Abstract
Purpose
Primary chemotherapy brings the opportunity for an early and accurate assessment of response and offers an ideal model to search for new predictors of response. HER-2/neu is one of the most studied genes for this purpose.
Patients and methods
Her-2/neu was tested in a non-randomized series of 300 patients with operable breast carcinomas treated with primary CMF. Response was assessed by mammography. Disease-free survival (DFS) and overall survival (OS) were calculated after a mean follow-up of 116 months. Statistical analysis was performed to study the association between HER-2/neu status and response to CMF.
Results
Overexpression/amplification was found in 23.66% cases. Univariate analysis showed that response was similar in HER-2/neu positive and negative tumors (51.38 vs. 47.36%, P = 0.6). Triple negative tumors (ER, PR and HER-2/neu negative) presented the highest response rate (64.9%). By multivariate analysis, response was significantly correlated to higher nuclear grade and negative estrogen receptor status (P = 0.02 and 0.007, respectively). Patients with HER-2/neu positive tumors presented shorter survival rates (P = 0.06). Patients with response to CMF showed a better survival over non-responders independent of Her-2/neu status. Patients with the combination of response to CMF and Her-2/neu negative tumors presented the best outcome. On the other hand, the association of no response to CMF and positive Her-2/neu score was statistically related to poor DFS and OS.
Conclusions
CMF indication is independent of Her-2/neu status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic treatment has improved survival in breast cancer. However, prediction of response in a particular patient is not possible because of the absence of reliable predictive markers. Primary chemotherapy brings the opportunity for an early and accurate assessment of response. It offers an ideal model to test chemo-sensitivity in vivo and also to find predictive markers (Bonadonna et al. 1998; Fisher et al. 1998). Until recently, CMF has been the treatment of choice for breast cancer and it is still recommended by St Gallen expert consensus (Goldhirsch et al. 2005).
Amplification/overexpression of Her-2/neu is present in about 20–25% of invasive breast carcinomas. Several preclinical studies suggested that its overexpression played a direct role in pathogenesis and aggressiveness of tumors (Bargmann et al. 1986). Her-2/neu status has been studied as a predictor of poor prognosis in both lymph node positive and negative cases (Paik et al. 1990; Van de Vijver et al. 1988; Moreno et al. 1997; Reed et al. 2000). Several studies have also reported the relationship between Her-2/neu status and response to chemotherapy or hormonotherapy (Allred et al. 1992; Wright et al. 1992).
Herein we report our results about the relationship between Her-2/neu and CMF in a series of 300 patients with operable breast carcinomas treated with primary CMF in a single institution, after a mean follow-up of 116 months.
Patients and methods
HER-2/neu status was assessed in sections of paraffin-embedded surgical specimens of a series of operable breast cancers. Patients had been included in a protocol of primary chemotherapy approved by our local Research Ethics Committee (number 3490) from January 8, 1990 to December 31, 1999. The study enrolled 305 women with operable breast cancer T > 3 cm classified T2-3/N0-1M0. Palpable tumors were required to be assessable by mammography in two dimensions. The criteria for exclusion were: locally advanced or metastatic breast cancer, multicentricity, age under 18 years or over 65 years, pregnancy, history of prior malignancies or severe concomitant systemic disease.
The general characteristics of the series, response and survival results have been previously published (Falo et al. 2005).
Primary CMF protocol
Chemotherapy
Patients were treated with CMF. The CMF consisted of cyclophosphamide, methotrexate and fluorouracil at doses of 600/40/600 mg/m2 intravenously on days 1 and 8 of each treatment cycle, every 28 days for three courses. In the presence of tumor progression primary chemotherapy was discontinued.
Assessment of response
The size of primary tumor was measured by palpation on the first day of each treatment cycle and before surgery. Response was assessed by mammography by measuring the product of the two largest tumor diameters. The response was classified as complete (cCR) in the absence of evidence of tumor in the breast and the axilla. Partial response (cPR) was defined as a reduction ≥50%. Tumor progression was defined as an increase by at least 25%. Cases with complete clinical response that presented breast cancer cells on the surgical specimen, were considered as partial response for statistical purposes (Moreno et al. 2002).
