Abstract
Purpose: We demonstrated that chromosome 8p deletion is associated with metastasis of human hepatocellular carcinoma (HCC). This study assesses the value of circulating plasma DNA level and its allelic imbalance (AI) on chromosome 8p in the prediction of HCC prognosis. Methods: Blood samples were collected from 79 patients with HCC before operation, 20 patients with liver cirrhosis, and 20 healthy volunteers. The HCC and adjacent non-tumor liver tissues were obtained from surgical specimens. Plasma DNA was extracted and quantified. Two microsatellite markers on chromosome 8p, D8S258 and D8S264, were selected and used in the AI analysis. Results: The circulating plasma DNA level was found to closely associate with tumor size (P=0.008) and TNM stage (P=0.040), negatively associate with the 3-year disease-free survival (DFS) (P=0.017) and overall survival (OS) (P=0.001). AI at D8S258 in plasma DNA was significantly correlated with tumor differentiation (P=0.050), TNM stage (P=0.010), and vascular invasion (P=0.023), negatively correlated with the 3-year DFS (P=0.005) and OS (P=0.036). However, AI at D8S264 was only closely associated with 3-year DFS (P=0.014). Combined detection of AI at D8S258 and circulating plasma DNA level was independently associated with DFS (P=0.018) and OS (P=0.002) of patients with HCC. For patients with both AI at D8S258 and a higher level of plasma DNA, the 3-year DFS and 3-year OS rates were decreased remarkably (P=0.014 and 0.044). Conclusion: Combination of circulating plasma DNA level and AI at D8S258 might be an independent predictor for prognosis of HCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common and aggressive malignancies worldwide, and it has been ranked the second cancer killer in China since 1990 s. The age-standardized mortality rate in China is as high as 34.7/100,000, which accounted for 53% of all liver cancer deaths worldwide (Pisani et al. 1999). Although encouraging long-term survival of HCC patients has been obtained in some clinical centers, recurrence after surgical resection remains one of the major obstacles to further prolonging the survival of HCC patients, and the overall dismal outcome of patients with HCC has not been completely changed (Greenlee et al. 2001; Tang 2001). The extremely poor prognosis of HCC is largely due to a high rate of recurrence after surgery or intra-hepatic metastases that develop through invasion of the portal vein or spread to other parts of the liver (Yuki et al. 1990; Genda et al. 1999).
A large number of molecular biological factors have been shown to associate with the invasion and metastasis of HCC, and have potential prognostic significance (Qin and Tang 2002). Since patients with cancers, particularly with high metastatic potential, tend to have higher levels of DNA in plasma, and the circulating DNA from plasma samples of cancer patients displays neoplastic characteristics, plasma DNA may be a good target to study instead of tumor tissues DNA for its easy accessibility, simple manipulation, and prognostic information available before operation (Anker et al. 2003; Stroun et al. 1989). A few reports have showed the existence of circulating DNA in HCC patients (Wong et al. 2003; Chang et al. 2002; Niu et al. 2003). However, it is still unclear whether the circulating plasma DNA level and its genetic aberrations could be used in predicting the clinical outcome of HCC patients.
In our previous work, using comparative genomic hybridization (CGH) technique, we compared the differences of chromosomal aberrations between the primary HCC tumors and their matched metastatic lesions, and found chromosome 8p deletions might contribute to HCC metastasis (Qin et al. 1999). This result was further confirmed by comparison between nude mice models of HCC with different metastatic potentials (Qin et al. 2001). With a genome-wide microsatellite analysis of primary and the matched metastatic HCC tissue, a more accurate location was identified on D8S258 and D8S264 (Zhang et al. 2003). These findings provide new targets for exploring predictive markers for the recurrence and prognosis of HCC.
To explore a non-invasive, facile, and practical method for assessing the prognosis of HCC patients, in this study, we evaluate the values of circulating plasma DNA level and its allelic imbalance (AI) on chromosome 8p in the prognostic prediction of HCC patients.
Patients and methods
Patients and their clinicopathological characteristics
Seventy-nine patients who received surgical treatment for HCC at Liver Cancer Institute (at Zhongshan Hospital) of Fudan University during August–December 2001 were enrolled in this study. The mean age of the patients was 51 years old (range 21–83 years). All the patients had a normal liver function preoperatively. All the tumors were histopathologically diagnosed as HCC (Table 1). TNM stages of the patients were classified according to the UICC TNM classification of primary liver cancer (6th edition) (Sobin and Wittekind 2002). All the 79 patients gave informed consent and were followed up till January 2005.
