Abstract
Objectives
To evaluate the usefulness of tumor-marker measurements and to identify prognostic factors in patients with cancer of unknown primary (CUP), receiving platinum-based combination chemotherapy and to verify the adjustment of previously reported prognostic models in this population.
Methods
We conducted univariate and multivariate analyses in consecutive patients with CUP receiving platinum-based combination chemotherapy. Previously reported prognostic models were then validated in this population.
Results
A total of 93 patients were analyzed and the response rate to platinum-based chemotherapeutic regimens among the 93 patients was 39.8%. The median time to progression and overall survival period were 4.1 and 12.4 months, respectively. The ST-439 level was significantly higher in patients with histologically confirmed adenocarcinoma than in patients with poorly differentiated adenocarcinoma or poorly differentiated carcinoma. A multivariate analysis indicated that performance status, the number of involved organs, and the serum lactate dehydrogenase level were the prognostic factors of the outcome. Both the previously reported prognostic models for predicting the duration of survival in this population were shown to be valid.
Conclusion
Tumor-marker measurements are not helpful in the management of patients with CUP. Previously reported prognostic models may be useful for selecting indication for chemotherapy or for stratifying the patients in clinical trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer of unknown primary (CUP) represents a group of heterogeneous malignancies and is defined by the presence of a metastatic disease without an identifiable primary tumor site on presentation. CUP accounts for approximately 2–3% of all newly diagnosed patients with solid malignancies. Approximately half of these patients will be diagnosed as having adenocarcinoma, 30% as having poorly differentiated adenocarcinoma or carcinoma, 15% as having squamous cell carcinoma, and the remaining 5% as having undifferentiated neoplasms (Greco and Hainsworth 2005).
Serum tumor markers for human chorionic gonadotropin β subunit (β-HCG), α-fetoprotein (AFP), and prostate-specific antigen (PSA) are useful for identifying treatable germ cell tumors or metastatic prostate cancer. In female patients, carbohydrate antigen 125 (CA125) can be of some help in diagnosing peritoneal carcinoma, which is usually treated as ovarian cancer (Greco and Hainsworth 2005; Varadhachary et al. 2004). A few studies have reported that common serum tumor markers like carcinoembryonic antigen (CEA), carbohydrate antigen 15-3 (CA15-3), and carbohydrate antigen 19-9 (CA19-9) were not generally useful in diagnostic or prognostic tests (Greco and Hainsworth 2005; Varadhachary et al. 2004; Pavlidis et al. 2003). However, routine measurements of various tumor markers in CUP and the role of tumor-marker measurements, besides indicating favorable subsets, have not been previously studied.
The prognosis of CUP is generally poor, with a median survival period of approximately 6–12 months. Some favorable subsets of patients with either clinical or pathologic features require specific treatment approaches and have the potential for prolonged survival (Greco and Hainsworth 2005; Pavlidis et al. 2003). However, most patients fit into an unfavorable subset that does not benefit from specific treatments and that potentially includes patients with broadly heterogeneous malignancies. In the 1980s, the use of platinum agents was shown to produce better responses and prolonged survival. With the introduction of new anti-neoplastic agents (i.e., paclitaxel, docetaxel, gemcitabine, and irinotecan), platinum-based combination chemotherapy provided treatment options for a large group of patients. However, since all of the studies were performed in non-randomized control settings, the benefits of the current therapy remains limited (Greco and Hainsworth 2005; Pavlidis et al. 2003). Many investigators have called for designed randomized trials in CUP patients belonging to the unfavorable subset, thereby generating the need for methods to select indication for chemotherapy or stratifying randomized trials, appropriately (van der Gaast et al. 1995; Culine et al. 2002).
