Abstract
Background
We aimed to construct a prognostic model to predict survival in patients with advanced pancreatic cancer (APC) receiving palliative chemotherapy using readily available pretreatment factors.
Methods
The model was constructed using data from 306 consecutive patients with APC who received palliative chemotherapy between January 2006 and March 2013. The predictive accuracy of the model was assessed using a concordance index (c-index) and calibration curves.
Results
Among the 12 potential prognostic factors investigated, multivariate analysis identified the following six independent negative prognostic factors—performance status (PS), the presence of distant metastatic disease, the status of initially unresectable disease, carcinoembryonic antigen (CEA) level, carbohydrate antigen 19-9 (CA19-9) level, and neutrophil–lymphocyte ratio (NLR). A prognostic index (PI) based on the coefficients of these factors was constructed as follows—PI = 2 (if PS 2–3) + 1 (if distant metastatic disease) + 1 (if initially unresectable disease) + 1 (if CEA level ≥5.0 ng/ml) + 1 (if CA 19-9 level ≥1,000 U/ml) + 2 (if NLR ≥5). The patients were classified into three prognostic groups—favorable (PI 0–1, n = 73), intermediate (PI 2–3, n = 145), and poor (PI 4–8, n = 88). The median overall survival times for each prognostic group were 16.5, 12.3, and 6.2 months, respectively (P < 0.001). Bootstrapping verified the good fitness of this model for predicting 1-year survival, and the c-index was 0.658.
Conclusions
This simple prognostic model could help clinicians to estimate survival in patients with APC who receive palliative chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer, particularly pancreatic ductal adenocarcinoma, is among the most intractable types of cancer and the 5-year survival rate rarely exceeds 5 % [1]. Despite significant recent improvements in diagnostic techniques and therapeutic modalities, the prognosis of patients with pancreatic cancer remains poor because the majority of patients present with advanced disease or experience recurrence despite curative surgery. Palliative chemotherapy has been shown to prolong survival in patients with advanced pancreatic cancer (APC), but their prognosis is generally very poor. Therefore, it is important to identify prognostic factors and predict patient survival to help clinicians implement better therapeutic strategies.
Several clinical and laboratory factors have been identified as independent prognostic factors in patients with APC [2–8]. Recently, we reported that an increased neutrophil–lymphocyte ratio (NLR) and the status of initially unresectable disease are associated with a poor prognosis in patients with APC [9, 10]. NLR is an easily obtained clinical biomarker and numerous studies have shown that an increased NLR is strongly associated with poor cancer-specific survival in various cancers [11–16]. Thus, it is necessary to integrate several prognostic factors for each patient to more accurately predict survival, and prognostic models for predicting survival have been proposed for various cancers [17–20]. However, few studies have reported clinically available prognostic models for predicting survival in patients with APC receiving palliative chemotherapy.
The aim of this study was to construct a prognostic model to predict survival in patients with APC receiving palliative chemotherapy using readily available clinical and laboratory factors that are assessed during routine clinical management.
Patients and methods
Patients
We evaluated data from 306 consecutive patients who received first-line palliative chemotherapy for unresectable or recurrent pancreatic cancer at Kyoto University Hospital or Kitano Hospital between January 2006 and March 2013. All patients were histopathologically or cytologically diagnosed with pancreatic ductal adenocarcinoma and underwent at least one chemotherapy cycle. Chemotherapeutic regimens included gemcitabine monotherapy [21], S-1 monotherapy [22], gemcitabine plus S-1 combination therapy [23], and gemcitabine plus erlotinib combination therapy [24]. The standard doses and regimen schedules were adjusted at the discretion of the treating physicians according to the incidence of adverse events or the general condition of the individual patient. Patient characteristics evaluated for prognostic significance were age; sex; Eastern Cooperative Oncology Group (ECOG) performance status (PS) score [25]; presence of distant metastatic disease; status of recurrent or initially unresectable disease; the levels of lactate dehydrogenase (LDH), albumin, C-reactive protein (CRP), carcinoembryonic antigen (CEA), and carbohydrate antigen 19-9 (CA 19-9); NLR; and platelet–lymphocyte ratio (PLR). LDH, albumin, CRP, NLR, and PLR were obtained 0–7 days before the initiation of chemotherapy. CEA and CA 19-9 were assessed by the most recent values obtained before chemotherapy initiation. NLR and PLR were derived by respectively dividing either neutrophil or platelet counts with the lymphocyte count. These cut-off values were decided according to published studies [9, 10, 26–28]. This study was approved by the institutional review boards of both hospitals.
