Abstract
Caveolae are uniform vesicular invaginations of the cell membrane. Caveolin-1 is responsible for the formation of caveolae and plays a key role in membrane traffic and signal transduction. The contribution of caveolin-1 to carcinogenesis has been widely investigated; however, the expression pattern of caveolin-1 is controversial both in gastrointestinal and extraintestinal cancers. Most of the results based on cancer cell line experiments suggest that caveolin-1 might act as a tumor-suppressor gene. On the contrary, several studies on the expression of caveolin-1 in tumor tissues indicate a possible tumor-promoting effect of caveolin-1. In this article we summarize the divergent results of caveolin-1 expression in gastrointestinal and extraintestinal cancer regarding possible future therapeutic implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Caveolae
Caveolae ("little caves") are uniform vesicular invaginations of 50–100 nm of the plasma membrane and were first described morphologically by Yamada (1955), and Palade and Bruns (1968). On electron microscopic images, these ultrastructures can be identified by their flask-shape morphology; however, the cytosolic surface of each caveola has a striated coat that is apparent in rapid-freeze deep-etch images (Liu et al. 2002). Caveolins reside in the filaments forming this coat. Caveolae are found in many cell types, but are notably abundant in fibroblasts, adipocytes, endothelial cells, type-I pneumocytes, epithelial cells, and smooth and striated muscle cells (Marx 2001). Caveolae have been previously implicated only in transcytosis and potocytosis. To our current knowledge, these organelles play an important role in a number of cellular signaling processes as well, including apoptosis, and in the pathogenesis of several human diseases, such as cancer (Timme et al. 2000; Galbiati et al. 1998).
The caveolin gene family
There are three caveolin genes (caveolin-1, caveolin-2, and caveolin-3) expressed in mammals with totally five different isoforms (Razani et al. 2001). Most of the tissues express at least one of these isoforms. Since caveolin-1 and caveolin-2 are usually co-expressed and caveolin-2 is rapidly degraded in cells not expressing caveolin-1, caveolin-2 was proposed to function as an accessory protein to caveolin-1. In the caveolae of skeletal and heart muscle cells, caveolin-1 is replaced by caveolin-3 (Marx 2001).
Human caveolin-1 and caveolin-2 are co-localized to 7q31 in a genomic region that is frequently lost in cancers (Engelman et al. 1999). Caveolin-3 is mapped to a different chromosome. The point mutation of this region (3p25) is responsible for the development of the autosomal-dominant form of limb-girdle muscular dystrophy (Galbiati et al. 2001; Razani et al. 2000).
Caveolin-1
Caveolin-1, a protein of 21–24 kDa, was first described as the major substrate for tyrosine phophorylation when cells were transformed by the Rous sarcoma virus (Glenney and Soppet 1992). Caveolin-1 acts as the driving force for caveolae formation and regulates the import and export of cellular cholesterol due to its ability to form homo-oligomers and its interaction with cholesterol and glycosphingolipids (Razani et al. 2001). Caveolin-1, however, also plays a key role in membrane traffic by attracting proteins to caveolae as a molecular motor that powers membrane invagination and budding (Liu et al. 2002). Through its special motif, the scaffolding domain, caveolin-1 interacts with lipid-modified signaling molecules such as G-protein alpha subunits, H-ras, Src-family tyrosine kinases, endothelial nitric oxide synthase, epidermal growth factor receptor (EGF-R), mitogen-activated protein kinase (MAPK), transforming growth factor-β/SMAD, and the Wnt/β-catenin/lef-1 pathway (Li et al. 1996; Garcia-Cardena et al. 1996; Mineo et al. 1996; Galbiati et al. 2000). Based on the observation that this scaffolding domain organizes the subcellular distribution of these signaling molecules in a highly dynamic manner, the "caveolae signaling hypothesis" has been proposed, which states that caveolar localization of various inactive signaling molecules provides a compartmental basis for their subsequent regulated activation, and explains "cross-talk" between different signaling pathways (Razani et al. 2001).
