Abstract
Vascular anomalies include a heterogeneous group of disorders that are categorized as vascular tumors or vascular malformations. Treatment options include resection, embolization, laser therapy, and sclerotherapy or medical treatment such as propranolol, steroids, interferon, and cytostatic chemotherapy. Mammalian target of rapamycin seems to play a key role in the signal pathway of angiogenesis and subsequently in the development of vascular anomalies. Recently, the successful use of sirolimus has been reported in children with lymphatic malformations and kaposiform hemangioendotheliomas. We report on six patients with different vascular anomalies (kaposiform hemangioendothelioma n = 2, combined lymphatico-venous malformation n = 2, pulmonary lymphangiectasia n = 1, and orbital lymphatic malformation n = 1) who were treated with peroral sirolimus. Three of the children initially presented with a Kasabach-Merrit phenomenon. Median duration of treatment was 10 months; two children are still on treatment. Three children each achieved complete and partial remission. Kasabach-Merrit phenomenon resolved within 1 month in all patients. Treatment with sirolimus was tolerated well; only mild reversible leukopenia was observed.
Conclusion: Sirolimus proved to be effective in children with complicated lymphatic or lymphatico-venous malformations and kaposiform hemangioendotheliomas. Treatment was tolerated well with acceptable side effects. The optimum length of treatment and possible long-term side effects have to be evaluated.
What is Known: • Vascular anomalies including vascular tumors and vascular malformations may lead to life-threatening conditions. • Some patients are refractory to established treatment and/or are not available for local invasive procedures. |
What is New: • We reviewed the literature focusing treatment of vascular anomalies in children and adolescents. • Our data support recent studies that sirolimus is an effective treatment option in patients with complicated vascular tumors and malformations. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular anomalies are a heterogeneous group of disorders originating from blood and/or lymphatic vessels. According to the most recent classification adopted by the International Society for the Study of Vascular Anomalies (ISSVA), these lesions are divided into true vascular tumors resulting from endothelial proliferation and vascular malformations resulting from defective embryonic vasculogenesis [1, 9, 10, 25]. The majority of vascular anomalies follow a benign clinical course: For example, most children with infantile hemangioma (IH) typically experience a phase of proliferation during the first year of life followed by a phase of stabilization and spontaneous involution with complete resolution over the next years [10]. However, a small portion of children with IH may develop overwhelming proliferation that may cause sometimes organ dysfunction with life-threatening clinical deterioration. Other vascular tumors, such as kaposiform hemangioendotheliomas (KHE), are infiltrative lesions that are frequently associated with a coagulopathy called Kasabach-Merrit phenomenon (KMP), thus resulting in significant morbidity and even mortality [3, 10]. Vascular malformations, particularly lymphatic malformations, venous malformations, or combined lesions, may also be associated with significant morbidity caused by disfiguration such as soft tissue hypertrophy, bony abnormalities, organ compromise, or chylous pleural effusions [10].
The optimum care of children with vascular anomalies requires close collaboration of different medical specialities including surgery, conventional and interventional radiology, hematology–oncology, and dermatology [10, 18]. Treatment options include resection, embolization, laser therapy, and sclerotherapy [10, 25]. Complicated vascular anomalies such as proliferative vascular tumors or refractory vascular malformations have been treated with different medical therapies. Corticosteroids are frequently used as first-line therapy with varying results [21]. Second-line therapy includes conventional chemotherapy (e.g., vincristine or cyclophosphamide) [6, 16] or interferon [5]. However, these treatment approaches were associated with limited responses and with significant side effects. Propranolol has emerged as a highly effective and well-tolerated therapy for IH [17]. Recent in vitro studies demonstrated the antiangiogenic properties of propranolol via downregulation of vascular endothelial growth factor (VEGF) pathways [19].