Statistical analysis
Overall survival was defined as the period from the date of chemotherapy initiation to the date of death from any cause or censored on the last follow-up visit. In patients with recurrent disease, the date of chemotherapy initiation after the relapse of cancer was chosen. Data from patients who were alive at the end of the follow-up period (March 2014) were regarded as censored. Overall survival and 1- and 2-year survival rates were analyzed using Kaplan–Meier curves, and P values were calculated using the log-rank tests. A P value <0.05 was considered statistically significant. The Cox proportional hazards model was used to estimate the hazard ratio (HR). The regression coefficient (β) for each independent prognostic factor was derived from the Cox regression equation (HR = e β) and rounded to score as described elsewhere [29].
The model performance was assessed with respect to calibration and discrimination [30]. Calibration was examined using calibration curves of the relationship between the observed 1-year survival rate and the predicted probabilities for each group. We used bootstrapping with 200 repetitions to obtain relatively unbiased estimates. Discrimination was evaluated by the concordance index (c-index) [31]. Statistical analyses were performed with StatView version 5.0 (SAS institute Inc., Cary, NC, USA) and R version 2.15.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Of the 306 patients, 249 (81.4 %) died and the median follow-up time was 10.8 months (range 1.7–72.1). The clinical characteristics of the patients are shown in Table 1. The median age was 67 years (range 31–86). Gemcitabine monotherapy was most commonly used as the first-line regimen (n = 180; 58.8 %). Other regimens included gemcitabine plus S-1 combination therapy (n = 96; 31.4 %), S-1 monotherapy (n = 28; 9.1 %), and gemcitabine plus erlotinib combination therapy (n = 2; 0.7 %).
Identification of prognostic factors by multivariate analysis
The results of univariate and multivariate analyses are summarized in Table 2. Of the 12 factors tested in the univariate analysis, the following 10 factors were identified as significant (at P < 0.05)—ECOG PS score (0–1 or 2–3), the presence of distant metastatic disease (non-distant metastatic or distant metastatic disease), the status of recurrent or initially unresectable disease (recurrent or initially unresectable disease), LDH level (<250 or ≥250 U/l), albumin level (<3.5 or ≥3.5 g/dl), CRP level (<0.5 or ≥0.5 mg/dl), CEA level (<5.0 or ≥5.0 ng/ml), CA 19-9 level (<1,000 or ≥1,000 U/ml), NLR (<5 or ≥5), and PLR (<150 or ≥150). After multivariate analysis, the following six factors remained as independent negative prognostic factors—ECOG PS score (2–3), the presence of distant metastatic disease (distant metastatic disease), the status of recurrent or initially unresectable disease (initially unresectable disease), CEA level (≥5.0 ng/ml), CA 19-9 level (≥1,000 U/ml), and NLR (≥5).
Establishment of the prognostic model
The prognostic model was based on the regression coefficients of the six significant independent negative prognostic factors, and the significant prognostic factors were assigned a prognostic index (PI) score (Table 3). If a factor was not independently significant, a score of 0 was assigned. The value of each significant prognostic factor was summed to obtain a PI as follows—PI = 2 (if PS 2–3) + 1 (if distant metastatic disease) + 1 (if initially unresectable disease) + 1 (if CEA level ≥5.0 ng/ml) + 1 (if CA 19-9 level ≥1,000 U/ml) + 2 (if NLR ≥5). PI was distributed between 0 and 8 (0, n = 13; 1, n = 60; 2, n = 78; 3, n = 67; 4, n = 63; 5, n = 11; 6, n = 12; 7, n = 1; 8, n = 1). Patients were categorized into the following three prognostic groups—favorable (PI 0–1, n = 73), intermediate (PI 2–3, n = 145), and poor (PI 4–8, n = 88).