Identifying prognostic models for patients with CUP is a challenge because of the vast heterogeneous nature of CUP. Previous studies used multivariate Cox regression analysis to identify prognostic factors for estimating the survival. Many prognostic factors were identified, including age, performance status, smoking history, number of metastatic sites, the presence of liver metastasis, and elevated serum alkaline phosphatase (ALP), or lactate dehydrogenase (LDH) levels (van der Gaast et al. 1995; Culine et al. 2002; Hainsworth et al. 1992; Abbruzzese et al. 1995; van de Wouw et al. 2004). Two studies described simple prognostic models for predicting survival. The previous study presented, but did not validate, a prognostic model based on the performance status and serum-ALP level (van der Gaast et al. 1995). Another study reported an externally validated prognostic model based on the performance status and serum-LDH level (Culine et al. 2002). However, these models may not be widely accepted in practical settings or clinical trials because both models were based on data from European populations and because CUP includes patients with heterogeneous cancers. Thus, these models may not be applicable to other populations or institutions. Consequently, these prognostic models should be verified in different populations; such an effort might contribute to advances in treatment strategies for CUP.
The aims of this study were as follows: (1) to evaluate the usefulness of various tumor-marker measurements for primary unknown cancer patients receiving platinum-based combination chemotherapy, (2) to identify predictive factors for response to chemotherapy and prognostic factors in this population, and (3) to verify the adjustment of previously reported simple prognostic models.
Patients and methods
This study retrospectively analyzed a total of 93 consecutive patients with CUP, who were treated with platinum-based combination chemotherapy between November 1997 and December 2005 at the National Cancer Center Hospital, Tokyo. All patients were diagnosed as having CUP if no primary tumor site could be identified after a thorough history and physical examination, complete blood cell counts and blood chemistry using routine tumor-marker measurements, chest radiography, a computed tomography scan between the neck and pelvis, upper gastrointestinal endoscopy, lower gastrointestinal endoscopy or barium enema imaging, urologist examination (male patients), mammography and gynecologist examination (female patients), and radiologic work-up for any symptomatic areas. All the pathological specimens were carefully evaluated by two or three pathologists to confirm the epithelial origin of the disease and to exclude other malignancies and specific tumor sites. All patients were examined for the presence of routine tumor markers, including AFP, β-HCG, protein induced by vitamin K absence-2, CEA, sialyl-specific embryonic antigen, cytokeratin 19 fragment (Cyfra), squamous-cell carcinoma antigen, CA19-9, CA15-3, sialyl Tn antigen, national cancer center-ST439 (ST-439), neuron-specific enolase (NSE), and progastrin-releasing peptide. In addition, the presence of PSA was examined in men and the presence of CA125 was examined in woman. Patients with squamous cell carcinoma or neuroendocrine carcinoma and patients with carcinomas belonging to any of the favorable subsets requiring well-defined treatments were excluded from the present study. All patients were required to provide written informed consent to review medical chart and imaging, which approved by the institutional review board at the National Cancer Center.
We used the World Health Organization criteria to assess the response to treatment of patients with measurable lesions (Miller et al. 1981). We also used Response Evaluation Criteria in Solid Tumors to evaluate the response to treatment (Therasse et al. 2000).
Time to progression was measured from the first day of treatment with platinum-based combination chemotherapy until disease progression, and the overall survival time was measured from the first day of treatment until death. Event-free cases at the final day of the follow-up period were censored in time to event analyses. The median time to progression and the median overall survival period were estimated using the Kaplan–Meier method, and differences between survival curves were assessed using a log-rank test. Observed differences in proportion were tested using the Fisher exact test. A multivariate logistic regression analysis was performed to determine the predictive factors for response to chemotherapy. A Cox regression analysis using a stepwise procedure was used to evaluate prognostic factors that were significantly related to survival in the univariate analysis performed in this study as well as the previously reported factors, including age, performance status, smoking history, number of metastatic sites, the presence of liver metastasis, and elevated serum-ALP and -LDH levels (van der Gaast et al. 1995; Culine et al. 2002; Hainsworth et al. 1992; Abbruzzese et al. 1995; van de Wouw et al. 2004). Statistical analysis was performed using SPSS 12.0 J (SPSS Inc., Chicago, IL, USA), the significance level for the results was set at 0.05 (two-sided) and the multiplicity of the statistical test was not corrected.