Sample collection
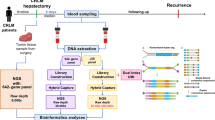
The peripheral blood samples (5 ml) were collected from the HCC patients before surgical operation, and put into an EDTA tube. Blood samples from 20 patients with compensated liver cirrhosis and 20 healthy volunteers were collected as control. The blood samples were centrifuged at 3000 g for 10 min to separate buffy coats and plasma. An additional centrifugation for 10 min was proceeded to produce cell-free plasma. The HCC and the adjacent non-tumor liver tissues were obtained from the surgical specimen immediately after surgical resection and frozen in liquid nitrogen. Before DNA extraction, a fragment of each sample of tumor tissue was fixed in formalin for histopathologic diagnosis. Tissue and blood samples were then stored at −80°C prior to DNA extraction.
Tissue microdissection and DNA extraction
To get rid of the contaminant and obtain more purified tissues, tumor and adjacent non-tumor liver tissues were microdissected from two to three 10 μm serial sections of fresh frozen blocks by laser captured microdissection according to the protocols of Leica laser microdissection system (Leica, Germany). The tissues were then treated with SDS and proteinase K followed by phenol and chloroform extraction to extract the DNA.
Plasma DNA quantification
Plasma and control leukocyte DNA were extracted with QIAamp® DNA Blood Midi Kit (Qiagen, Hilden, Germany) according to the blood and body fluid protocol of the manufacturer. Two milliliters of plasma were used, and a DNA elution volume of 300 μl was obtained after extraction. Ten microliters of plasma DNA was mixed with equivalence 1:3,000 SYBR green I (fluorescence dye). The mixture was analyzed by ultraviolet transilluminator system and photographed under stimulating wave of 345 nm and absorbing wave of 500 nm. The diagram was analyzed by software system to read its intensity (Vitzthum et al. 1999). The quantity of plasma DNA was calculated by regression equation which was made by intensity of standard DNA content.
Allelic imbalance analysis
Based on the results of previous studies, two microsatellite markers on chromosome 8p, D8S258 and D8S264, were chosen to perform the AI analysis. The forward primer for D8S258 was 5′-CTGCCAGGAATCAACTGAG-3′ labeled by HEX, the reverse primer was 5′-TTGACAGGGACCCACG-3′); the forward primer for D8S264 was 5′-ACATCTGCGTCGTCTTCATA-3′ labeled by 6-FAM, the reverse primer was 5′-CCAACACCTGAGTCAGCATA-3′. PCR amplification (DNA Engine, Tetrad, Watertown, MA, USA) was carried out in a 10 μl of final volume containing 30 ng of DNA template, 0.25 unit of Taq polymerase in PCR buffer (1.5 mM MgCl2, 50 mM KCl, 20 mM Tris–HCl, pH 8.4 (ShenYou Co. Ltd., Shanghai, China)), 200 μM of each deoxynucleotide triphosphate, and 0.2 μM of each primer. The PCR protocol began with 3 min at 96°C, followed by a 10 cycle touch-down procedure: 30 s at 94°C, 40 s at 63°C (decrease 0.5°C each cycle), 40 s at 72°C, then 20 cycles of 30 s at 94°C, 40 s at 58°C, 40 s at 72°C, followed by a final extension of 5 min. The amplified fragments were analyzed on an ABI prism 377 DNA sequencer (Applied Biosystems, Foster City, CA, USA). Molecule weight marker ROX-250 (Applied Biosystems, Foster City, CA, USA) was used in each lane. This technique allowed a sensitive and quantitative estimation of allele ratio by measuring the peak height of both alleles. The AI assay was based on the detection of an alteration of the allele ratio in tumor or plasma DNA, compared with the allele ratio in the paired normal liver or blood cell DNA. Any change of the allele ratio in tumor or plasma DNA was referred to as AI. The presence of an additional peak which referred to as microsatellite instability was not analyzed in this study. The extent of AI was calculated as a percentage: AI% = absolute value ((Bb/Ba) - (Tb/Ta)) × 100/(Bb/Ba) in which Ba and Bb represent the height of the two alleles in the liver or in the blood, and Ta and Tb in the tumor or in the plasma. A cutoff value (of the intensity of the AI) greater than 20% for each microsatellite indicated the presence of significant AI (Beau-Faller et al. 2003). This analysis was performed in a blinded way by an investigator who was not aware of the clinical data, and each result of amplification was confirmed by at least two independent PCR analyses.
Statistical analysis
Results were expressed as means with ranges. Fisher’s exact test was used to compare qualitative data. A P-value <0.05 was considered significant. Continuous variables were transformed into dichotomous variables before the survival analysis. Student’s t-test was used for mean comparisons. Area under curve (AUC) of receiver operator characteristic curve (ROC) was calculated to evaluate the diagnostic efficacy. Patients’ survival was calculated from the data of operation to the data of death or to the data of point, which was the major clinical endpoint. Survival curves were estimated according to the method of Kaplan–Meier and compared with a log-rank test. To identify the factors that might be of independent significance in influencing the disease-free survival (DFS) or overall survival (OS), a Cox stepwise proportional risk regression model was fitted. All statistical calculations were performed with Stata (version 7) statistical software.