Local treatment
Surgery was planned after three to 4 weeks after the third course of CMF. Conservative surgery with wide tumorectomy was performed when radical and aesthetic criteria allowed it. Selected cases with great response required radiological guided surgery. A few cases were submitted to conservative surgery with the aid of plastic surgery. The remaining patients underwent modified radical mastectomy. In all patients, three level axillary dissection was performed.
Radiotherapy was delivered to all patients subjected to breast-conservative surgery, to mastectomy patients classified as T3 or T4, and to those cases with more than four metastasic axillary lymph nodes. Patients with surgical margins less than 10 mm had a boost of 20 Gy instead of 10 Gy on the tumor bed. Radiotherapy started 3–4 weeks after adjuvant chemotherapy was completed.
Adjuvant treatment
Adjuvant treatment started 15 days after surgery. Cases with response (complete or partial) received three more courses of CMF. Patients without response were treated with doxorubicin at doses of 75 mg/m2 in four courses. Tamoxifen was not the standard of care at that time.
Follow-up
After the completion of the treatment program, physical examination, and hematological tests and blood biochemistry were performed every 3 months for the first 3 years, every 4 months in the 4th and 5th year, and every 6 months thereafter. Mammography was performed once a year starting 6 months from the end of breast irradiation therapy. Other studies were performed annually and included chest X-ray and bone X-ray or bone scans. After the 10th year the follow-up became annual.
Her-2/neu assessment
Her-2/neu was detected on formalin-fixed, paraffin-embedded surgical specimens by immunohistochemistry (IHC) and by fluorescence in situ hybridization (FISH), according to the algorithm of our institution (Falo et al. 2003).
The algorithm consisted on testing all the cases by IHC with the monoclonal antibody CB-11 from Biogenex™ (mab CB-11). The negative cases were submitted to a second immunohistochemical run with the HercepTest kit (DAKO™). Finally, those cases considered positive with the HercepTest (score 2+ and score 3+) that had been negative with the monoclonal antibody CB-11 were selected to quantify amplification by FISH with the Oncor Ventana Inform Her-2/neu gene detection system™.
Positive cases were defined as a positive immunostaining with the mab CB-11 or as amplified by FISH.
Tumour subclassification
Genomic expression profiling studies on breast tumors have identified distinct subtypes of breast carcinomas that are associated with different responses to chemotherapy and to different clinical outcomes (Van’t veer et al. 2002; Sorlie et al. 2001).
Taking into account the recent subclassification of breast carcinomas according to gene expression profiling, we have subdivided our series into the three main groups, i.e., triple negative [estrogen receptor (ER), progesterone receptor (PR) and HER-2/neu], luminal tumors (ER or PR positive, HER-2/neu negative) and HER-2/neu positive tumors.
Statistical analyses
Disease characteristics according to Her-2/neu status were compared using chi square tests.
In the univariate analysis, Her-2/neu amplification/overexpression has been related to response using chi square tests. Corrections for hormonal receptor expression have been done.
In the multivariate analysis, interrelationship between the different predictive factors (including nuclear grade, hormonal receptor and HER-2/neu status) was determined using a logistic regression test. The variable to predict was tumor reduction ≥50%, including complete tumor remission.
Disease-free survival (DFS) and overall survival (OS) have been calculated according to the method of Kaplan-Meier. Her-2/neu amplification/overexpression has been related to survival (DFS and OS) using the Cox-regression analysis. Survival curves were performed according to triple negative, luminal and HER-2/neu positive tumors using the cox-regression model as a survival function for patterns 1–3. Finally, survival was related both to HER-2/neu status and to response using the Log-rank test between groups.
All P values were two-sided and a value of P < 0.05 was considered statistically significant. Data were analyzed with SPSS™ (version 13.0 Chicago, IL).
Results
Her-2/neu status
Three hundred tumors were available for determination. After the application of the mentioned algorithm, 71 cases (23.66%) were considered positive. Disease characteristics according to Her-2/neu status are pictured in Table 1. HER-2/neu positive tumors were significantly associated with negative hormonal receptors (P = 0.02) and with the mean value of pathological involved lymph nodes (P = 0.05).