Results
Patient characteristics
A total of 93 patients including 48 men, were included in the analysis. The median age was 60 years (range, 28–76 years), and the median performance status was 1 (range, 0–3). The histologic types consisted of 48 patients with adenocarcinoma, 21 patients with poorly differentiated adenocarcinoma, and 27 patients with poorly differentiated carcinoma. The median number of involved organs was 1 (range, 1–5). Most patients (78%) had lymph-node metastasis, but liver metastasis (n=15), lung metastasis (n=16), bone metastasis (n=18), and brain metastasis (n=4) were also seen. Almost all the patients (91 of 93 patients) exhibited an elevated serum tumor marker. The median number of elevated serum tumor markers was 5 (range, 0–11); the serum tumor marker characteristics are listed in Table 1. Elevations in Cyfra and ST-439 were significantly associated with histologically confirmed adenocarcinoma in a univariate analysis (P=0.04 and P=0.005, respectively), and an elevation in ST-439 was associated with histologically confirmed adenocarcinoma in a multivariate analysis (P=0.006).
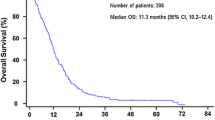
A total of 340 courses of platinum-based combination chemotherapy were administered and the median number of administered courses was 4 (range, 1–6). Approximately two-thirds of the patients in this study received a taxanes plus platinum regimen (37 patients received paclitaxel plus carboplatin, 36 patients received docetaxel plus cisplatin) and the remaining 20 patients received irinotecan plus carboplatin. The response rate of the 93 patients was 39.8% (95% confidence interval, 29.9–49.7%). No treatment-related deaths occurred in this study. The median time to progression and overall survival period were 4.1 and 12.4 months, respectively (Fig. 1). At the time of the analysis, 64 of the 93 patients had died.
Prediction of response to treatment and prognostic models
Table 2 shows the relationship between patient characteristics, including the presence of elevated tumor markers, and response to platinum-based combination chemotherapy. No significant predictive factors of response to chemotherapy were seen in the univariate and multivariate analyses. The results of the univariate analysis for prognostic factors are listed in Table 3. Poor performance status (>1), number of involved organs (>2), and elevated serum-LDH and -NSE levels were significantly associated with survival in this univariate analysis. The multivariate analysis indicated that performance status, number of involved organs, and elevated serum-LDH levels were the prognostic factors (P=0.01, P=0.033, P=0.006, respectively).
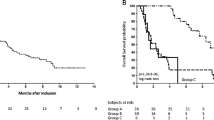
All 93 patients with a complete data set were analyzed to verify the previously reported prognostic models. The prognostic model by Culine et al. significantly divided these patients into two groups with median survival times of 21.0 and 10.1 months, respectively (P=0.003, Fig. 2). The other prognostic model by Van der Gaast et al. significantly divided these patients into three groups with median survival times of 19.6, 12.2, and 6.7 months, respectively (Fig. 3).
Overall survival according to a previously reported prognostic model using performance status and serum LDH level (Culine et al. 2002). The solid line indicates good risk patients (performance status of 0 or 1 and a normal LDH level), and the dotted line represents poor-risk patients (performance status >1 or elevated LDH level). Vertical bars indicate censored cases
Overall survival according to a previously reported prognostic model using performance status and serum-ALP level (van der Gaast et al. 1995). The solid line indicates patients with a performance status of 0 and an ALP level <1.25 N, the broken line indicates patients with a performance status ≥1, or an ALP level ≥1.25 N, and the dotted line represents patients with a performance status ≥1 and an ALP level ≥1.25 N. Vertical bars indicate censored cases
Discussion
Based on the results of the present study, various routine tumor-marker measurements were not useful for predicting either the response to chemotherapy or survival in patients with CUP. Poor performance status, the number of involved organs, and an elevated serum-LDH level were the prognostic factors for survival in patients receiving platinum-based combination chemotherapy, including taxanes or irinotecan. In addition, the previously reported prognostic models were validated in this population. To our knowledge, this is the first report to verify prognostic models for patients with CUP.