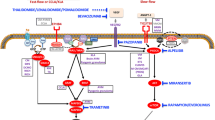
The mammalian target of rapamycin (mTOR) activates protein synthesis, resulting in numerous cellular processes including cell proliferation and increased angiogenesis, thus playing a key role in the pathogenesis of various vascular anomalies [10]. Sirolimus, also known as rapamycin, is a US Food and Drug Administration (FDA)-approved mTOR inhibitor used as immunosuppressive agent in pediatric renal transplant recipients [7]. The first report on the successful use of sirolimus as antiangiogenetic agent in a child with refractory KHE and KMP was published in 2010 [2]. Since clinical data on the use of sirolimus in patients with vascular anomalies are still rare [10, 13, 15, 28], we present our experience of six children treated with sirolimus for various vascular anomalies.
Patients and methods
A retrospective chart review was performed including six children (four males, two females) treated with sirolimus for various vascular anomalies in our institution between 2010 and 2014. Two children were diagnosed with KHE (plus KMP), two with combined lymphatico-venous malformations, one with pulmonary lymphangiectasia with bilateral chylous pleural effusions, and one with orbital lymphatic malformation. Diagnosis was made at birth in 4/6 children and at the age of 10 and 13 months, respectively (Table 1). Diagnosis was established by clinical evaluation, routine laboratory parameters (e.g., blood counts, serumparameters, and coagulation profile), and magnetic resonance imaging (MRI) in 4/6 patients; biopsy was done in 2/6 children (patients 3 and 4).
Sirolimus was given at 0.05 mg/kg twice daily perorally and subsequently adjusted to achieve serum levels between 5 and 15 ng/ml. Serum levels of sirolimus were measured monthly during treatment.
Informed consent was obtained from the parents or the legal guardians.
Results
Treatment characteristics are summarized in Table 1. Median duration of treatment was 10 months (range 3 to 53 months). Four of six children had received unsuccessful pre-treatment with different treatment modalities. All patients showed clinical response to sirolimus. Complete remission was achieved in three children after 6, 3, and 19 months of treatment respectively. Three children showed partial response, and two of them are still on treatment for 14 and 53 months respectively. In the three children with KMP, platelet counts and coagulation profiles normalized within 1 month.
Patient 1 presented with an unresectable lymphatic malformation of the left orbit at the age of 10 months. Sclerotherapy with OK-432 (lyophilized incubation mixture of group A Streptococcus pyogenes of human origin) was ineffective, and the lesion remained stable for many years. At the age of 9 years, rapid progression of the lesion occurred, thus endangering the vision of the left eye. After a 6-month treatment with sirolimus, partial response was achieved and the lesion could be resected completely (Fig. 1a–c). Patient 2 was diagnosed at the age of 13 months with a combined lymphatico-venous malformation of the left orbit with extension into the cheek, palatine, and pharynx. She was refractory to multimodal treatment including prednisone, interferon, propranolol, and bevacizumab. Several life-threatening episodes with enlargement and bleeding of the lesion occurred. Sirolimus was introduced at the age of 7 years leading to partial response and clinical stabilization. The girl is still treated since 53 months. Patient 3 was a newborn girl with KHE (plus KMP) originating from the neck and upper back that was refractory to pretreatment with prednisone, cyclophosphamide, and vincristine. Diagnosis of KHE was confirmed by biopsy. Treatment with sirolimus leads to normalization of KMP after 1 month and complete disappearance of the KHE after 6 months. This patient has been reported recently [13]. Patient 4 presented at birth with bilateral chylous pleural effusions and trisomy 21 after preterm delivery at 34 weeks’ gestation. Treatment with octreotide infusions, parenteral nutrition, and a medium-chain triglyceride-rich diet was ineffective. Computed tomography (CT) scan revealed suspicion of pulmonary lymphangiectasia that was confirmed subsequently by lung biopsy. Treatment with sirolimus was introduced, pleural effusions disappeared 1 month later, and normal feeding could be started. Duration of sirolimus treatment was 3 months. Patient 5 was diagnosed at birth for combined lymphatico-venous malformation (plus KMP) in the left cervical region. MRI revealed a solid and cystic lesion between the left clavicle, larynge, and the cervical spine measuring 4.6 cm in diameter. Treatment with sirolimus leads to normalization of KMP within 1 month and shrinkage of the lesion to less than 1.3 cm within 8 months. Complete remission could be achieved after 19 months of treatment (Fig. 2a, b). Patient 6 presented at birth with a KHE in the left-sided upper abdomen (plus KMP) measuring 7.6 × 6 × 9.1 cm. Treatment with sirolimus was started on the 6th day of life, leading to normalization of KMP within 1 month and shrinkage of the lesion to 2.9 × 2.6 × 3.2 cm after 9 months (Fig. 3a, b). The patient is still under treatment with sirolimus.