Results
Circulating plasma DNA level and its clinical implication
Compared with the healthy volunteers (mean 17.6±9.5 ng/ml), a significant higher circulating plasma DNA level was found in the patients with HCC (mean 47.1±43.7 ng/ml, P=0.000), or liver cirrhosis (mean 30.0±13.3 ng/ml, P=0.002). However, no significant difference was found in the circulating plasma DNA levels between the patients with HCC and liver cirrhosis (P=0.191).
When we used 36.6 ng/ml (mean healthy volunteers + 2 SD) (Thijssen et al. 2002) as cutoff point to assess whether there is any potential value as diagnostic aid for HCC, the sensitivity and specificity in distinguishing the HCC patients from the healthy volunteers were 51.9 and 95%, respectively, and AUC of ROC was 0.80 with a 95% CI from 0.70 to 0.89. In distinguishing the HCC patients from the healthy volunteers and the patients with compensated liver cirrhosis, the sensitivity and specificity were 51.9 and 77.5%, respectively, and AUC of ROC was 0.70 with a 95% CI from 0.60 to 0.79.
Using 36.6 ng/ml as the cutoff point of plasma DNA level, the 79 HCC patients were divided into high plasma DNA level (HDNA) group (≥36.6 ng/ml) and low plasma DNA level (LDNA) group (<36.6 ng/ml). Significant differences were found in tumor size (P=0.008) and TNM stage (P=0.040) between the two groups of HCC patients (Table 1).
Allelic imbalance study and its clinical implication
Allelic imbalances at D8S258 and D8S264 microsatellite markers were analyzed both in circulating DNA in plasma (by comparing with the paired leukocyte DNA) and in the HCC tissue (comparing with the adjacent non-tumor liver tissue) in this cohort of 79 HCC patients. AI was observed in 84.8% (67/79) of the HCC patients in plasma and/or tumor tissues. Among the 79 HCC patients, AI at D8S258 was detected in the plasma of 49 (62.0%) patients and in the HCC tissues of 45 (57.0%) patients, while AI at D8S264 was detected in the plasma of 47 (59.5%) patients and in HCC tissues of 42 (53.2%) patients. A high coincidence was found between the AI detected in plasma DNA and corresponding tumor DNA, either at D8S258 (87.3%, 69/79, P=0.000) or at D8S264 (83.5%, 66/79, P=0.000). The specificity of this AI assay was also very satisfactory because none of the normal control samples tested was positive at any loci.
Allelic imbalance at D8S258 in plasma DNA was found to associate with tumor differentiation (P=0.050), vascular invasion (P=0.023), and TNM stage (P=0.010), while AI at D8S264 did not show any significant relationship with the clinicopathological features. Combination of AI at D8S258 and a higher level of plasma DNA added weight to the prognostic predictive value; better significant difference was found to associate with tumor size (P=0.000), vascular invasion (P=0.016), and TNM stage (P=0.002). Besides, 17 out of 30 (57%) patients with TNM stage III/IV HCC, and 11 of the 49 (22%) patients with TNM I/II HCC were found both with AI at D8S258 and a higher level of plasma DNA (Table 2).
The relationship between the level/AI of plasma DNA and prognosis of HCC patients
The 3-year DFS rates for HDNA group and LDNA group were 22 and 47%, respectively (P=0.008), while the 3-year OS rates were 24 and 61%, respectively (P=0.000). In multivariate analysis, HDNA was independently associated with a poorer DFS and OS (P=0.004 and 0.000) (Table 3).
Allelic imbalance at D8S258 in plasma DNA was closely associated with 3-year DFS (P=0.004) and 3-year OS (P=0.022), while AI at D8S264 in plasma DNA was only associated with 3-year DFS (P=0.011). In multivariate analysis, neither AI at D8S258 nor AI at D8S264 was an independent predictor for DFS or OS. However, when AI at D8S258 combined with a higher level of plasma DNA, better significant difference was found to associate with 3-year DFS (P=0.000) and 3-year OS (P=0.000) (Table 3), and this combination was an independent predictor for both DFS (P=0.018) and OS (P=0.002) in the multivariate analysis (Figs. 1, 2).
Furthermore, among patients with TNM stage I or II, the 3-year DFS and 3-year OS of patients having AI at D8S258 together with a higher level of plasma DNA were 18% (2/11) and 45% (5/11), which were much lower than that of the other patients [the 3-year DFS was 50% (19/38) (P=0.060), the 3-year OS was 63% (24/38) (P=0.293)]. Among patients with TNM stage III or IV, the 3-year DFS and 3-year OS of patients having AI at D8S258 together with a higher level of plasma DNA were even lower at 0% (0/17) and 12% (2/17) than that of the other patients [the 3-year DFS was 31% (4/13) (P=0.014), the 3-year OS was 31% (4/13) (P=0.044)].
