic grade, and patient survival was not well established in our study.
Dysregulation of C/EBPα has been noted due to gene mutation in AML (Frohling et al. 2004; Pabst et al. 2001; Preudhomme et al. 2002). But in solid tumors, epigenetic silencing from DNA hypermethylation has been found causing low C/EBPα expression in lung cancer (Tada et al. 2006) and head and neck squamous cell carcinoma (Bennett et al. 2007). We also performed methylation analysis on C/EBPα promoter, but unfortunately we did not find a significant difference of methylation status between tumor and nontumor specimens. Tumor suppressive activity of C/EBPα might also be blocked by dephosphorylation through phosphatidylinositol 3-kinase signaling (Datta et al. 2007), which we did not explore. Another limitation of this study is the liver factors such as cirrhosis or alcohol exposure were merely reported as absence or presence but not stratified for degree of fibrosis or consumption amount. The sample size also should be increased enough to clarify the relationship of C/EBPα expression with histologic grade, and grade with patient survival.
In conclusion, we demonstrated that C/EBPα protein was decreased in HCC, a phenomenon not observed in preceding hepatitis or cirrhosis. We also showed that loss of C/EBPα expression was associated with advanced tumor stage and shortened patient survival, thus confirming the tumor-suppressive function and a prognostic value of C/EBPα in HCC. Taken together, this molecule may be a useful prognostic marker or a potential therapeutic target in HCC regardless of different etiology.
References
American Joint Committee on Cancer (1997) AJCC cancer staging manual. Lippincott-Raven, Philadelphia
Bennett KL, Hackanson B, Smith LT, Morrison CD, Lang JC, Schuller DE et al (2007) Tumor suppressor activity of CCAAT/enhancer binding protein alpha is epigenetically down-regulated in head and neck squamous cell carcinoma. Cancer Res 67:4657–4664. doi:10.1158/0008-5472.CAN-06-4793
Bosch FX, Ribes J, Diaz M, Cleries R (2004) Primary liver cancer: worldwide incidence and trends. Gastroenterology 127:S5–S16. doi:10.1053/j.gastro.2004.09.011
Bridle K, Cheung TK, Murphy T, Walters M, Anderson G, Crawford DG et al (2006) Hepcidin is down-regulated in alcoholic liver injury: implications for the pathogenesis of alcoholic liver disease. Alcohol Clin Exp Res 30:106–112. doi:10.1111/j.1530-0277.2006.00002.x
Costa DB, Li S, Kocher O, Feins RH, Keller SM, Schiller JH et al (2007) Immunohistochemical analysis of C/EBPalpha in non-small cell lung cancer reveals frequent down-regulation in stage II and IIIA tumors: a correlative study of E3590. Lung Cancer 56:97–103. doi:10.1016/j.lungcan.2006.11.023
D’Alo F, Johansen LM, Nelson EA, Radomska HS, Evans EK, Zhang P et al (2003) The amino terminal and E2F interaction domains are critical for C/EBP alpha-mediated induction of granulopoietic development of hematopoietic cells. Blood 102:3163–3171. doi:10.1182/blood-2003-02-0479
Datta J, Majumder S, Kutay H, Motiwala T, Frankel W, Costa R et al (2007) Metallothionein expression is suppressed in primary human hepatocellular carcinomas and is mediated through inactivation of CCAAT/enhancer binding protein alpha by phosphatidylinositol 3-kinase signaling cascade. Cancer Res 67:2736–2746. doi:10.1158/0008-5472.CAN-06-4433
Diehl AM, Johns DC, Yang SQ, Lin HZ, Yin M, Matelis LA et al (1996) Adenovirus-mediated transfer of CCAAT/enhancer-binding protein-alpha identifies a dominant antiproliferative role for this isoform in hepatocytes. J Biol Chem 271:7343–7350. doi:10.1074/jbc.271.13.7343
Farazi PA, DePinho RA (2006) Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 6:674–687. doi:10.1038/nrc1934
Flodby P, Antonson P, Barlow C, Blanck A, Porsch-Hallstrom I, Xanthopoulos KG (1993) Differential patterns of expression of three C/EBP isoforms, HNF-1, and HNF-4 after partial hepatectomy in rats. Exp Cell Res 208:248–256. doi:10.1006/excr.1993.1244
Flodby P, Barlow C, Kylefjord H, AhrlundRichter L, Xanthopoulos KG (1996) Increased hepatic cell proliferation and lung abnormalities in mice deficient in CCAAT/enhancer binding protein alpha. J Biol Chem 271:24753–24760. doi:10.1074/jbc.271.40.24753
Frohling S, Schlenk RF, Stolze I, Bihlmayr J, Benner A, Kreitmeier S et al (2004) CEBPA mutations in younger adults with acute myeloid leukemia and normal cytogenetics: prognostic relevance and analysis of cooperating mutations. J Clin Oncol 22:624–633. doi:10.1200/JCO.2004.06.060
Gery S, Tanosaki S, Bose S, Bose N, Vadgama J, Koeffler HP (2005) Down-regulation and growth inhibitory role of C/EBPalpha in breast cancer. Clin Cancer Res 11:3184–3190. doi:10.1158/1078-0432.CCR-04-2625
Halmos B, Huettner CS, Kocher O, Ferenczi K, Karp DD, Tenen DG (2002) Down-regulation and antiproliferative role of C/EBPalpha in lung cancer. Cancer Res 62:528–534