Tumour subclassification
Of the 284 breast carcinomas available for the subclassification, 57 (20%) corresponded to the triple negative subtype, 156 (55%) were luminal and 71 (25%) were HER-2/neu positive tumors. These figures are in agreement of those published in the literature (Sorlie et al. 2001).
Response
The overall response rate for the whole series was 48.3%.
Univariate analysis
No statistical differences in terms of response rate, were found according to the HER-2/neu status. Response was similar for HER-2/neu negative and positive tumors (47.59 vs. 50.70% respectively, P = 0.06).
The relation between HER-2/neu and response was corrected by hormonal receptor status (Fig. 1). The highest response rates were seen in the triple negative tumors (64.9%), compared to HER-2/neu positive tumors (50.7%) and luminal tumors (39.7%).
Multivariate analysis
By multivariate analysis, no association was observed between HER-2/neu status and tumor response (P = 0.94). The only independent predictors of response were estrogen receptor status and nuclear grade (P = 0.007 and 0.02, respectively).
Survival
Events
After a mean follow-up of 116 months (range 63+ to 173+), there have been 117 relapses (21 local, 78 systemic, and 18 local and systemic) and 75 deaths. Events according to Her-2/neu status are shown in Table 2.
Local recurrence and failure
Local recurrence is defined as recurrence on the ipsilateral breast, or on the ipsilateral chest wall, or in the ipsilateral draining lymph nodes in the absence of systemic disease. Local failure is defined as local recurrence independent of systemic disease.
At the time of the present analysis, there have been 21 local recurrences and 39 local failures.
Patients with Her-2/neu positive tumors presented an increased rate of local failure compared with patients with Her-2/neu negative tumors: 19.7 versus 10.9%, respectively. This difference reached standard levels of significance (P = 0.04).
Disease free survival, disease free of local recurrence, disease free of local failure and overall survival
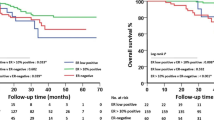
The DFS, DFS of local recurrence, DFS of local failure and OS curves are provided in Figs. 2, 3, 4 and 5, respectively.
Patients with Her-2/neu positive tumors presented a poorer outcome compared to patients with negative tumors. DFS at 8 years was 53.3 versus 61.5% (P = 0.29); disease free of local recurrence was 86.6 versus 92.6% (P = 0.22); disease free of local failure was 77.6 versus 88.2% (P = 0.04): and OS was 63.3 versus 75.8% (P = 0.06).
Survival rates were analyzed taking into account the three subtypes of breast cancer, i.e., basal, luminal and HER-2/neu positive tumors. No significant statistical differences were seen between the three groups (P = 0.6).
Survival related to combined Her-2/neu status and response to chemotherapy
To evaluate survival according to Her-2/neu status and response to chemotherapy, four groups were defined as follows: group 1 (n = 120): non-response and Her-2/neu negative; group 2 (n = 35): non-response and Her-2/neu positive; group 3 (n = 109): response and Her-2/neu negative; group 4 (n = 36): response and Her-2/neu positive.
Events
The number of recurrences by subgroups was the following: group 1: 50 (41.6%); group 2: 19 (54.2%); group 3: 35 (32.1%); group 4: 13 (36.1%). The number of deaths by subgroups was distributed as follows: group 1: 34 (28.3%); group 2: 15 (42.8%); group 3: 16 (14.6%); group 4: 10 (27.7%).
Disease free and overall survival, according to response and HER-2/neu status
The DFS and OS curves according to Her-2/neu status and response are provided in Figs. 6 and 7, respectively. Patients with response presented better survival rates than non-responders, regardless of HER-2/neu status.
The best outcome (DFS and OS) was achieved in patients with both response and Her-2/neu negative tumors (group 3). On the opposite, non-responders with Her-2/neu positive tumors (group 4) presented the worst survival rates.
Discussion
Several studies suggested that Her-2/neu was a factor of resistance to tamoxifen (Wright et al. 1992) and to CMF-like regimens (Allred et al. 1992; Gusterson et al. 1992) whereas its overexpression benefited from optimal doses of antracyclines (Muss et al. 1994). Those studies, however, lacked from an optimal methodology for Her-2/neu assessment and were not directly designed to answer that question. Other works do not support chemoresistance linked to CMF (Ménard et al. 2001; Miles et al. 1999) and indicate that the benefit of antracyclines is only marginal (Paik et al. 2000), although some recent papers still support the idea of CMF resistance related to overexpression of Her-2/neu and p21 cip1(Yang et al. 2003).
