Abstract
Purpose of Review
The purpose of this review is to describe up-to-date diagnosis and management of vascular malformations.
Recent Findings
Traditional treatment modalities include surgery, sclerotherapy, and laser therapy. Landmark research suggests that lymphatic malformations and other vascular malformations are caused by somatic mutations such as PIK3CA, and may be treated successfully medically.
Summary
Therapy targeting the phosphatidylinositide-3-kinase/protein kinase B/mechanistic target of rapamycin signaling pathway (mTOR) may be helpful in treating some vascular anomalies. Multimodal therapy such as intralesional injection of n-butyl cyanoacrylate followed by surgical resection may be successful in treating other lesions. In carefully selected cases in which fetal imaging detects vascular anomalies, an ex utero intrapartum treatment (EXIT) may be beneficial. Multidisciplinary collaboration is key to the successful management of patients with vascular malformations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular malformations are a spectrum of head and neck lesions within the broader category of vascular anomalies which also includes vascular tumors with the most common of those being infantile hemangioma (Table 1 [1•). Vascular malformations occur in 4.5% of children and have an equal gender distribution [2]. The most common vascular malformations are venous malformations (40%), followed by capillary malformations (32%), combined low-flow malformations (15%), lymphatic malformations (12%), and arteriovenous malformations (3%) [4]. Accurate diagnosis helps guide caregiver education and treatment and depends on clinical presentation, lesion growth rate, radiologic characteristics, and pathophysiology. The optimal treatment of patients with vascular malformations requires multidisciplinary collaboration among specialists in otolaryngology, interventional radiology, hematology oncology, dermatology, pediatrics, and plastic surgery.
Presentation
Clinical history and physical exam are the most important components in determining the type of vascular malformation. Imaging can also be helpful with magnetic resonance imaging with gadolinium as the study of choice, but the benefits must be balanced with the risks of sedation. Biopsy is less commonly needed for accurate diagnosis. Accurate diagnosis depends on the context of presentation and growth pattern. Was the lesion present at birth or did it present in infancy? Did the lesion manifest in the setting of an acute infection or trauma? Did the lesion rapidly child and slowly stop growing or did it grow with the child? Did the lesion change in color or have associated ulceration or bleeding?
Vascular malformations are disorders of abnormal vascular morphogenesis. They may fail to regress, may grow with the child, and have normal endothelial mitotic activity [5]. Vascular malformations may have components from the capillary, venous, arterial, or lymphatic systems. In contrast, benign vascular tumors such as infantile hemangiomas exhibit cellular proliferation and typically rapidly enlarge and then slowly regress. Unlike infantile hemangiomas, vascular malformations are not positive for GLUT-1 on immunohistochemistry. Vascular malformations are further categorized into low-flow and high-flow lesions based on blood vessel type and radiographic appearance. Low-flow lesions are capillary, venular, venous, or lymphatic malformations while high-flow lesions are arteriovenous malformations or fistulae [6].
Capillary Malformations
Capillary malformations occur in 0.3% of children. These skin lesions are often red or purple and are made up of dilated channels of abnormal capillaries. They are present at birth. Although a genetic cause of isolated capillary malformations is not clear, RASA1 mutations have been linked to capillary malformation-arteriovenous malformation [7].
Sturge-Weber syndrome (SWS) is associated with capillary malformations or port wine stains in the distribution of the ophthalmic (V1) and maxillary (V2) divisions of the trigeminal nerve, leptomeningeal angiomatosis, and choroid angioma. SWS is the most common syndrome associated with capillary malformations (CMs) [8]. A somatic mutation in the GNAQ gene is likely the cause of SWS [9]. Patients with signs of SWS should undergo MRI of the brain and consultation with ophthalmology and neurology as they may present with seizures, mental retardation, and glaucoma [8].
While surgery is reserved for lesions that are more advanced, flash pulse dye laser (FPDL) and potassium-titanyl-phosphate (KTP) lasers are typically used to treat flat capillary malformations [8].
Venous Malformations
Venous malformations are the most common vascular malformation and are generally present at birth and grow proportionally with the child. Deep lesions may not be diagnosed until rapid expansion due to triggers such as infection, trauma, or hormonal changes of puberty and pregnancy. Lesions overlying the skin or mucosa often appear blue or purple. Vascular malformations (VMs) are compressible and can develop tender phleboliths. These lesions can fluctuate in size with blood pressure changes, dependent positioning, or with a Valsalva maneuver.
Plain X-rays can be useful to diagnose phleboliths. MRI with gadolinium is the imaging study of choice. Abnormal venous morphogenesis creates a low-flow venous malformation system that can lead to thrombosis or local intravascular coagulopathy (LIC).