Discussion
The first discovery of extracellular nucleic acids in the circulation was reported in 1948 by Mandel and Métais (1948), who demonstrated the presence of both DNA and RNA in the plasma of healthy and sick individuals. This work was particularly remarkable as it was reported only 4 years after the demonstration of DNA as the material of inheritance (Avery et al. 1995), and it even preceded the landmark paper by Watson and Crick (1953) on the double helical structure of DNA. However, their work did not attract much attention, and further development of the field had to wait some 30 years until Leon showed that cancer patients had much higher concentration of circulating DNA than those with non-malignant diseases (Leon et al. 1977). They also showed that, in some cases, the level of circulating DNA decreased after successful anti-cancer therapy. Since then, several follow-up studies have been done to establish the possible value of free circulating DNA as a prognostic factor in patients with pancreatic carcinoma (Castells et al. 1999), esophageal adenocarcinoma (Kawakami et al. 2000), lung cancer (Sozzi et al. 2001), melanoma (Taback et al. 2001), and nasopharyngeal carcinoma (Lin et al. 2004).
Although isolation and quantification of total plasma DNA in cancer patients are routinely feasible at present, the mechanism leading to the presence of free tumor DNA in cancer patients’ plasma remains enigmatic. It can be presumed that circulating DNA in healthy subjects derives from lymphocytes or other nucleated cells. Yet, it is not well understood why cancer patients have such large quantities of plasma DNA, nor where this genetic material derives from. The most common hypothesis advanced for circulating DNA in the plasma of cancer patients is that it is due to the lysis of circulating cancer cells or micrometastases shed by the tumor. This is clearly not the case since there are not enough circulating cells to justify the amount of DNA found in the plasma. Another hypothesis is that tumor DNA shed in the blood stream could be due either to DNA leakage resulting from tumor necrosis or apoptosis, or to a new mechanism of active release. But an argument against this hypothesis is that after radiation therapy, the plasma DNA levels decrease remarkably instead of increasing, as one might expect. As a third possibility, it may be hypothesized that the tumor actively releases DNA into the blood stream. Although the phenomena that cells from leukemia patients release more DNA than lymphocytes from healthy donors has been observed, it is still worth remembering some data on this subject (Anker et al. 1999).
Both plasma and serum samples have frequently been used in the analysis of cell-free DNA. Of particular interest is that the concentrations in serum samples have been significantly higher than those in matched plasma samples, which is mainly generated in vitro by lysis of white blood cells; this makes it not suitable for serum to monitor the concentration of cell-free DNA (Lee et al. 2001). A recent work performed a quantitative comparison of matched serum and plasma DNA in patients with colorectal liver metastases. Serum and plasma DNA level were not correlated. Furthermore, while serum DNA was significantly associated with the presence of metastases, only plasma DNA was predictive of recurrence. It was thus concluded that serum DNA might represent an indirect but tumor-related process, and that plasma DNA better reflects the in vivo levels of circulating DNA (Thijssen et al. 2002). Different blood processing protocol may also influence the level of circulating DNA (Taback et al. 2004). The plasma obtained by centrifugation alone, at various speeds, without subsequent microcentrifugation, has been shown to have substantial amounts of cellular components which may led to the detection of aberrantly high total concentrations of plasma DNA (Chiu et al. 2001). Therefore, in our study, an additional centrifuge for 10 min was performed to produce cell-free plasma. In order to exclude interference of cell death caused by hepatitis or liver resection, normal liver function tests are required for patients’ blood samples collection before operation.
Regarding the diagnostic potential of circulating plasma DNA level in HCC, unfortunately, according to our results, quantitative assessment of circulating plasma DNA is not sensitive or specific enough for diagnostic use, since levels overlap considerably between the HCC patients and those with non-tumor liver cirrhosis. However, we found that the mean circulating plasma DNA level was significantly associated with tumor size and TNM stage, which indicates that large or invasive tumor may release much circulating DNA, and higher level of plasma circulating DNA may be associated with poor prognosis. It is further confirmed by the multivariate analysis which showed that HDNA was an independent predictor for poorer DFS and OS.
Alterations of human chromosome 8p have been frequently detected in many tumor types (Qin 2002; Yokota et al. 1999; Perinchery et al. 1999). Some studies have shown that loss of 8p is associated with the advance of tumors, and plays an important role in the tumor progression of many tumors including ovarian (Wright et al. 1998), colorectal (Takanishi et al. 1997), bladder (Muscheck et al. 2000; Wagner et al. 1997), and breast cancers (Yokota et al. 1999). Recently, some studies have also shown that loss of 8p may be associated with the metastasis of laryngeal carcinoma (Kujawski et al. 1999), bladder cancer (Ohgaki et al. 1999), renal cell carcinoma (Bissig et al. 1999), colorectal carcinoma (Parada et al. 1999), lung cancer (Petersen et al. 2000), mantle cell lymphoma (Martinez-Climent et al. 2001), and the poor prognosis of colorectal cancer patients (Halling et al. 1999). Bockmuhl et al. (2001) found that 8p23 allelic loss was an independent prognostic marker for disease-free interval, and was associated with poor prognosis in head and neck squamous cell carcinoma, and could be useful in refining diagnosis of these tumors. Therefore, 8p might harbor some tumor suppressor genes that are important in the progression, especially in the metastasis of cancers, which was confirmed by irradiated MMCT technique (Nihei et al. 1996, 2002).