In light of the aforementioned evidence, caveolin-1 has been speculated to have a role in cellular transformation and tumorigenesis. Nevertheless, the in vitro and in vivo animal experiments and the studies on human specimens have generated a controversial picture. As listed below, several research groups have implied that caveolin-1 might fulfill a tumor-suppressor role (Engelman et al. 1998; Bender et al. 2001; Wiechen et al. 2001; Cui et al. 2001; Racine et al. 1999); however, others have reported a positive association of caveolin-1 with tumorigenesis and progression, thereby suggesting a tumor-promoting function (Kato et al. 2002; Yang et al. 1998; Yang et al. 1999; Ito et al. 2002, Ho et al. 2002). These conflicting results refer to the complex physiological functions of caveolin-1 and its contribution to carcinogenesis.
In the subset of gastrointestinal cancers, there have been few studies performed on the expression of caveolin-1; however, even in this subgroup of cancers, the reported behavior of caveolin-1 is contradictory.
In this article we give a brief overview of the divergent results of caveolin-1 expression in various cancers with special emphasis on its expression in gastrointestinal cancers.
Is caveolin-1 a tumor-suppressor gene?
Extraintestinal cancers
There are several bodies of evidence that caveolin-1 gene functions as a tumor-suppressor gene. Based on the association of the loss of heterozygosity (LOH) in the D7S522 region with carcinogenesis, Zenklusen et al. (1995) indicated that a novel tumor-suppressor gene is likely to reside within the 7q31.1 region. Engelman et al. (1999) also proposed caveolin-1 to be the tumor-suppressor gene at the D7S522 locus in human chromosome 7. In accordance with this hypothesis, caveolin-1 mRNA and protein levels were reduced in the oncogenically transformed NIH 3T3 fibroblasts (Koleske et al. 1995). Furthermore, the targeted down-regulation of the caveolin-1 gene in NIH 3T3 cell lines resulted in the anchorage-independent growth of these cells (Galbiati et al. 1998). Furthermore, Lee et al. (1998) found significantly reduced caveolin-1 expression in breast cancer cells compared with that of normal breast tissue, and when caveolin-1 cDNA was transfected to cancer cells, the overexpression of caveolin-1 resulted in substantial growth inhibition. In breast cancer cell lines with caveolin-1 down-regulated by the recently identified sporadic P132L mutation, cellular transformation was enhanced by the activation of the MAP kinase pathway (Hayashi et al. 2001). In prostate and breast cancer, the hypermethylation of the promoter region in the caveolin-1 gene was reported which is deemed an early event of tumorigenesis (Engelman et al. 1999; Cui et al. 2001). In lung cancer, a marked reduction in the expression of caveolin-1 was reported both in cancer cell lines and tumor specimens in contrast to the expression status of the normal bronchial epithelium (Racine et al. 1999; Wikman et al. 2002). The down-regulation of caveolin-1 was demonstrated both in ovarian cancer cell lines and ovarian carcinoma specimens (Wiechen et al. 2001; Davidson et al. 2001) and the in vitro reexpression of caveolin-1 in the ovarian carcinoma cell line OVCAR-3 resulted in the suppression of tumor cell survival (Wiechen et al. 2001).
Gastrointestinal cancers
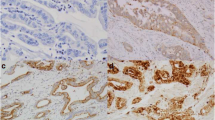
In gastric cancer, M. Juhász et al. (submitted) assessed the expression of caveolin-1 both in mRNA and protein level. Caveolin-1 mRNA expression was found to be down-regulated in gastric cancer as compared with that of the normal gastric mucosa. Caveolin-1 immunoreactivity was also markedly reduced in gastric cancer specimens in contrast to normal gastric epithelium. In the non-epithelial cell compartment of gastric cancer, caveolin-1 expression was more intense in well-differentiated tumors than in undifferentiated and metastatic gastric cancers. Comparing gastric cancers of the diffuse and the intestinal type, no difference could be detected concerning caveolin-1 mRNA expression. On the contrary, only gastric cancers of the intestinal type expressed caveolin-1 by means of immunohistochemistry.
Bender et al. (2001) evaluated the expression of caveolin-1 and its role in cellular transformation in colon cancer. In human colon carcinoma cell lines, both the mRNA and the protein levels of caveolin-1 were reduced. In tumorous colon mucosa, caveolin-1 protein levels were significantly decreased in 67% of the colon cancer patients. In order to assess the role of caveolin-1 in tumor formation, the HT29 and DLD1 colon carcinoma cells were transfected with caveolin-1. When injected to nude mice, the reexpression of caveolin-1 led to decreased tumorigenicity in 77% of the HT29 cancer cells, and in 71% of the DLD1 cancer cells. These results seem to confirm the tumor-suppressor role of caveolin-1 and indicate that the down-regulation of the caveolin-1 gene is not necessarily an irreversible process.