Information on the association between Her-2/neu and response to taxane-based regimens is increasing. Preliminary results from small phase II studies in metastatic breast cancer, showed better response rates in patients with positive tumors (Baselga et al. 1997). These results are being consolidated in further studies (Konecny et al. 2004; Martin et al. 2005).
In the current series, tumor response was measured after primary CMF, which brings the advantage of a direct and earlier assessment of response. By univariate analysis we found no statistical significant differences in the rate of response between Her-2/neu negative and positive tumors (47.59 vs. 50.70%, P = 0.6). Correction of Her-2/neu status for hormone receptor expression was done. Our analysis confirmed that triple negative tumors present the highest response rate to CMF (64.9%) In the multivariate analysis, response to CMF was confirmed to be independent of HER-2/neu status (P = 0.94), whereas it was significantly related to estrogen receptor status and nuclear grade (P = 0.007 and 0.02, respectively).
Patients with response to primary CMF improved survival over non-responders both in Her-2/neu positive and negative tumors. These results are in accordance with those of other studies on adjuvant chemotherapy as those of Ménard and Miles but contradict those initial studies of Gusterson and Muss.
As in other studies from the literature (Slamon et al. 1987), in our work, Her-2/neu status is an indicator of poor outcome. Patients with HER-2/neu positive tumors presented a shorter DFS and OS, even if the differences did not reach stastistical levels of significance (P = 0.29 and 0.06, respectively). In terms of local failure, patients with HER-2/neu positive tumors presented a double rate of events (19.7 vs. 10.9, P = 0.04). The higher local failure rate has been previously related to resistance to radiotherapy (Haffty et al. 1996), however it has not been confirmed in recent studies (Buchholz et al. 2004).
An interesting point of our study is the analysis of survival combining Her-2/neu status and tumor response. Patients with response to CMF and Her-2/neu negative tumors presented the best survival rates. On the opposite, patients without response and Her-2/neu positive tumors presented the worst for both DFS and OS. This combination seems more valuable for prediction of prognosis than Her-2/neu status or response alone, although a larger series is necessary to support this hypothesis.
In summary, the present study is a retrospective study developed during the 1990’s, when CMF was the treatment of choice in breast cancer, which cannot be considered a standard of primary chemotherapy nowadays. However this study gives one of the most solid data sets addressing the question as to whether HER-2/neu positive breast cancers are resistant to CMF. By univariate and multivariate analysis, we report no statistical differences in the overall response rate to primary CMF between HER-2/neu positive and negative tumors. Accordingly, the indication of CMF in breast cancer must be independent of Her-2/neu status.
References
Allred DC, Clark GM, Tandon AK et al (1992) HER-2/neu in node-negative breast cancer: prognostic significance of overexpression influenced by the presence of in situ carcinoma. J Clin Oncol 10:599–605
Bargmann CI, Hung MC, Weinberg (1986) The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature 319:226–230
Baselga J, Seidman AD, Rosen PP et al (1997) HER2 overexpression and paclitaxel sensitivity in breast cancer: therapeutic implications. Oncology 11:43–48
Bonadonna G, Valagussa P, Brambilla C et al (1998) Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol 16:93–100
Buchholz TA, Huang EH, Berry D et al (2004) HER2/neu positive disease does not increase risk of locorregional recurrence for patients treated with neoadjuvant doxorrubicin-based chemotherapy, mastectomy, and radiotherapy. Int J Radiat Oncol Biol Phys 59:1337–1342
Falo C, Moreno A, Lloveras B et al (2003) Algorithm for the diagnosis of HER-2/neu status in breast infiltrating carcinomas. Am J Clinical Oncol 26:465–470
Falo C, Moreno A, Benito E et al (2005) Primary chemotherapy with cyclophosphamide, methotrexate, and 5-fluorouracil in operable breast carcinoma. Cancer 12:657–663