Chromosome 8p deletion is also one of the recurrent chromosomal aberrations in HCC, detected either by CGH or by microsatellite analysis (Guan et al. 2000; Li et al. 2001; Wang et al. 2001). In our previous study, we compared the differences in genomic alterations between matched primary and metastatic HCC by CGH, and found that the significant deletion of chromosome 8p might contribute to the development of HCC metastasis (Qin et al. 1999). This result was further confirmed by comparison between nude mice models of HCC with different metastatic potentials (Qin et al. 2001). With a genome-wide microsatellite analysis of primary and the matched metastatic HCC tissue, a more accurate location was identified on D8S258 and D8S264 (Zhang et al. 2003).
Using these two microsatellite markers, we performed the AI analysis and found 84.81% (67/79) of the HCC patients could detect AI at plasma and/or tumor tissues which suggested that these two microsatellite markers are highly informative in our cohort. Moreover, a high coincidence found between the AI detected in plasma DNA and tumor DNA either at D8S258 or at D8S264 indicated that the presence of AI in plasma was generally associated with the presence of the AI in its matched tumor; AI in the plasma DNA can represent the AI in the tumor tissues. The reason for the inconsistent AI status in some cases (i.e., AI was detected in the tumor tissues, while it was not in their corresponding plasma DNA) may be due to the low amount of circulating plasma DNA released by those cases, compared to the normal released DNA, which lead to the false negative results (Chan et al. 2003; Silva et al. 2002). On the other hand, in some other patients, the AI was detected in circulating plasma DNA but not in HCC tissue, and the possible reason may be due to the heterogeneity of tumor cells; only some of them had microsatellite polymorphisms, and the circulating DNA was mainly released by the micrometastatic tumor cells with AI but not primary tumor (Lin et al. 2002; Dahse et al. 2002; Utting et al. 2002). Another explanation is the clones with polymorphism were ruined precedently, and could be detected only in circulating DNA (Goessl 2003; Sozzi et al. 1999).
In this study, we found tumor with poor differentiation or vascular invasion may cause frequently AI at D8S258, which was closely related to TNM stage, and finally cause poor prognosis. However, according to the multivariate analysis, AI at D8S258 was not an independent predictor for DFS or OS. Although HDNA was formerly found to be an independent predictor for decreased DFS and OS of HCC patients, it is not specific for HCC. Thus, we try to determine whether or not the combination of AI at D8S258 and plasma DNA level could have more prognostic value for HCC patients. We found AI at D8S258 together with high level of plasma DNA was closely associated with both DFS and OS. This combination was also an independent predictor for DFS and OS. Since quantification and AI analysis of circulating plasma DNA in cancer patients are practical and routinely feasible at present, such plasma analysis might be used as a non-invasive follow-up tool by monitoring genetic changes in the circulating DNA of HCC patients.
Of particular interest, AI at D8S258 together with high level of plasma DNA could be detected in 8 of 24 patients with serum AFP level less than 20 μg/l, and 7 of which were finally found to have a recurrence in 3 years. This data imply that the puzzle of AFP negative HCC patients in monitoring recurrence might be compensated by nucleic acid-based assays on circulating plasma DNA. And, we also found the 3-year DFS and 3-year OS of patients having AI at D8S258 together with high level of plasma DNA are significantly poorer. These data suggest that nucleic acid-based assays on circulating plasma DNA might be supplementary to traditional TNM staging. However, further work is needed to confirm whether nucleic acid-based assay on circulating plasma DNA could be used in molecular staging of liver cancer and monitoring HCC progression at different stages of disease.