Using human cancer cDNA arrays, Terris et al. (2002) have characterized the gene expression profile of the intraductal papillary-mucinous tumors of the pancreas. Along with 57 other genes, caveolin-1 was also found to be down-regulated in this rare type of pancreatic cancer. In a recent study by Murakami et al. the expression of caveolin-1 was also analyzed in extrahepatic bile duct carcinoma (Murakami et al. 2003). Using immunohistochemistry caveolin-1 expression was observed in 22 of 60 carcinomas, and interestingly, the expression of caveolin-1 in these cancer cells was associated with a favorable prognosis; thus, the survival of patients with caveolin-1 expression in the cancer cells was significantly longer compared with cancers devoid of caveolin-1 expression. This again supports the hypothesis that caveolin-1 may act as a tumor-suppressing gene in various cancers.
Is caveolin-1 a tumor promoting gene?
Extraintestinal cancers
Several researchers, however, question this putative tumor-suppressor role of caveolin-1. Numerous studies performed on human tumorous tissues and not on cancer cell lines exhibit a completely opposite pattern of caveolin-1 expression. In the papillary carcinoma of the thyroid, caveolin-1 expression was shown to be elevated as compared with the normal thyroid mucosa (Ito et al. 2002). Interestingly, the expression status of caveolin-1 differed among the various histological types of thyroid carcinomas indicating that the expression of caveolin-1 might be specific not only according to localization but also according to histological type.
Caveolin-1 was also found to be overexpressed in breast and prostate cancer (Yang et al. 1998). Moreover, caveolin-1 was shown to be a metastases-related gene and a reliable prognostic marker for prostate cancer patients undergoing radical prostatectomy (Yang et al. 1999; Tahir et al. 2001). In bladder cancer, caveolin-1 was associated with tumor dedifferentiation in a subset of high-grade bladder cancer (Rajjayabun et al. 2001). Ho et al. (2002) reported that the reexpression of caveolin-1 gene enhanced the invasive capability of lung cancer cell lines, and they revealed an increased caveolin-1 immunoreactivity in primary lung adenocarcinoma cells giving metastases to regional lymph nodes, and found caveolin-1 to be an independent negative prognostic marker.
Hurlstone et al. (1999) found no in vivo expression of caveolin-1 in normal breast ductal epithelial cells, no mutation of caveolin-1 in human cancers, no methylation of CpG islands in either primary tumors or tumor-derived cell lines, and no correlation of LOH at the caveolin-1 locus with the expression of caveolin-1, thereby conflicting the results of many other studies.
Gastrointestinal cancers
Kato et al. (2002) evaluated the association of caveolin-1 immunoreactivity with the clinicopathological parameters and prognosis of patients with esophageal squamous cell carcinoma. Caveolin-1 expression was observed in 45% of patients. The expression of caveolin-1 was correlated with the pathologic stage, the lymph node, and distant metastases. The survival rate of patients with caveolin-1 positive tumors was significantly worse than that of patients with caveolin-1 negative tumors. Tumor size, lymphatic metastasis and the positive surgical margin proved to be independent negative prognostic factors in esophageal squamous cell carcinoma.
Using human cancer cDNA arrays, Hu et al. (2001) have performed the profiling of the differentially expressed cancer-related genes in esophageal squamous cell carcinoma. Caveolin-1 was found to be one of the 13 up-regulated gene in HKESC-1 and HKESC-2 esophageal squamous cell carcinoma cell lines. This result is completely in line with the aforementioned results of Kato et al. (2002) obtained by immunohistochemistry.
With the help of immunohistochemistry, Fine et al. (2001) studied the expression of caveolin-1 and caveolin-2 in normal epithelium, adenoma, and adenocarcinoma of the colon. The expression of caveolin-1 was elevated in 78% of the adenocarcinomas while only 6% of normal epithelium and adenoma cases presented positive stainings. Caveolin-1 exhibited a statistically significant correlation with adenocarcinoma but not with any other clinicopathological factors. Caveolin-2 expression could be detected neither in the adenocarcinoma nor in the adenoma and the normal epithelium of the colon.