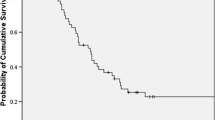
The median overall survival time of the 306 patients was 11.3 months (95 % confidence interval [CI], 10.2–12.4) and the 1-year survival rate was 45.9 % (95 % CI 40.3–51.5) (Fig. 1; Table 4). On the other hand, the median overall survival times for the favorable, intermediate, and poor prognosis groups were 16.5 (95 % CI 13.3–18.7), 12.3 (95 % CI 10.9–13.7), and 6.2 (95 % CI 4.4–8.0) months, respectively, whereas the 1-year survival rates were 67.3 % (95 % CI 56.3–78.3), 51.3 % (95 % CI 42.9–59.7), and 19.1 % (95 % CI 10.5–27.7), respectively (P < 0.001; Fig. 2; Table 4). In addition, the 2-year survival rates were 38.9 % (95 % CI 26.9–50.9), 14.2 % (95 % CI 7.7–20.7), and 3.1 % (95 % CI 0–7.2), respectively (Table 4). If the favorable prognosis group was set as reference, the HRs of the intermediate and poor prognosis group were 2.06 (95 % CI 1.46–2.91) and 3.95 (95 % CI 2.69–5.81), respectively.
Validation of the prognostic model
This model was well calibrated to predict 1-year survival, where overestimation (2.4 % and 0.2 % in the favorable and poor prognosis groups, respectively) and underestimation (3.6 % in the intermediate prognosis group) were observed (Fig. 3). The c-index of the model was 0.658.
Discussion
In the present study, we developed and validated a novel prognostic model to predict the survival of patients with APC receiving palliative chemotherapy. Based on six readily available prognostic factors, the patients were well classified into favorable, intermediate, and poor prognostic groups, where they had significantly different median overall survival times of 16.5, 12.3, and 6.2 months, respectively.
Systemic palliative chemotherapy is the standard treatment for patients with APC. However, despite recent advances in this field [21–24, 32–35], the prognosis of patients with APC remains poor. Several clinical and laboratory factors are known to be associated with the prognosis of patients with APC [2–10]. A single prognostic factor may not be sufficient to predict survival; thus, it is reasonable to integrate several factors and to develop a prognostic model using statistical methods. However, there are few clinically available prognostic models for predicting survival in patients with APC receiving palliative chemotherapy.
Therefore, we constructed a simple prognostic model using readily available factors in daily clinical practice. First, we explored the clinical and laboratory factors that could predict the survival of patients with APC and identified the following six independent prognostic factors that are routinely assessed in daily clinical practice—ECOG PS score, the presence of distant metastatic disease, the status of recurrent or initially unresectable disease, CEA level, CA 19-9 level, and NLR. In agreement with previous studies [2–7], we confirmed that the ECOG PS score, the presence of distant metastatic disease, CA 19-9 level, and NLR have prognostic significance in patients with APC. In particular, NLR is an easily obtained clinical biomarker and increasing evidence demonstrates that NLR is a promising prognostic marker in various cancers including pancreatic cancer [11–16, 36–38]. The HR for NLR observed in this study was comparable with the data from other groups, suggesting that our study cohort was representative of patients with APC who receive palliative chemotherapy in daily clinical practice.
Simplicity is the strength of our model. All the prognostic factors used in this model are routinely collected in daily pretreatment assessment, making it feasible for clinicians to estimate patient survival with this approach. The calibration curves demonstrated that the probabilities predicted from this model were within approximately 5 % of the actual probability. Moreover, the c-index of 0.658 suggests that this model has a clinically relevant ability to discriminate the prognosis of patients with APC receiving palliative chemotherapy. To make this model much simpler, we derived a modified PI by excluding ECOG PS score and allocating the flat coefficients as follows—PI = 1 (if distant metastatic disease) + 1 (if initially unresectable disease) + 1 (if CEA level ≥5.0 ng/ml) + 1 (if CA 19-9 level ≥1,000 U/ml) + 1 (if NLR ≥5). Using the PI, the patients were classified into three prognostic groups—favorable (PI 0–1; n = 75), intermediate (PI 2–3; n = 169) and poor (PI 4–5; n = 62). The median overall survival times for favorable, intermediate and poor prognosis groups were 16.2 (95 % CI 12.9–19.6), 11.7 (95 % CI 10.0–13.4), and 6.0 (95 % CI 4.9–7.1) months, respectively, whereas the 1-year survival rates were 65.4 % (95 % CI 54.4–76.4), 47.8 % (95 % CI 40.0–55.6), and 17.0 % (95 % CI 7.2–26.8), respectively (P < 0.001). Furthermore, this model was well calibrated to predict 1-year survival, in which overestimation (3.4 % in the favorable prognosis group) and underestimation (1.6 % in the poor prognosis group and 2.0 % in the intermediate prognosis groups) were observed. The c index of this modified model (0.641) was slightly lower than that of original PI model (0.658); however, considering the advantage of the simplicity, this modified model might be also helpful in daily clinical practice.