Treatment with sirolimus was tolerated very well, mild reversible leukopenia was seen in all patients, and no severe infections occurred during treatment.
Discussion
For many decades, medical treatment of vascular anomalies was focused on the treatment of vascular tumors, namely complicated hemangiomas. The first reports on application of corticosteroids with a response rate of 30 % date back to the late 1960s. Despite controversial publications thereafter, a substantial number of patients did not seem to benefit from this therapy with a suspected inhibitory effect on angiogenesis, but yet not clarified mechanism of action and serious side effects [8, 21, 30]. Treatment of life-threatening hemangiomas and other vascular tumors with cytostatics, such as cyclophosphamide and vincristine, not only yields a variable response but also significant short-term and long-term side effects, especially in children under 18 months of age [6, 16, 18]. Interferon-α is known to suppress angiogenic growth factors’ activity and has been applied successfully in different vascular anomalies, which failed to respond to other therapies [5, 24, 29]. A serious limitation is the danger of irreversible spastic diplegia in infants [27]. A revolution in the conservative management of vascular anomalies was achieved with the application of propranolol in complicated IH [17, 22]. The effectiveness of this non-selective β-blocker is attributed to its complex mechanism of action—vasoconstriction, inhibition of angiogenesis, and induction of apoptosis [17]. Satisfactory therapeutic results along with the minimal side effects turned propranolol into a first-line therapy for IH [11].
In 1996, a novel classification and nomenclature of vascular anomalies was introduced [1, 10, 12], proposing a differentiation between vascular tumors subdivided with regard to their degree of cellular proliferative activity and invasive potential and vascular malformations. As these distinct entities differ in their biologic behavior, different therapeutic approaches are also required. Therefore, accurate diagnosis of vascular anomalies (tumors vs. malformations), often presenting with overlapping clinical features and imaging findings, is essential to the choice of an appropriate therapeutic option [18].
Since 2010, sporadic reports on the use of sirolimus in the management of KHE, a vascular tumor of borderline malignancy, confirm its effectiveness in this entity [2, 10, 14, 23]. In 2011, Hammill et al. reported on five patients with complicated lymphatic malformations and one patient with KHE who were treated with sirolimus after failing multiple other therapies [10]. There are only sporadic further case reports on the use of sirolimus in children with different vascular anomalies, namely KHE and/or lymphatic malformations [13, 15, 23, 28]. In accordance to these data, two patients with KHE and KMP in our series (patients 3 and 6) improved dramatically under sirolimus without experiencing any significant complications. Li Kai et al. reported another six cases with KHE and KMP and refractory to at least two medical therapies, which responded to sirolimus with shrinkage of the tumor mass and resolution of KMP [14]. Uno et al. applied another mTOR inhibitor, everolimus, in a patient with KHE and KMP with satisfactory result and minimal side effects, following unsuccessful therapy with propranolol, prednisolone, and cytostatics [26].