Suzuoki et al. (2002) investigated the association of caveolin-1 expression with clinicopathological parameters and clinical outcome in patients with pancreatic adenocarcinoma undergoing surgical resection. Positive caveolin-1 immunoreactivity was detected in 41% of the pancreatic adenocarcinoma patients, whereas the normal ductal epithelium showed only subtle or no immunostaining. Caveolin-1 expression exhibited a statistically significant correlation with tumor size, grade of differentiation, and poor prognosis. Caveolin-1 was shown to be an independent negative prognostic factor in patients with pancreatic adenocarcinoma.
Conclusion
The initial studies of caveolin-1 expression indicated a potential tumor-suppressor role for caveolin-1. These previous results were based mainly on experiments with cancer cell lines. As the evaluation of caveolin-1 was extended to tumorous tissue specimens, several researchers reported the up-regulation of caveolin-1 expression in both gastrointestinal and extraintestinal cancers; however, this question cannot be simplified to the antagonism of the results obtained from cancer cell lines and tumor tissues since conflicting results have been reported from either side. This currently unexplained contradiction is apparent in the subset of gastrointestinal cancers as well (Table 1). In the slight majority of studies on the expression of caveolin-1 in gastrointestinal cancers, caveolin-1 is overexpressed in contrast to normal mucosa and was found to be a negative prognostic factor. The remaining studies demonstrated an expression status indicating a potential tumor-suppressor role of caveolin-1. These contradictory findings are especially true for the expression of caveolin-1 in pancreatic cancers which is associated with a poor prognosis as opposed to the findings by Murakami et al. (2003) who reported a favorable prognosis for caveolin-1 expression in bile duct cancers (Suzuoki et al. 2002); thus, the expression of caveolin-1 can vary in the different organs, and in the same localization, depending on the histological type as seen in pancreatic, gastric, and thyroid cancer. Furthermore, depending on the primary origin of the cancers, the prognostic implications can vary extensively. However, the various studies addressing caveolin-1 expression in gastrointestinal cancers employed different methodological approaches which need to be taken into account when assessing the role of caveolin-1 expression in these malignancies; thus, while the increased expression of caveolin-1 in esophageal cancer was observed in cell lines by DNA array analysis (Hu et al. 2001), other groups used immunohistochemistry and Western blot analysis in order to assess the protein levels of caveolin-1 expression in the cancer cells (Table 1). Using immunohistochemistry the expression of caveolin-1 was increased in esophageal, colon pancreatic, and bile duct cancers. This technique allows the direct identification of the cellular origin of caveolin-1 in the cancer cells; however, other studies reported decreased caveolin-1 expression using PCR or Northern blot analysis (Table 1).
Lee et al. (2000) suggested that the diverse effects of caveolin-1 may be mediated by different regions of the caveolin-1 molecule, and may depend on the expression levels of coexpressed molecules such as c-Src and Grb7. In an attempt to somehow match these divergent results, a new concept of the contribution of caveolin-1 to neoplastic transformation has emerged. According to this hypothesis, caveolin-1 seems to behave in a tissue-dependent manner and might have a role in not only one but in distinct stages of carcinogenesis.
The elucidation of the role of caveolin-1 in tumor formation has important future therapeutic perspectives. The former anticancer treatment strategies comprised mainly cytotoxic compounds. The current drug development is focusing instead on targeted therapeutics that act on specific molecular targets responsible for the malignant phenotype. One possible approach is the pharmacological targeting of signal transduction pathways that play a key role in oncogenic cellular transformation and malignant progression. Indeed, through the inhibition of signal transduction by the negative regulation of the hsp90 molecular chaperone with 17-allylamino-17-demethoxygeldanamycin, the expression of caveolin-1 could be down-regulated in human colon cancer cells (Clarke et al. 2000). This promising preclinical result projects the oncoming anticancer treatment modalities in cancers with up-regulated caveolin-1 expression.