In our cohort, all the patients received gemcitabine or S-1-based regimen as first-line chemotherapy. Gemcitabine has been recognized as a key drug for patients with APC over the past few decades [21]. Recently, three randomized Phase III studies have demonstrated the survival benefit of gemcitabine plus erlotinib combination therapy, fluorouracil/leucovorin plus irinotecan plus oxaliplatin (FOLFIRINOX), and nab-paclitaxel plus gemcitabine combination therapy compared with gemcitabine monotherapy, and these regimens are now recognized as the standard first-line chemotherapeutic regimens for APC [24, 33, 35]. On the other hand, fluoropyrimidine-based chemotherapy, particularly S-1, has been a therapeutic alternative for patients with APC in Japan [22, 23, 32]. S-1 is an oral fluoropyrimidine that comprises tegafur, 5-chloro-2, 4-dihydroxypyridine, and potassium oxonate [39]. A recent randomized Phase III study reported that the survival benefit of S-1 monotherapy is comparable to that of gemcitabine monotherapy for patients with APC, although the superiority of gemcitabine plus S-1 combination therapy compared with gemcitabine monotherapy was not demonstrated [34]. Thus, all the patients in our cohort received gemcitabine or S-1-based regimen as the first-line chemotherapy. Because most of the patients (99.3 %) in our cohort received gemcitabine monotherapy, S-1 monotherapy, or gemcitabine plus S-1 combination therapy, it is unlikely that differences in the chemotherapeutic regimen affected our results.
Hamada et al. recently reported a nomogram for predicting survival in patients with unresectable pancreatic cancer receiving gemcitabine-based chemotherapy [7]. In their study, the following six factors were used to estimate the survival—age, sex, ECOG PS score, tumor size, regional lymph node metastasis, and distant metastasis. Using these six factors, a nomogram was designed to predict the survival probabilities at 6, 12, and 18 months as well as the median survival time. The c-index of our model was 0.658 compared with 0.686 using their nomogram; however, we believe that our model is easier to use in daily clinical practice and has an encouraging level of predictive accuracy.
This study had some limitations. First, this model was derived from retrospective data, making it susceptible to a data collection bias. Second, the portability of this model to other cohorts needs to be externally validated.
In conclusion, we developed and validated a simple prognostic model based on six readily available pretreatment factors for patients with APC receiving palliative chemotherapy. This prognostic model can help clinicians to predict the survival of patients with APC receiving palliative chemotherapy, thereby facilitating the adoption of better therapeutic strategies in daily clinical practice.