VMs are more commonly sporadic and unifocal. They are also associated with autosomal dominant genetic anomalies such as glomuvenous malformations (GVM) and multiple cutaneous and mucosal venous malformations (VMCM) GVM is associated with loss-of-function mutations in glomulin while VMCM is associated with mutations in the receptor tyrosine kinase TIE2 (or TEK) [10]. Blue rubber bleb nevus syndrome (BRBNS) or “Bean syndrome” is associated with rubbery, compressible mucocutaneous blue blebs as well as visceral and gastrointestinal tract VMs. BRBNS or “Bean syndrome” is associated with a somatic mutation in the TEK gene [11]. VMs are also seen in Klippel-Trenaunay syndrome (KTS). KTS consists of a combination of multiple lymphatic, venous, and capillary malformations as well as soft tissue or skeletal hypertrophy of the affected site [12]. The categories of venous malformations are summarized with causal genes in Table 2.
Patients with multiple venous malformations, large-volume (> 10cm2) VMs, phleboliths, or KTS need an extensive preoperative work-up as they can develop severe coagulopathies [13]. Testing for LIC with pro-thrombin time, activated partial thromboplastin time, fibrinogen levels, D-dimer, and complete blood count should be performed in patients with these risk factors. Elevated D-dimer levels are highly specific for LIC [14, 15].
Multiple modalities are used to treat venous malformations (Table 3). Small, asymptomatic VMs can be observed while superficial, well-circumscribed, symptomatic lesions can be treated surgically or with Nd:YAG laser therapy [10]. A combination of glue (n-butyl cyanoacrylate) embolization immediately followed by surgical resection has shown promising results in appropriately selected cases [16]. More extensive lesions can be reduced with sclerotherapy using agents such as ethanol, bleomycin, doxycycline, and picinabil (OK-432) or a combined approach with sclerotherapy and surgery. Patients with VMs and elevated D-dimers (≥ 5 times normal levels) may benefit from low-dose aspirin or low molecular weight heparin (LMWH) to prevent further formation of phleboliths [15]. Systemic treatment options such as rapamycin are currently under investigation.
Lymphatic Malformations
Lymphatic malformations are an abnormal collection of low-flow lymphatic channels occurring in 1 out of 500 to 4000 live births. Microcystic components and the presence of mucosal disease are associated with worse prognosis. Lymphatic malformations (LMs) can be associated with syndromes such as Noonan’s and Klippel-Trenaunay [3, 17]. MRI is often useful to evaluate the extent of the lesion and to distinguish between LMs and VMs with VMs demonstrating hyperintensity on T2 sequences or greater enhancement with gadolinium than LMs.
Lesions are classified as microcystic if they are less than 2 cm in diameter, macrocystic if they are greater than 2 cm in diameter, or mixed if components of both exist [3]. The de Serres proposal for staging lymphatic malformations divides LMs into 5 stages to help determine prognosis and response to treatment: stage 1, lesions that are unilateral infrahyoid; stage 2, unilateral suprahyoid; stage 3, unilateral suprahyoid and infrahyoid; stage 4, bilateral suprahyoid; and stage 5, bilateral suprahyoid and infrahyoid [18•].
In addition to improving symptoms such as pain and swelling, treatment objectives include restoring or maintaining functional and aesthetic appearance. Conservative therapy includes treating superimposed infections with antibiotics and steroids, therapies to address lymphatic blebs which may be associated with intermittent bleeding and decreased quality of life, and optimizing function including breathing and swallowing. Sclerotherapy has been used to treat macrocystic LMs with small risks of cranial nerve injury, skin necrosis, skin discoloration, chronic pain, and edema as well as agent-specific complications. Doxycycline can cause electrolyte abnormalities and discolored teeth in young patients. OK-432 is a sclerosant mixture of group A Streptococcus pyogenes and benzylpenicillin, which is currently not FDA approved in the USA. It has the potential for shock-like symptoms in those with penicillin allergies. Bleomycin use can cause pulmonary fibrosis and interstitial pneumonia and it has dose limitations. The use of absolute ethanol is falling out of favor as it can cause respiratory depression, arrhythmias, seizures, and rhabdomyolysis [17].
Treatment with sirolimus to block the PIK3CA pathway has emerged as a promising medical therapy especially for microcystic disease traditionally more resistant to therapies. There are ongoing clinical trials and further investigation is necessary to determine optimal treatment duration. Sirolimus therapy also requires ongoing laboratory surveillance of liver function.
Arteriovenous Malformations
Arteriovenous malformations have anomalous connections between the venous and arterial system. In contrast, arteriovenous fistulas (AVFs) cause direct shunting of blood. AVMs can have a quiescent stage but they can also undergo expansion due to a variety of triggers such has puberty. Arteriovenous malformations (AVMs) can be diffusely infiltrative and destructive, and have a high recurrence rate, especially when there are multiple arterial feeders involved [2].The skin overlying an AVM can be pulsatile and warm with a palpable thrill and a slight blush. Skin changes of infiltrative lesions include ulceration and bleeding. AVMs can also show rapid growth with hormonal changes such as puberty [2].