Serum tumor markers are substances that can be measured quantitatively using laboratory methods and that can be used to detect cancer and possibly the organ where it resides, as well as being useful for monitoring responses to therapy. Several tumor markers like PSA, CA125, AFP, and β-HCG have been useful for screening for cancer, monitoring treatment, and detecting recurrence. Although a positive correlation exists between tumor mass and the marker level, most tumor markers are elevated in various types of cancer and sometimes even in benign conditions. At present, the role of serum tumor markers in the management of various cancers might be limited (Canil and Tannock 2002).
A few studies have examined tumor markers in patients with CUP, but their implications for the management of CUP are controversial. Koch and McPherson suggested that a CEA above 10 ng/ml indicated that the tumor site was more likely to be an endodermally derived organ, like a breast or ovary, containing a mucinous carcinoma (Koch and McPherson 1981). However, a previous study reported that CEA, CA19-9, CA15-3, and CA125 levels were not correlated with histologic type, the number of involved organs, or the disease site but that an elevated CEA level was a significant prognostic factor for survival (Milovic et al. 2002). Yet another study reported that elevated CA19-9 levels were related to histologic adenocarcinoma and the presence of liver metastasis (Pavlidis et al. 1994). However, CEA, CA19-9, CA15-3, CA125, β-HCG, and AFP were not reported as predictive factors for response to chemotherapy or survival in two other reports (Pavlidis et al. 1994; Currow et al. 1996). In our study, almost all of the patients exhibited several elevated serum tumor markers, suggesting that patients with CUP exhibit a non-specific over-expression of serum tumor marker. Based on an analysis of our study and a previous one (Pavlidis et al. 1994), we concluded that the routine measurement of tumor markers does not offer any diagnostic or therapeutic assistance to patients with CUP, except for identifying some specific cancers such as germ-cell tumors, prostate cancer, and peritoneal carcinoma.
We retrospectively analyzed 93 consecutive patients with CUP, who had been treated with platinum-based combination chemotherapy. In this study, the response rate and the median survival period were 39.8% and 12.4 months, respectively; these results are similar to those of the previous reports on taxanes-plus-platinum-based combination chemotherapy (Pavlidis et al. 2003; Greco et al. 2000; Greco and Hainsworth 2005; Briasoulis et al. 2000). Since all the patients in this study received new-generation anticancer drugs plus platinum agents, the median survival time was longer than those of the previous studies reporting prognostic models (van der Gaast et al. 1995; Culine et al. 2002). In a previous prognostic report conducted in France, 10% of the patients only received the best supportive care and most of the patients (41%) received doxorubicin-plus-etoposide-plus-cyclophosphamide-plus-platinum agent therapy (van der Gaast et al. 1995). Patients in another study were treated with either bleomycin-plus-etoposide-plus-cisplatin therapy or etoposide-plus-cisplatin therapy (Culine et al. 2002). Although there are many differences in the patient characteristics, chemotherapy regimens, and treatment results among these previous reports and ours, it is noteworthy that the prognostic factors for survival are similar (van der Gaast et al. 1995; Culine et al. 2002; van de Wouw et al. 2004). In addition, the two previously reported prognostic models fitted our results for Japanese patients with CUP quite well. Though the investigators published a regression tree analysis in 1,000 consecutive patients in a US population (Hess et al. 1999), that kind of analysis requires a much larger data set and was not feasible in this study. Even using a simple prognostic assessment, however, the verification of prognostic models using an independent data set may be useful for establishing therapeutic strategies for patients with CUP.
Concerning the indications for chemotherapy, previous studies have suggested that patients in the poor-risk group should not be offered chemotherapy routinely, since the median survival time of poor-risk patients is less than 4 months (van der Gaast et al. 1995; Culine et al. 2002). However, in our study, the median survival times of the poor-risk patients according to the prognostic models were 10.1 and 6.7 months, respectively. Whether, poor-risk patients should be offered palliative therapy or chemotherapy may be difficult to determine without conducting a randomized trial. Since survival times have been prolonged by advances in new anticancer drugs, the utility of platinum-based combination chemotherapy or single-agent chemotherapy for poor-risk CUP patients should be carefully considered in a prospective trial.