Haratake J, Takeda S, Kasai T, Nakano S, Tokui N (1993) Predictable factors for estimating prognosis of patients after resection of hepatocellular carcinoma. Cancer 72:1178–1183. doi :10.1002/1097-0142(19930815)72:4<1178::AID-CNCR2820720408>3.0.CO;2-Q
Hendricks-Taylor LR, Darlington GJ (1995) Inhibition of cell proliferation by C/EBP alpha occurs in many cell types, does not require the presence of p53 or Rb, and is not affected by large T-antigen. Nucleic Acids Res 23:4726–4733. doi:10.1093/nar/23.22.4726
Hirohashi S, Ishak KG, Kojiro M, Wanless IR, Theise ND, Tsukuma H et al (2000) Hepatocellular carcinoma. In: Hamilton SR, Aaltonen LA (eds) World Health Organization classification of tumors: pathology and genetics of tumors of the digestive system. IARC Press, Lyon, pp 165–166
Lai CL, Wu PC, Lam KC, Todd D (1979) Histologic prognostic indicators in hepatocellular carcinoma. Cancer 44:1677–1683. doi :10.1002/1097-0142(197911)44:5<1677::AID-CNCR2820440522>3.0.CO;2-D
Mann CD, Neal CP, Garcea G, Manson MM, Dennison AR, Berry DP (2007) Prognostic molecular markers in hepatocellular carcinoma: a systematic review. Eur J Cancer 43:979–992. doi:10.1016/j.ejca.2007.01.004
Morrison C, Marsh W, Frankel WL (2002) A comparison of CD10 to pCEA, MOC-31, and hepatocyte for the distinction of malignant tumors in the liver. Mod Pathol 15:1279–1287. doi:10.1097/01.MP.0000037312.69565.24
Muller C, Calkhoven CF, Sha X, Leutz A (2004) The CCAAT enhancer-binding protein alpha (C/EBPalpha) requires a SWI/SNF complex for proliferation arrest. J Biol Chem 279:7353–7358. doi:10.1074/jbc.M312709200
Nonami T, Harada A, Kurokawa T, Nakao A, Takagi H (1997) Hepatic resection for hepatocellular carcinoma. Am J Surg 173:288–291. doi:10.1016/S0002-9610(96)00399-6
Pabst T, Mueller BU, Zhang P, Radomska HS, Narravula S, Schnittger S et al (2001) Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBP alpha), in acute myeloid leukemia. Nat Genet 27:263–270. doi:10.1038/85820
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Preudhomme C, Sagot C, Boissel N, Cayuela J-M, Tigaud I, de Botton S et al (2002) Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: a study from the Acute Leukemia French Association (ALFA). Blood 100:2717–2723. doi:10.1182/blood-2002-03-0990
Ramji DP, Foka P (2002) CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J 365:561–575
Schuster MB, Porse BT (2006) C/EBPalpha: a tumour suppressor in multiple tissues? Biochim Biophys Acta 1766:88–103
Shim M, Powers KL, Ewing SJ, Zhu S, Smart RC (2005) Diminished expression of C/EBPalpha in skin carcinomas is linked to oncogenic Ras and reexpression of C/EBPalpha in carcinoma cells inhibits proliferation. Cancer Res 65:861–867
Suriawinata A, Xu RL (2004) An update on the molecular genetics of hepatocellular carcinoma. Semin Liver Dis 24:77–88. doi:10.1055/s-2004-860865
Tada Y, Brena RM, Hackanson B, Morrison C, Otterson GA, Plass C (2006) Epigenetic modulation of tumor suppressor CCAAT/enhancer binding protein alpha activity in lung cancer. J Natl Cancer Inst 98:396–406
Tan EH, Hooi SC, Laban M, Wong E, Ponniah S, Wee A et al (2005) CCAAT/enhancer binding protein alpha knock-in mice exhibit early liver glycogen storage and reduced susceptibility to hepatocellular carcinoma. Cancer Res 65:10330–10337. doi:10.1158/0008-5472.CAN-04-4486
Timchenko NA, Harris TE, Wilde M, Bilyeu TA, BurgessBeusse BL, Finegold MJ, Darlington GJ (1997) CCAAT/enhancer binding protein alpha regulates p21 protein and hepatocyte proliferation in newborn mice. Mol Cell Biol 17:7353–7361
Tomizawa M, Watanabe K, Saisho H, Nakagawara A, Tagawa M (2003) Down-regulated expression of the CCAAT/enhancer binding protein alpha and beta genes in human hepatocellular carcinoma: a possible prognostic marker. Anticancer Res 23:351–354
Wang H, Iakova P, Wilde M, Welm A, Goode T, Roesler WJ et al (2001) C/EBPalpha arrests cell proliferation through direct inhibition of Cdk2 and Cdk4. Mol Cell 8:817–828. doi:10.1016/S1097-2765(01)00366-5
Xu LX, Sui YF, Wang WL, Liu YF, Gu JR (1994) Immunohistochemical demonstration of CCAAT/enhancer binding protein (C/EBP) in human liver tissues of various origin. Chin Med J (Engl) 107:596–599
Xu L, Hui L, Wang S, Gong J, Jin Y, Wang Y et al (2001) Expression profiling suggested a regulatory role of liver-enriched transcription factors in human hepatocellular carcinoma. Cancer Res 61:3176–3181
Acknowledgments
The study was supported by the National Science Council under the grant of NSC 94-2320-B-039-019.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tseng, HH., Hwang, YH., Yeh, KT. et al. Reduced expression of C/EBPα protein in hepatocellular carcinoma is associated with advanced tumor stage and shortened patient survival. J Cancer Res Clin Oncol 135, 241–247 (2009). https://doi.org/10.1007/s00432-008-0448-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-008-0448-5