MRI can be helpful to evaluate the extent of the lesion and demonstrates characteristic arterial flow voids. Angiography can determine the feeding vessels and also help guide treatment [2]. AVM treatment consists of embolization and/or surgical resection. Embolization with agents such as ethanol, polyvinyl alcohol, coils, and Onyx can lead to complications such as ulceration, soft tissue necrosis, and nerve injury. Often, preoperative embolization is done 24 to 48 h prior to surgical resection to help prevent blood loss [2, 8]. Diffuse AVMs have a high recurrence rate and are unfortunately often debilitating.
Current Research
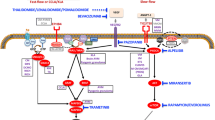
Recent research suggests that LMs and other vascular malformations are caused by somatic mutations such as PIK3CA. PIK3CA encodes the catalytic subunit of phosphatidylinositol 3-kinase which can enhance tumor growth in association with other mutations. Five different Pik3CA mutations (p.C420R, p.E542K, p.E545K, p.H1047R, and p.H1047L) were found to commonly occur in patients with isolated LMs as well as LM as part of a syndrome, such as Klippel-Trenaunay syndrome (KTS), fibro-adipose vascular anomaly (FAVA), congenital lipomatous overgrowth with vascular, epidermal, and skeletal anomalies syndrome (CLOVES) [19•].
The PIK3CA signaling pathway (PI3K/AKT/mTOR) or the phosphatidylinositide-3-kinase/protein kinase B/mechanistic target of rapamycin signaling pathway plays a role in cell proliferation and growth [20]. In theory, therapy targeting the PI3K/AKT/mTOR may reduce complications from vascular malformations with somatic activating mutations in that pathway. For instance, COX-2 inhibitors such as aspirin are thought to inhibit AKT and inhibitors of mTOR including sirolimus (rapamycin) and everolimus are available and have an established safety profile [20]. Several case reports have suggested that sirolimus has been safe and effective in treating patients with vascular anomalies but larger controlled studies are needed [21, 22] as the natural course of some of these lesions may be to get smaller with time.
While exclusive medical therapy may be the future, current treatment options often involve multimodal therapy. For instance, intralesional injection of n-butyl cyanoacrylate (n-BCA) followed by surgical resection may be used as a single-stage modality to treat venous malformations. Direct fluoroscopic guidance of n-BCA for percutaneous embolization into the venous channels results in an acute inflammatory reaction and a demarcation line between the VM and healthy tissue. In a case series, this technique, with selective motor nerve monitoring, has facilitated the safe and complete removal of venous malformations with limited nerve dissection, and maximal tissue and functional preservation [16].
Finally, recent advances in prenatal MRI and fetal ultrasound have allowed EXIT of patients who are found to have vascular malformations involving the airway detected on fetal imaging. In select cases, the EXIT procedure can allow for a controlled, partial fetal delivery to allocate time to establish the fetal airway, while fetal oxygenation is maintained through utero-placental circulation [23, 24]. Figure 1 shows intrapartum and postpartum images of a patient with a large lymphatic malformation that was delivered via the EXIT procedure.
A patient with a lymphatic malformation diagnosed on fetal imaging. a MRI imaging of the infiltrative hyperintense T2/hypointense T1, heterogeneously enhancing mass involving the dorsal paraspinal soft tissues, the right posterior cervical space, carotid space, parotid space, supratemporal and infratemporal masticator space, and parapharyngeal and retropharyngeal spaces with associated mass effect on the oropharyngeal airway. b An ultrasound probe being used to confirm the location of the endotracheal tube during the EXIT procedure. c A laryngoscopy showing involvement of the hypopharyngeal wall and aryepiglottic fold with LM. d The patient at 6 weeks of age. The lymphatic malformation got smaller in subsequent visits
Conclusions
The diagnosis and treatment of patients with vascular malformations requires multidisciplinary collaboration. Diagnosis can often be made clinically with MRI with gadolinium as the radiologic study of choice. Treatment involves education of the natural history of the lesion and often “active observation” may be the best initial treatment if a child has minimal functional or cosmetic impairment. New knowledge on the genetics of these conditions suggests promise for medical therapies for lesions resistant to more traditional therapies.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
• Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136(1):e203–14. ISSVA classification schemes.
Hoff SR, Rastatter JC, Richter GT. Head and neck vascular lesions. Otolaryngol Clin N Am. 2015;48(1):29–45.