Abbreviations
- HCC:
-
hepatocellular carcinoma
- AI:
-
allelic imbalance
- AFP:
-
alpha fetoprotein
- DFS:
-
disease-free survival
- OS:
-
overall survival
- HDNA:
-
high plasma DNA level
- LDNA:
-
low plasma DNA level
References
Anker P, Mulcahy H, Chen XQ, Stroun M (1999) Detection of circulating tumour DNA in the blood (plasma/serum) of cancer patients. Cancer Metastasis Rev 18:65–73
Anker P, Mulcahy H, Stroun M (2003) Circulating nucleic acids in plasma and serum as a noninvasive investigation for cancer: time for large-scale clinical studies? Int J Cancer 103:149–152
Avery OT, MacLeod CM, McCarty M (1995) Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from Pneumococcus type III. 1944. Mol Med 1:344–365
Beau-Faller M, Gaub MP, Schneider A, Ducrocq X, Massard G, Gasser B, Chenard MP, Kessler R, Anker P, Stroun M, Weitzenblum E, Pauli G, Wihlm JM, Quoix E, Oudet P (2003) Plasma DNA microsatellite panel as sensitive and tumor-specific marker in lung cancer patients. Int J Cancer 105:361–370
Bissig H, Richter J, Desper R, Meier V, Schraml P, Schaffer AA, Sauter G, Mihatsch MJ, Moch H (1999) Evaluation of the clonal relationship between primary and metastatic renal cell carcinoma by comparative genomic hybridization. Am J Pathol 155:267–274
Bockmuhl U, Ishwad CS, Ferrell RE, Gollin SM (2001) Association of 8p23 deletions with poor survival in head and neck cancer. Otolaryngol Head Neck Surg 124:451–455
Castells A, Puig P, Mora J, Boadas J, Boix L, Urgell E, Sole M, Capella G, Lluis F, Fernandez-Cruz L, Navarro S, Farre A (1999) K-ras mutations in DNA extracted from the plasma of patients of patients with pancreatic carcinoma: diagnostic utility and prognostic significance. J Clin Oncol 17:578–584
Chan AK, Chiu RW, Lo YM (2003) Cell-free nucleic acids in plasma, serum and urine: a new tool in molecular diagnosis. Ann Clin Biochem 40:122–130
Chang YC, Ho CL, Chen HH, Chang TT, Lai WW, Dai YC, Lee WY, Chow NH (2002) Molecular diagnosis of primary liver cancer by microsatellite DNA analysis in the serum. Br J Cancer 87:1449–1453
Chiu RW, Poon LL, Lau TK, Leung TN, Wong EM, Lo YM (2001) Effects of blood processing protocols on fetal and total DNA quantification in maternal plasma. Clin Chem 47:1607–1613
Dahse R, Utting M, Werner W, Schimmel B, Claussen U, Junker K (2002) TP53 alterations as a potential diagnostic marker in superficial bladder carcinoma and in patients serum, plasma and urine samples. Int J Oncol 20:107–115
Genda T, Sakamoto M, Ichida T, Asakura H, Kojiro M, Narumiya S, Hirohashi S (1999) Cell motility mediated by rho and rho-associated protein kinase plays a critical role in intrahepatic metastasis of human hepatocellular carcinoma. Hepatology 30:1027–1036
Goessl C (2003) Noninvasive molecular detection of cancer: the bench and the bedside. Curr Med Chem 10:691–706
Greenlee RT, Hill-Harmon MB, Murray T, Thun M (2001) Cancer Statistics, 2001. CA Cancer J Clin 51:15–36
Guan XY, Fang Y, Sham JS, Kwong DL, Zhang Y, Liang Q, Li H, Zhou H, Trent JM (2000) Recurrent chromosome alterations in hepatocellular carcinoma detected by comparative genomic hybridization. Genes Chromosomes Cancer 29:110–116
Halling KC, French AJ, McDonnell SK, Burgart LJ, Schaid DJ, Peterson BJ, Moon-Tasson L, Mahoney MR, Sargent DJ, O’Connell MJ, Witzig TE, Farr GH Jr, Goldberg RM, Thibodeau SN (1999) Microsatellite Instability and 8p Allelic Imbalance in Stage B2 and C Colorectal Cancers. J Natl Cancer Inst 91:1295–1303
Kawakami K, Brabender J, Lord RV, Groshen S, Greenwald BD, Krasna MJ, Yin J, Fleisher AS, Abraham JM, Beer DG, Sidransky D, Huss HT, Demeester TR, Eads C, Laird PW, Ilson DH, Kelsen DP, Harpole D, Moore MB, Danenberg KD, Danenberg PV, Meltzer SJ (2000) Hypermethylated APC DNA in plasma and prognosis of patients with esophageal adenocarcinoma. J Natl Cancer Inst (Bethesda) 92:1805–1811
Kujawski M, Sarlomo-Rikala M, Gabriel A, Szyfter K, Knuutila S (1999) Recurrent DNA copy number losses associated with metastasis of larynx carcinoma. Genes Chromosomes Cancer 26:253–257
Lee TH, Montalvo L, Chrebtow V, Busch MP (2001) Quantitation of genomic DNA in plasma and serum samples: higher concentrations of genomic DNA found in serum than in plasma. Transfusion 41:276–282
Leon SA, Shapiro B, Sklaroff DM, Yaros MJ (1977) Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res 37:646–650