Fisher B, Bryant J, Wolmark N et al (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16:2672–2685
Goldhirsch A, Glick JH, Gelber RD et al (2005) Meeting Highlights: International Expert consensus on the primary therapy of early breast cancer 2005. Ann of Oncol 16:1569–1583
Gusterson BA, Gelber RD, Goldhirsch A et al (1992) Prognostic importance of c-erbB-2 expression in breast cancer. J Clin Oncol 10:1049–1056
Haffty BG, Brown F, Carter D et al (1996) Evaluation of HER-2/neu oncoprotein expression as a prognostic indicator of local recurrence in conservatively treated breast cancer: a case-control study. Int J Radiat Oncol Biol Phys 35:751–757
Konecny GE, Thomssen Ch, Lück HJ et al (2004) HER-2/neu gene amplification and response to paclitaxel in patients with metastatic breast cancer. J Natl Cancer Inst 96:1141–1151
Martin M, Pienkowski T, Mackey J et al (2005) Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352:2302–2313
Ménard S, Valagussa P, Pilotti S et al (2001) Response to cyclophosphamide, methrotrexate and fluorouracil in lymph node-positive breast cancer according to HER-2 overexpression and other tumor biologic variables. J Clin Oncol 19:329–335
Miles DW, Harris WH, Gillett CE et al (1999) Effect of c-erbB(2) and estrogen receptor status on survival of women with primary breast cancer treated with adjuvant cyclophosphamide/methotrexate/fluorouracil. Int J Cancer 84:354–359
Moreno A, Lloveras B, Figueras A et al (1997) Ductal carcinoma in situ of the breast: correlation between histologic classifications and biologic markers. Mod Pathol 10(11):1088–1092
Moreno A, Escobedo A, Benito E, Serra JM, Guma A, Riu F (2002) Pathologic changes related to CMF primary chemotherapy in breast cancer. Pathological evaluation of response predicts clinical outcome. Breast Cancer Res Treat 75(2):119–125
Muss HB, Thor AD, Berry DA et al (1994) C-erbB-2 expression and response to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med 330:1260–1266
Paik S, Hazan R, Fisher ER et al (1990) Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of c-erbB-2 protein overexpression in primary breast cancer. J Clin Oncol 8:103–112
Paik S, Bryant J, Tan-Chiu E et al (2000) HER2 and choice of adjuvant chemotherapy for invasive breast cancer: National Surgical Adjuvant Breast And Bowel Project Protocol B-15. J Natl Cancer Inst 92:1991–1998
Reed W, Hannisdal E, Boehler PJ et al (2000) The prognostic value of p53 and c-erbB-2 immunostaining is overrated for patients with lymph node negative breast carcinoma: a multivariate analysis of prognostic factors in 613 patients with a follow-up of 14–30 years. Cancer 88:804–813
Slamon DJ, Clark GM, Wong SG et al (1987) Human breast cancer. Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182
Sorlie T, Perou CM, Tibshirani R et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874
Van de Vijver MJ, Peterse JL, Mooi WJ et al (1988) Neu-protein overexpression in breast cancer. Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med 319:1239–1245
van’t Veer LJ, Dai H, van de Vijver MJ et al (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature (Lond) 415:530–536
Wright C, Nicholson S, Angus B et al (1992) Relationship between c-erB-2 protein product expression and response to endocrine therapy in advanced breast cancer. Br J Cancer 65:118–121
Yang W, Klos Ks, Zhou X et al (2003) ErbB2 overexpression in human breast carcinoma is correlated with p21Cip1 up-regulation and tyrosine-15 hyperphosphorilation of p34Cdc2: poor responsiveness to chemotherapy with cyclophosphamide, methotrexate, and 5-fluorouracil is associated with ErbB2 overexpression and with p21Cip1 overexpression. Cancer 98:1123–1130
Author information
Authors and Affiliations
Corresponding author
Additional information
We have not received any financial support for the development of this study. The authors thank Edurne Arriola for the assistance in the correction of the paper.
Rights and permissions
About this article
Cite this article
Falo, C., Moreno, A., Varela, M. et al. HER-2/neu status and response to CMF: retrospective study in a series of operable breast cancer treated with primary CMF chemotherapy. J Cancer Res Clin Oncol 133, 423–429 (2007). https://doi.org/10.1007/s00432-006-0176-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-006-0176-7