In contrast to vascular tumors, vascular malformations are mainly treated with various interventional procedures to achieve local control, including a broad spectrum ranging from surgical resection to embolization, sclerotherapy, and laser therapy [1, 4, 18]. Nevertheless, some of these lesions are refractory to these methods [18]. Presuming the non-proliferative nature of abnormal growth in vascular malformations, medical treatment with antiproliferative agents is not expected to produce a significant response in these lesions. Surprisingly, antiangiogenetic therapy with sirolimus proved to be effective also in some children with vascular malformations [10, 18, 20, 25] so that, for the first time, a medical treatment of vascular malformations, particularly lymphatic malformations, became available. Four of our six patients were diagnosed with lymphatic malformations, two of them with combined lymphatico-venous lesions. In four of the cases, complete remission could be achieved. The other two demonstrated significant improvement, currently with ongoing therapy. Given that all patients reached serum levels between 5 and 15 ng/ml, no association was seen between serum levels and grade of response. Three of six patients were treated >12 months, and one of them showed slight growth retardation (decline of length from the 50th to the 10th percentile) during treatment period. Although data are currently limited to sporadic case reports and small case series, introduction of mTOR inhibitors in the management of complicated vascular tumors and malformations provides promising results, namely high response rate and a satisfactory safety profile. Our experience supports these clinical data.
However, some questions regarding the preliminary results for successful implementation of mTOR inhibitors in vascular anomalies are a matter of discussion:
-
The exact mechanism of action of mTOR inhibitors in vascular anomalies is still poorly understood; antiproliferative/antiangiogenetic agent is supposed to be of limited value against lesions of low proliferative activity and mainly vascular malformations. It could be speculated that abnormal growth in some malformations is also attributed to angiogenetic factors, thus presenting with features of both congenital malformation and proliferating lesion, therefore a target for sirolimus.
-
As complete remission in some cases has not been achieved during the period of observation (see patients 2 and 6), the duration of sirolimus therapy and timing for additional intervention in children with partial response remain elusive.
Conclusion
Sirolimus emerges as a fascinating new medical treatment option for patients with complicated vascular tumors (KHE) and malformations, particularly of lymphatic origin. Due to our experience with these six patients, we suggest to use sirolimus as first-line treatment in similar cases. Nevertheless, a multidisciplinary approach is still a conditio sine qua non for the successful management of these patients. Further analysis of mTOR inhibitors’ contribution to the therapy of vascular anomalies would benefit from a larger body of systematized scientific data.
Abbreviations
- CT:
-
Computed tomography
- FDA:
-
Food and Drug Administration
- IH:
-
Infantile hemangioma
- ISSVA:
-
International Society for the Study of Vascular Anomalies
- KHE:
-
Kaposiform hemangioendothelioma
- KMP:
-
Kasabach-Merrit phenomenon
- MRI:
-
Magnetic resonance imaging
- mTOR:
-
Mammalian target of rapamycin
- VEGF:
-
Vascular endothelial growth factor
References
Adams DM, Wentzel MS (2008) The role of the hematologist/oncologist in the care of patients with vascular anomalies. Pediatr Clin N Am 55:339–355
Blatt J, Stavas J, Moats-Staats B et al (2010) Treatment of childhood kaposiform hemangioendothelioma with sirolimus. Pediatr Blood Cancer 55:1396–1398
Blei F (2015) Kaposiform hemangioendothelioma: therapeutic efficacy for an enigmatic diagnosis. Pediatr Blood Cancer 62:551–552
Blei F (2012) Medical management of vascular anomalies. Facial Plast Surg 28:575–583
Ezekowitz RA, Mulliken JB, Folkman J (1992) Interferon alfa-2a therapy for life-threatening hemangiomas of infancy. N Engl J Med 326:1456–1463
Fahrtash F, McCahon E, Arbuckle S (2010) Successful treatment of kaposiform hemangioendothelioma and tufted angioma with vincristine. J Pediatr Hematol Oncol 32:506–510