Li SP, Wang HY, Li JQ, Zhang CQ, Feng QS, Huang P, Yu XJ, Huang LX, Liang QW, Zeng YX (2001) Genome-wide analyses on loss of heterozygosity in hepatocellular carcinoma in Southern China. J Hepatol 34:840–849
Lin JC, Chen KY, Wang WY, Jan JS, Wei YH (2002) PCR detection of circulating tumor cells in nasopharyngeal carcinoma patients with distant metastasis: effect of enzyme and sampling. Head Neck 24:591–596
Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, Jiang RS (2004) Quantification of plasma Epstein–Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med 350:2461–2470
Mandel P, Métais P (1948) Les acides nucléiques du plasma sanguin chez l’homme. CR Acad Sci Paris 142:241–243
Martinez-Climent JA, Vizcarra E, Sanchez D, Blesa D, Marugan I, Benet I, Sole F, Rubio-Moscardo F, Terol MJ, Climent J, Sarsotti E, Tormo M, Andreu E, Salido M, Ruiz MA, Prosper F, Siebert R, Dyer MJ, Garcia-Conde J (2001) Loss of a novel tumor suppressor gene locus at chromosome 8p is associated with leukemic mantle cell lymphoma. Blood 98:3479–3482
Muscheck M, Sukosd F, Pesti T, Kovacs G. (2000) High density deletion mapping of bladder cancer localizes the putative tumor suppressor gene between loci D8S504 and D8S264 at chromosome 8p23.3. Lab Invest 80:1089–1093
Nihei N, Kouprina N, Larionov V, Oshima J, Martin GM, Ichikawa T, Barrett JC (1996) Mapping of metastasis suppressor gene(s) for rat prostate cancer on the short arm of human chromosome 8 by irradiated microcell-mediated chromosome transfer. Genes Chromosomes Cancer 17:260–268
Nihei N, Kouprina N, Larionov V, Oshima J, Martin JM, Ichikawa T, Barrett JC (2002) Functional evidence for a metastasis suppressor gene for rat prostate cancer within a 60-kilobase region on human chromosome 8p21-p12. Cancer Res 62:367–370
Niu Q, Tang ZY, Qin LX, Ma ZC, Zhang LH (2003) Loss of heterozygosity at D14S62 and D14S51 detected by a simple and non-radioactive method in plasma DNA is a potential marker of metastasis and recurrence after curative hepatic resection in hepatocellular carcinoma. Hepatogastroenterology 50:1579–1582
Ohgaki K, Iida A, Ogawa O, Kubota Y, Akimoto M, Emi M (1999) Localization of tumor suppressor gene associated with distant metastasis of urinary bladder cancer to a 1-Mb interval on 8p22. Genes Chromosomes Cancer 25:1–5
Parada LA, Maranon A, Hallen M, Tranberg KG, Stenram U, Bardi G, Johansson B (1999) Cytogenetic analyses of secondary liver tumors reveal significant differences in genomic imbalances between primary and metastatic colon carcinomas. Clin Exp Metastasis 17:471–479
Perinchery G, Bukurov N, Nakajima K, Chang J, Hooda M, Oh BR, Dahiya R (1999) Loss of two new loci on chromosome 8 (8p23 and 8q12-13) in human prostate cancer. Int J Oncol 14:495–500
Petersen S, Aninat-Meyer M, Schluns K, Gellert K, Dietel M, Petersen I (2000) Chromosomal alterations in the clonal evolution to the metastatic stage of squamous cell carcinomas of the lung. Br J Cancer 82:65–73
Pisani P, Parkin DM, Bray F, Ferlay J (1999) Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 83:18–29
Qin LX (2002) Chromosomal aberrations related to metastasis of human solid tumors. World J Gastroenterol 8:769–776
Qin LX, Tang ZY (2002) The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol 8:193–199
Qin LX, Tang ZY, Sham JS, Ma ZC, Ye SL, Zhou XD, Wu ZQ, Trent JM, Guan XY (1999) The association of chromosome 8p deletion and tumor metastasis in human hepatocellular carcinoma. Cancer Res 59:5662–5665
Qin LX, Tang ZY, Ye SL, Liu YK, Ma ZC, Zhou XD, Wu ZQ, Lin ZY, Sun FX, Tian J, Guan XY, Pack SD, Zhuang ZP (2001) Chromosome 8p deletion is associated with metastasis of human hepatocellular carcinoma when high and low metastatic models are compared. J Cancer Res Clin Oncol 127:482–488
Silva JM, Rodriguez R, Garcia JM, Munoz C, Silva J, Dominguez G, Provencio M, Espana P, Bonilla F (2002) Detection of epithelial tumour RNA in the plasma of colon cancer patients is associated with advanced stages and circulating tumour cells. Gut 50:530–534
Sobin LH, Wittekind CH (2002) TNM classification of malignant tumours, 6th edn. Wiley-Liss, New York
Sozzi G, Musso K, Ratcliffe C, Goldstraw P, Pierotti MA, Pastorino U (1999) Detection of microsatellite alterations in plasma DNA of non-small cell lung cancer patients: a prospect for early diagnosis. Clin Cancer Res 5:2689–2692