References
Hidalgo M (2010) Pancreatic cancer. N Engl J Med 362(17):1605–1617
Ueno H, Okada S, Okusaka T et al (2000) Prognostic factors in patients with metastatic pancreatic adenocarcinoma receiving systemic chemotherapy. Oncology 59(4):296–301
Morizane C, Okusaka T, Morita S et al (2011) Construction and validation of a prognostic index for patients with metastatic pancreatic adenocarcinoma. Pancreas 40(3):415–421
Humphris JL, Chang DK, Johns AL et al (2012) The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol 23(7):1713–1722
Bauer TM, El-Rayes BF, Li X et al (2013) Carbohydrate antigen 19-9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: a pooled analysis of 6 prospective trials. Cancer 119(2):285–292
Stotz M, Gerger A, Eisner F et al (2013) Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 109(2):416–421
Hamada T, Nakai Y, Yasunaga H et al (2014) Prognostic nomogram for nonresectable pancreatic cancer treated with gemcitabine-based chemotherapy. Br J Cancer 110(8):1943–1949
Szkandera J, Stotz M, Absenger G et al (2014) Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br J Cancer 110(1):183–188
Xue P, Kanai M, Mori Y et al (2014) Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med 3(2):406–415
Xue P, Kanai M, Mori Y et al (2014) Comparative outcomes between initially unresectable and recurrent cases of advanced pancreatic cancer following palliative chemotherapy. Pancreas 43(3):411–416
Proctor MJ, McMillan DC, Morrison DS et al (2012) A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer 107(4):695–699
Cho IR, Park JC, Park CH et al (2014) Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer 17(4):703–710
Li MX, Liu XM, Zhang XF et al (2014) Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer 134(10):2403–2413
McNamara MG, Templeton AJ, Maganti M et al (2014) Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur J Cancer 50(9):1581–1589
Pinato DJ, Shiner RJ, Seckl MJ et al (2014) Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer 110(8):1930–1935
Xiao WK, Chen D, Li SQ et al (2014) Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer 14:117
Teramukai S, Ochiai K, Tada H et al (2007) PIEPOC: a new prognostic index for advanced epithelial ovarian cancer–Japan Multinational Trial Organization OC01-01. J Clin Oncol 25(22):3302–3306
Park MJ, Lee J, Hong JY et al (2009) Prognostic model to predict outcomes in nonsmall cell lung cancer patients treated with gefitinib as a salvage treatment. Cancer 115(7):1518–1530
Kanagavel D, Pokataev IA, Fedyanin MY et al (2010) A prognostic model in patients treated for metastatic gastric cancer with second-line chemotherapy. Ann Oncol 21(9):1779–1785
Halabi S, Lin CY, Kelly WK et al (2014) Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol 32(7):671–677
Burris HA 3rd, Moore MJ, Andersen J et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15(6):2403–2413
Ueno H, Okusaka T, Ikeda M et al (2005) An early phase II study of S-1 in patients with metastatic pancreatic cancer. Oncology 68(2–3):171–178
Nakamura K, Yamaguchi T, Ishihara T et al (2006) Phase II trial of oral S-1 combined with gemcitabine in metastatic pancreatic cancer. Br J Cancer 94(11):1575–1579
Moore MJ, Goldstein D, Hamm J et al (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada clinical trials group. J Clin Oncol 25(15):1960–1966
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–655
Tanaka T, Ikeda M, Okusaka T et al (2008) Prognostic factors in Japanese patients with advanced pancreatic cancer treated with single-agent gemcitabine as first-line therapy. Jpn J Clin Oncol 38(11):755–761
Papadoniou N, Kosmas C, Gennatas K et al (2008) Prognostic factors in patients with locally advanced (unresectable) or metastatic pancreatic adenocarcinoma: a retrospective analysis. Anticancer Res 28(1B):543–549
Haas M, Laubender RP, Stieber P et al (2010) Prognostic relevance of CA 19-9, CEA, CRP, and LDH kinetics in patients treated with palliative second-line therapy for advanced pancreatic cancer. Tumour Biol 31(4):351–357
Steyerberg EW (2009) Clinical Prediction Models. Springer, New York
Harrell FE, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387
Harrell FE, Califf RM, Pryor DB et al (1982) Evaluating the yield of medical tests. JAMA 247:2543–2546
Okusaka T, Funakoshi A, Furuse J et al (2008) A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol 61(4):615–621
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19):1817–1825
Ueno H, Ioka T, Ikeda M et al (2013) Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 31(13):1640–1648
Von Hoff DD, Ervin T, Arena FP et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369(18):1691–1703
Luo G, Guo M, Liu Z et al (2015) Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol 22(2):670–676
Inoue D, Ozaka M, Matsuyama M et al (2015) Prognostic value of neutrophil-lymphocyte ratio and level of C-reactive protein in a large cohort of pancreatic cancer patients: a retrospective study in a single institute in Japan. Jpn J Clin Oncol 45(1):61–66
Qi Q, Geng Y, Sun M, et al (2015) Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer. Pancreatology. [Epub ahead of print]
Shirasaka T (2009) Development history and concept of an oral anticancer agent S-1 (TS-1): its clinical usefulness and future vistas. Jpn J Clin Oncol 39(1):2–15
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kou, T., Kanai, M., Yamamoto, M. et al. Prognostic model for survival based on readily available pretreatment factors in patients with advanced pancreatic cancer receiving palliative chemotherapy. Int J Clin Oncol 21, 118–125 (2016). https://doi.org/10.1007/s10147-015-0864-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0864-x