Falger JC, Mueller T, Arbeiter K et al (2006) Conversion from calcineurin inhibitor to sirolimus in pediatric chronic allograft nephropathy. Pediatr Transplant 10:565–569
Fost NC, Esterly NB (1968) Successful treatment of juvenile hemangiomas with prednisone. J Pediatr 72:351–357
Garzon MC, Huang JT, Enjolras O et al (2007) Vascular malformations: part I. J Am Acad Dermatol 56:353–370
Hammill AM, Wentzel MS, Gupta A et al (2011) Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer 57:1018–1024
Holmes WJ, Mishra A, Gorst C et al (2011) Propranolol as first-line treatment for rapidly proliferating infantile haemangiomas. J Plast Reconstr Aesthet Surg 64:445–451
ISSVA Classification of Vascular Anomalies ©(2014) International Society for the Study of Vascular Anomalies. Available at “issva.org/classification”. Accessed April 2014
Jahnel J, Lackner H, Reiterer F et al (2012) Kaposiform hemangioendothelioma with Kasabach. Merritt phenomenon: from vincristine to sirolimus. Klin Padiatr 224:395–397
Kai L, Wang Z, Yao W et al (2014) Sirolimus, a promising treatment for refractory Kaposiform hemangioendothelioma. J Cancer Res Clin Oncol 140:471–476
Kim D, Benjamin L, Wysong A et al (2015) Treatment of complex periorbital venolymphatic malformation in a neonate with a combination therapy of sirolimus and prednisolone. Dermatol Ther Mar 5 (Epub ahead of print)
Lackner H, Urban C, Schwinger W et al (1995) Cyclophosphamide therapy in life-threatening inoperable cystic hygromas. Pediatr Surg Int 10:199–201
Leaute-Labreze C, Dumas de la Roque E, Hubiche T et al (2008) Propranolol for severe hemangiomas of infancy. N Engl J Med 358:2649–2651
Margolin JF, Soni HM, Pimpalwar S (2014) Medical therapy for pediatric vascular anomalies. Semin Plast Surg 28:79–86
Pan WK, Li P, Huang Q (2015) Propranolol induces regression of hemangioma cells via the down-regulation of the PI3K/Akt/eNOS/VEGF pathway. Pediatr Blood Cancer. doi:10.1002/pbc.25453
Reinglas J, Ramphal R, Bromwich M (2011) The successful management of diffuse lymphangiomatosis using sirolimus: a case report. Laryngoscope 121:1851–1854
Sadan N, Wolach B (1996) Treatment of hemangiomas of infants with high doses of prednisone. J Pediatr 128:141–146
Sans V, de la Roque ED, Berge J et al (2009) Propranolol for severe infantile hemangiomas: follow-up report. Pediatrics 124:423–431
Schroeder U, Lauten M, Stichtenoth G et al (2014) Laryngomalacia and complicated, life-threatening mTOR-positive Kaposiform hemangioendothelioma cured by Supraglottoplasty and sirolimus. Klin Padiatr 226:362–368
Timke C, Krause MF, Oppermann HC et al (2007) Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer 48:108–111
Trenor CC (2011) Sirolimus for refractory vascular anomalies. Pediatr Blood Cancer 57:904–905
Uno T, Ito S, Nakazawa A et al (2015) Successful treatment of Kaposiform hemangioendothelioma with everolimus. Pediatr Blood Cancer 62:536–538
Vesikari T, Nuutila A, Cantell K (1988) Neurologic sequelae following interferon therapy of juvenile laryngeal papilloma. Acta Paediatr Scand 77:619–622
Wang Z, Li K, Dong K et al (2013) Successful treatment of Kasabach-Merritt phenomenon arising from Kaposiform hemangioendothelioma by sirolimus. J Pediatr Hematol Oncol 37:72–73
White CW, Wolf SJ, Korones DN et al (1991) Treatment of childhood angiomatous diseases with recombinant interferon alfa-2a. J Pediatr 118:59–66
Zarem HA, Edgerton MT (1967) Induced resolution of cavernous hemangiomas following prednisolone therapy. Plast Reconstr Surg 39:76–83
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in our retrospective report were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Author’s contributions
HL and AK prepared the manuscript and searched the literature. DS, WS, MB, PS, MS, DFR, ES, EH and CU revised the manuscript. All authors contributed in the clinical management of the patients and reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Nadal
Rights and permissions
About this article
Cite this article
Lackner, H., Karastaneva, A., Schwinger, W. et al. Sirolimus for the treatment of children with various complicated vascular anomalies. Eur J Pediatr 174, 1579–1584 (2015). https://doi.org/10.1007/s00431-015-2572-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-015-2572-y