References
Bender FC, Reymond MA, Bron C, Quest AFG (2001) Caveolin-1 levels are down-regulated in human colon tumors, and ectopic expression of caveolin-1 in colon carcinoma cell lines reduces cell tumorigenicity. Cancer Res 60:5870–5878
Clarke PA, Hostein I, Banerji U, Stefano FD, Maloney A, Walton M, Judson I, Workman P (2000) Gene expression profiling of human colon cancer cells following inhibition of signal transduction by 17-allylamino-17-demethoxygeldanamycin, an inhibitor of the hsp90 molecular chaperone. Oncogene 19:4125–4133
Cui J, Rohr RL, Swanson G, Speights VO, Maxwell T, Brothman AR (2001) Hypermethylation of the caveolin-1 gene promoter in prostate cancer. Prostate 46:249–256
Davidson B, Nesland JM, Goldberg I, Kopolovic J, Gotlieb WH, Bryne M, Ben-Baruch G, Berner A, Reich R (2001) Caveolin-1 expression in advanced-stage ovarian carcinoma: a clinicopathological study. Gynecol Oncol 81:166–171
Engelman JA, Zhang XL, Lisanti MP (1998) Genes encoding human caveolin-1 and –2 are co-localized to the D7S522 locus (7q31.1), a known fragile site (FRA7G) that is frequently deleted in human cancers. FEBS Lett 436:403–410
Engelman JA, Zhang XL, Lisanti MP (1999) Sequence and detailed organization of the human caveolin-1 and -2 genes located near the D7S522 locus (7q31.1). Methylation of a CpG island in the 5' promoter region of the caveolin-1 gene in human breast cancer cell lines. FEBS Lett 448:221–230
Fine SW, Lisanti MP, Galbiati F, Li M (2001) Elevated expression of caveolin-1 in adenocarcinoma of the colon. Am J Clin Pathol 115:719–724
Galbiati F, Volonte D, Engelman JA, Watanabe G, Burk R, Pestell RG, Lisanti MP (1998) Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J 17:6633–6648
Galbiati F, Volonte D, Brown AMC, Weinstein DE, Ben-Ze'ev A, Pestell RG (2000) Caveolin-1 expression inhibits Wnt/β-catenin/Lef-1 signaling by recruiting β-catenin to caveolae membrane domains. J Biol Chem 275:23368–23377
Galbiati F, Razani B, Lisanti MP (2001) Caveolae and caveolin-3 in muscular dystrophy. Trends Mol Med 7:435–441
Garcia-Cardena G, Fan R, Stern DF (1996) Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J Biol Chem 271:27237–27240
Glenney JR, Soppet D (1992) Sequence and expression of caveolin, a protein component of caveolae plasma membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci USA 89:10517–10521
Hayashi K, Matsuda S, Machida K, Yamamoto T, Fukuda Y, Nimura Y, Hayakawa T, Hamaguchi M (2001) Invasion activating caveolin-1 mutation in human scirrhous breast cancer. Cancer Res 61:2361–2364
Ho CC, Huang PH, Huang HY, Chen YH, Yang PC, Hsu SM (2002) Up-regulated caveolin-1 accentuates the metastasis capability of lung adenocarcinoma by inducing filopodia formation. Am J Pathol 161:1647–1656
Hu YC, Lam KY, Law S, Wong J, Srivastava G (2001) Profiling of differentially expressed cancer-related genes in esophageal squamous cell carcinoma (ESCC) using human cancer cDNA arrays: overexpression of oncogene MET correlates with tumor differentiation in ESCC. Clin Cancer Res 7:3519–3525
Hurlstone AFL, Reid G, Reeves JR, Fraser J, Strathdee G, Rahilly M, Parkinson EK, Black DM (1999) Analysis of the caveolin-1 gene at human chromosome 7q31.1 in primary tumors and tumor-derived cell lines. Oncogene 18:1881–1890
Ito Y, Yoshida H, Nakano K, Yokozawa T, Hirai K, Matsuzuka F, Matsuura N, Kakudo K, Kuma K, Miyauchi A (2002) Caveolin-1 overexpression is an early event in the progression of papillary carcinoma of the thyroid. Br J Cancer 86:912–916
Kato K, Hida Y, Miyamoto M, Hashida H, Shinohara T, Itoh T, Okushiba T, Kondo S, Katoh H (2002) Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer 94:929–933
Koleske AJ, Baltimore D, Lisanti MP (1995) Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA 92:1381–1385
Lee SW, Reimer CL, Oh P, Campbell DB, Schnitzer JE (1998) Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene 16:1391–1397
Lee H, Volont D, Galbiati F, Lyengar P, Lublin DM, Bregman DB, Wilson MT, Campos-Gonzalez R, Bouzahzah B, Pestell RG, Scherer PE, Lisanti MP (2000) Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol 14:1750–1775