References
Abbruzzese JL, Abbruzzese MC, Hess KR, Raber MN (1995) Analysis of a diagnostic strategy for patients with suspected tumors of unknown origin. J Clin Oncol 13:2094–2103
Briasoulis E, Kalofonos H, Bafaloukos D, Samantas E, Fountzilas G, Xiros N, Skarlos D, Christodoulou C, Kosmidis P, Pavlidis N (2000) Carboplatin plus paclitaxel in unknown primary carcinoma: a phase II Hellenic Cooperative Oncology Group Study. J Clin Oncol 18:3101–3107
Canil CM, Tannock IF (2002) Doctors dilemma: incorporating tumor markers into clinical decision making. Semin Oncol 29:286–293
Culine S, Kramar A, Saghatchion M, Bugat R, Lesimple T, Latholary A, Merrouche Y, Laplanche A, Fisasi K (2002) Development and validation of a prognostic model to predict the length of survival in patients with carcinomas of an unknown primary site. J Clin Oncol 20:4679–4683
Currow DC, Findlay M, Cox K, Harnette PR (1996) Elevated germ cell markers in carcinoma of uncertain primary site do not predict response to platinum based chemotherapy. Eur J Cancer 32A:2357–2359
van der Gaast A, Verweij J, Planting AST, Hop WCJ, Stater G (1995) Simple prognostic model to predict survival in patients with undifferentiated carcinoma of unknown primary site. J Clin Oncol 13:1720–1725
Greco FA, Hainsworth JD (2005) Cancer of unknown primary site. In: Devita VT (ed) Cancer, principles and practice of oncology. 7th edn. Lippincott, Williams&Wilkins, Philadelphia, pp 2213–2236
Greco FA, Burris HA 3rd, Erland JB, Gray JR, Kalman LA, Schreeder MT, Hainsworth JD (2000) Carcinoma of unknown primary site. Cancer 89:2655–2660
Hainsworth JD, Johnson DH, Greco FA (1992) Cisplatin-based combination chemotherapy in the treatment of poorly differentiated carcinoma and poorly differentiated adenocarcinoma of unknown primary site: result of a 12-year experience. J Clin Oncol 10:912–922
Hess KR, Abbruzzese MC, Lenzi R, Raber MN, Abbruzzese JL (1999) Classification and regression tree analysis of 1000 consecutive patients with unknown primary carcinoma. Clin Cancer Res 5:3403–3410
Koch M, McPherson TA (1981) Carcinoembryonic antigen levels as an indicator of the primary site in metastatic disease of unknown origin. Cancer 48:1242–1244
Miller AB, Hoogstraten B, Staquet M (1981) Reporting results of cancer treatment. Cancer 147:207–214
Milovic M, Popov I, Jelic S (2002) Tumor markers in metastatic disease from cancer of unknown primary origin. Med Sci Monit 8:MT25–MT30
Pavlidis N, Kalef-Ezra J, Briassoulis E, Skarlos D, Kosmidis P, Saferiadis K, Bairaktari E, Bafaloukos D, Maravegias A, Theoharis D, Fountzilas G (1994) Evaluation of six tumor markers in patients with carcinoma of unknown primary. Med Pediatr Oncol 22:162–167
Pavlidis N, Briasoulis E, Hainsworth J, Greco FA (2003) Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer 39:1990–2005
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Varadhachary GR, Abbruzzese JL, Lenzi R (2004) Diagnostic strategies for unknown primary cancer. Cancer 100:1776–1785
van de Wouw AJ, Jansen RLH, Griffioen AW, Hillen HFP (2004) Clinical and immunohistochemical analysis of patients with unknown primary tumor. A search for prognostic factors in UPT. Anticancer Res 24:297–302
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yonemori, K., Ando, M., Shibata, T. et al. Tumor-marker analysis and verification of prognostic models in patients with cancer of unknown primary, receiving platinum-based combination chemotherapy. J Cancer Res Clin Oncol 132, 635–642 (2006). https://doi.org/10.1007/s00432-006-0110-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-006-0110-z