Manning SC, Perkins J. Lymphatic malformations. Curr Opin Otolaryngol Head Neck Surg. 2013;21(6):571–5.
Fraulin FO, Flannigan RK, Sharma VK, McPhalen DF, Harrop RA. The epidemiological profile of the Vascular Birthmark Clinic at the Alberta Children’s Hospital. Can J Plast Surg. 2012;20(2):67–70.
Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982 Mar;69(3):412–22.
Patel NA. Vascular anomalies. In: Moubayed SP, Mourad M, editors. Facial plastic and reconstructive surgery: high yield review. New Delhi: Jaypee Brothers Medical Publishers; 2017. Chapter 5.9.
Eerola I, Boon LM, Mulliken JB, Burrows PE, Dompmartin A, Watanabe S, et al. Capillary malformation–arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73(6):1240–9.
Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr. 2012;2012:645678.
Sundaram SK, Michelhaugh SK, Klinger NV, Kupsky WJ, Sood S, Chugani HT, et al. GNAQ mutation in the venous vascular malformation and underlying brain tissue in Sturge-Weber syndrome. Neuropediatrics. 2017;48(5):385–87.
Amato MV, Patel NA, Hu S, Pantelides H. Sporadic multifocal venous malformations of the head and neck. Case Rep Otolaryngol. 2015;2015:508149.
Soblet J, Kangas J, Natynki M, Mendola A, Helaers R, Uebelhoer M, et al. Blue rubber bleb nevus (BRBN) syndrome is caused by somatic TEK (TIE2) mutations. J Invest Dermatol. 2017;137(1):207–16.
Marler JJ, Mulliken JB. Current management of hemangiomas and vascular malformations. Clin Plast Surg. 2005;32(1):99–116. ix
Hung JW, Leung MW, Liu CS, Fung DH, Poon WL, Yam FS, et al. Venous malformation and localized intravascular coagulopathy in children. Eur J Pediatr Surg. 2017;27(2):181–4.
Dompmartin A, Ballieux F, Thibon P, Lequerrec A, Hermans C, Clapuyt P, et al. Elevated D-dimer level in the differential diagnosis of venous malformations. Arch Dermatol. 2009;145(11):1239–44.
Zhuo KY, Russell S, Wargon O, Adams S. Localised intravascular coagulation complicating venous malformations in children: associations and therapeutic options. J Paediatr Child Health. 2017;53(8):737–41.
Tieu DD, Ghodke BV, Vo NJ, Perkins JA. Single-stage excision of localized head and neck venous malformations using preoperative glue embolization. Otolaryngol Head Neck Surg. 2013;148(4):678–84.
Perkins JA, Manning SC, Tempero RM, Cunningham MJ, Edmonds JL Jr, Hoffer FA, et al. Lymphatic malformations: review of current treatment. Otolaryngol Head Neck Surg. 2010;142(6):795–803. e1
• de Serres LM, Sie KC, Richardson MA. Lymphatic malformations of the head and neck. A proposal for staging. Arch Otolaryngol Head Neck Surg. 1995;121(5):577–82. Staging system for lymphatic malformations that enabled more accurate outcomes studies.
• Luks VL, Kamitaki N, Vivero MP, Uller W, Rab R, Bovee JV, et al. Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. J Pediatr. 2015;166(4):1048–54 e1-5. PIK3CA somatic mutations cause many vascular malformations and this has an impact on treatment.
Keppler-Noreuil KM, Parker VE, Darling TN, Martinez-Agosto JA. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies. Am J Med Genet C Semin Med Genet. 2016;172(4):402–21.
Hammill AM, Wentzel M, Gupta A, Nelson S, Lucky A, Elluru R, et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer. 2011;57(6):1018–24.
Adams DM, Trenor CC 3rd, Hammill AM, Vinks AA, Patel MN, Chaudry G, et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. 2016;137(2):e20153257.
Dighe MK, Peterson SE, Dubinsky TJ, Perkins J, Cheng E. EXIT procedure: technique and indications with prenatal imaging parameters for assessment of airway patency. Radiographics. 2011;31(2):511–26.
Stefini S, Bazzana T, Smussi C, Piccioni M, Frusca T, Taddei F, et al. EXIT (Ex utero Intrapartum Treatment) in lymphatic malformations of the head and neck: discussion of three cases and proposal of an EXIT-TTP (Team Time Procedure) list. Int J Pediatr Otorhinolaryngol. 2012;76(1):20–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pediatric Otolaryngology
Rights and permissions
About this article
Cite this article
Patel, N.A., Perkins, J.A. & Bly, R.A. Vascular Malformations. Curr Otorhinolaryngol Rep 5, 245–250 (2017). https://doi.org/10.1007/s40136-017-0174-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-017-0174-0