Sozzi G, Conte D, Mariani L, Lo Vullo S, Roz L, Lombardo C, Pierotti MA, Tavecchio L (2001) Analysis of circulating tumor DNA in plasma at diagnosis and during follow-up of lung cancer patients. Cancer Res 61:4675–4678
Stroun M, Anker P, Maurice P, Lyautey J, Lederrey C, Beljanski M (1989) Neoplastic characterization of DNA from the plasma of cancer patients. Oncology 46:318–322
Taback B, Fujiwara Y, Wang HJ, Foshag LJ, Morton DL, Hoon DS (2001) Prognostic significance of circulating microsatellite markers in the plasma of melanoma patients. Cancer Res 61:5723–5726
Taback B, O’Day SJ, Hoon DS (2004) Quantification of circulating DNA in the plasma and serum of cancer patients. Ann N Y Acad Sci 1022:17–24
Takanishi DM Jr, Kim SY, Kelemen PR, Yaremko ML, Kim AH, Ramesar JE, Horrigan SK, Montag A, Michelassi F, Westbrook CA (1997) Chromosome 8 losses in colorectal carcinoma: localization and correlation with invasive disease. Mol Diagn 2:3–10
Tang ZY (2001) Hepatocellular carcinoma-cause, treatment and metastasis. World J Gastroenterol 7:445–454
Thijssen MA, Swinkels DW, Ruers TJ, de Kok JB (2002) Difference between free circulating plasma and serum DNA in patients with colorectal liver metastases. Anticancer Res 22:421–425
Utting M, Werner W, Dahse R, Schubert J, Junker K (2002) Microsatellite analysis of free tumor DNA in urine, serum, and plasma of patients: a minimally invasive method for the detection of bladder cancer. Clin Cancer Res 8:35–40
Vitzthum F, Geiger G, Bisswanger H, Brunner H, Bernhagen J (1999) A quantitative fluorescence based microplate assay for the determination of double-stranded DNA using SYBR Green I and a standard ultraviolet transilluminator gel imaging system. Anal Biochem 276:59–64
Wagner U, Bubendorf L, Gasser TC, Moch H, Gorog JP, Richter J, Mihatsch MJ, Waldman FM, Sauter G (1997) Chromosome 8p deletions are associated with invasive tumor growth in urinary bladder cancer. Am J Pathol 151:753–759
Wang G, Zhao Y, Liu X, Wang L, Wu C, Zhang W, Liu W, Zhang P, Cong W, Zhu Y, Zhang L, Chen S, Wan D, Zhao X, Huang W, Gu J (2001) Allelic loss and gain, but not genomic instability, as the major somatic mutation in primary hepatocellular carcinoma. Genes Chromosomes Cancer 31:221–227
Watson JD, Crick FH (1953) Molecular structure of nucleic acids: a structure for deoxyribose nucleic acid. Nature 171:737–738
Wong IH, Zhang J, Lai PB, Lau WY, Lo YM (2003) Quantitative analysis of tumor-derived methylated p16INK4a sequences in plasma, serum, and blood cells of hepatocellular carcinoma patients. Clin Cancer Res 9:1047–1052
Wright K, Wilson PJ, Kerr J, Do K, Hurst T, Khoo SK, Ward B, Chenevix-Trench G. Frequent (1998) Loss of heterozygosity and three critical regions on the short arm of chromosome 8 in ovarian adenocarcinomas. Oncogene 17:1185–1188
Yokota T, Yoshimoto M, Akiyama F, Sakamoto G, Kasumi F, Nakamura Y, Emi M (1999) Localization of a tumor suppressor gene associated with the progression of human breast carcinoma within a 1-cM interval of 8p22-p23.1. Cancer 85:447–452
Yuki K, Hirohashi S, Sakamoto M, Kanai T, Shimosato Y (1990) Growth and spread of hepatocellular carcinoma. A review of 240 consecutive autopsy cases. Cancer 66:2174–2179
Zhang LH, Qin LX, Ma ZC, Ye SL, Liu YK, Ye QH, Wu X, Huang W, Tang ZY (2003) Allelic imbalance regions on chromosomes 8p, 17p and 19p related to metastasis of hepatocellular carcinoma: comparison between matched primary and metastatic lesions in 22 patients by genome-wide microsatellite analysis. J Cancer Res Clin Oncol 129:279–286
Acknowledgments
This work was supported by the National Science Fund for Distinguished Young Scholars (30325041), the “863” R&D high-tech Key Project (2002BA711A02-4), Fund for Outstanding Scholars in New Era from Ministry of Education in China (2003), the Key Project from the Ministry of Education of China, Shanghai Science and Technology Developing Program (No. 03DZ14024, Shanghai, China).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, N., Qin, LX., Tu, H. et al. The prognostic value of circulating plasma DNA level and its allelic imbalance on chromosome 8p in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol 132, 399–407 (2006). https://doi.org/10.1007/s00432-005-0049-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-005-0049-5