Li S, Couet J, Lisanti MP (1996) Src tyrosine kinases, G alpha subunits, and H-ras share a common membrane-anchored scaffolding protein, caveolin: caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem 271:29182–29190
Liu P, Rudick M, Anderson RG (2002) Multiple functions of caveolin-1. J Biol Chem 277:41295–41298
Marx J (2001) Caveolae: a once-elusive structure gets some respect. Science 294:1862–1865
Mineo C, James GL, Smart EJ (1996) Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membranes. J Biol Chem 271:11930–11935
Murakami S, Miyamoto M, Hida Y, Cho Y, Fukunga A, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Itoh T, Shinohara T, Morikawa T, Okushiba S, Kondo S, Katoh H (2003) Caveolin-1 overexpression is a favourable prognostic factor for patients with extrahepatic bile duct carcinoma. Br J Cancer 88:1234–1238
Palade GE, Bruns RR (1968) Structural modification of plasmalemma vesicles. J Cell Biol 37:633–649
Racine C, Belanger M, Hirabayashi H, Boucher M, Chakir J, Couet J (1999) Reduction of caveolin 1 gene expression in lung carcinoma cell lines. Biochem Biophys Res Commun 255:580–586
Rajjayabun PH, Garg S, Durkan GC, Charlton R, Robinson MC, Mellon JK (2001) Caveolin-1 expression is associated with high-grade bladder cancer. Urology 58:811–814
Razani B, Schlegel A, Lisanti MP (2000) Caveolin proteins in signaling, oncogenic transformation and muscular dystrophy. J Cell Sci 113:2103–2109
Razani B, Schlegel A, Liu J, Lisanti MP (2001) Caveolin-1, a putative tumor suppressor-gene. Biochem Soc Transact 29:494–499
Suzuoki M, Miyamoto M, Kato K, Hiraoka K, Oshikiri T, Nakakubo Y, Fukunaga A, Shichinohe T, Shinohara T, Itoh T, Kondo S, Katoh H (2002) Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br J Cancer 87:1140–1144
Tahir SA, Yang G, Ebara S, Timme TL, Satoh T, Li L, Goltsov A, Ittmann M, Morrisett JD, Thompson TC (2001) Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res 61:3882–3885
Terris B, Blaveri E, Crnogorac-Jurcevic T, Jones M, Missiaglia E, Ruszniewski P, Sauvanet A, Lemoine NR (2002) Characterization of gene expression profiles in intraductal papillary-mucinous tumors of the pancreas. Am J Pathol 160:1745–1754
Timme TL, Goltsov A, Tahir S, Li L, Wang J, Ren C, Johnston RN, Thompson TC (2000) Caveolin-1 is regulated by c-myc and suppresses c-myc induced apoptosis. Oncogene 19:3256–3265
Wiechen K, Diatchenko L, Agoulnik A, Scharff KM, Schober H, Arlt K, Zhumabayeva B, Siebert PD, Dietel M, Schafer R, Sers C (2001) Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. Am J Pathol 159:1635–1643
Wikman H, Kettunen E, Seppanen JK, Karjalainen A, Hollmen J, Anttila S, Knuutila S (2002) Identification of differentially expressed genes in pulmonary adenocarcinoma by using cDNA array. Oncogene 21:5804–5813
Yamada E (1955) The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol 1: 445–458
Yang G, Truong LD, Timme TL, Ren C, Wheeler TM, Park SH, Nasu Y, Bangma CH, Kattan MW, Scardino PT, Thompson TC (1998) Elevated expression of caveolin is associated with prostate and breast cancer. Clin Cancer Res 4:1873–1880
Yang G, Truong LD, Wheeler TM, Thompson TC (1999) Caveolin-1 expression in clinically confined human prostate cancer: a novel prognostic marker. Cancer Res 59:5719–5723
Zenklusen JC, Thompson JC, Klein-Szanto AJP, Conti CJ (1995) Frequent loss of heterozygosity in human primary squamous cell and colon carcinomas at 7q31.1: evidence for a broad range tumor suppressor gene. Cancer Res 55:1347–1350
Acknowledgements
This work was supported by a scholarship to M.J. by the EAGE. M.E. is supported by the Heisenberg Program of the DFG (Eb 187/5-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Juhász, M., Chen, J., Tulassay, Z. et al. Expression of caveolin-1 in gastrointestinal and extraintestinal cancers. J Cancer Res Clin Oncol 129, 493–497 (2003). https://doi.org/10.1007/s00432-003-0468-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-003-0468-0