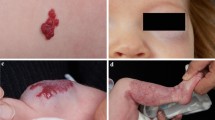
Abstract
With a prevalence of 2.6–4.5 %, infantile haemangiomas (IH) represent the most common tumour of infancy. While the majority of IH does not require therapy and regresses spontaneously, about 10 % of IH exhibit complications such as obstruction, ulceration or disfigurement. With the advent of oral propranolol, many conventional treatment options have become obsolete. This paper summarizes current recommendations for management of complicated IH. These recommendations have been written by an expert group after a consensus process including bibliographic review, several drafts of synthesis, meetings with quantitative voting system and redaction of an approved final manuscript.
Conclusion: Oral propranolol is the first-line agent for the treatment of complicated IH.
What is Known: • Infantile haemangiomas (IH) are the most common tumours of infancy. Within a very short period after its discovery and long before the publication of randomized controlled trials, propranolol has become the number one agent for the treatment of complicated IH. |
What is New: • We report IH treatment recommendations of an international, interdisciplinary team of experts, based on an up-to-date review of the literature. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infantile haemangiomas (IH) represent the most common tumours of infancy, affecting between 2.6 and 4.5 % of all infants [15, 42, 49]. Most haemangiomas are small and regress spontaneously without any need for intervention; however, 5–10 % of haemangiomas, often site dependent, can cause serious complications and require treatment. We review the pathogenesis, clinical course and complications of IH and give recommendations for treatment of complicated haemangiomas based on published evidence and the consensus agreement of a group of European experts.
Methods
Consensus was reached by a modified Delphi procedure where the voting process permits the expression of votes in a quantitative and qualitative way. After a thorough bibliographical review, statements on relevant topics were generated and circulated to all group members prior to a meeting in Castres/France on March 13/14, 2014, where all controversial issues were extensively discussed. The end result of all this is a definitive text reflecting the issues addressed in a manner consistent with the practice of evidence-based medicine. This final write-up also includes the opinions, experiences and convictions of participating experts (see Appendix 1—Final statements with agreement rates). The consensus paper was then prepared and again circulated to all members for revision and final approvement. Propranolol has meanwhile been approved by the FDA and EMA for the treatment of infantile haemangiomas, based on the results of a large confirmatory randomized controlled trial [40].
Risk factors and pathogenesis
The main risk factors for the development of IH are female sex, low birth weight and prematurity [15, 19, 25, 49]. Girls are affected 2.3–2.9 times more often than boys. Low birth weight seems to be the most significant risk factor: for every 500-g decrease in birth weight, the risk of IH increases by 40 % [15, 16]. Prospective studies [25, 49] have also identified multiple gestation, increased maternal age, in vitro fertilization, pre-eclampsia and placental anomalies (e.g. placenta previa, placental abruption, abnormal insertion of umbilical cord) as cofactors. A positive family history in a first-degree relative increases the risk of IH formation in an infant twofold [16].
The pathogenesis of IH is still incompletely understood [35]. Currently, three hypotheses (which are not mutually exclusive) are favoured:
-
1.
Somatic mutation of haemangioma stem cells and upregulation of VEGFR-signalling—Recent studies suggest that IH are derived from CD34+/CD133+endothelial progenitor cells (EPC, also referred to as haemangioma stem cells, HemSC) [12, 23]; they are capable of differentiating into GLUT1+ endothelial cells, and during regression of IH into adipocytes [23]. Mediators of EPC and vasculogenesis (e.g. vascular endothelial growth factor VEGF-A and hypoxia-inducible factor HIF-1α) are elevated in children with IH [35, 36].
-
2.
Placental theory—The histochemical markers characteristic of IH (GLUT1, Lewis Y, FcRIII, merosin) are similar to those expressed by placental tissue [12], which has led to the hypothesis that IH are derived from embolized placental cells [48]. In fact, the evolution of IH and placentae—rapid proliferation followed by gradual involution—are similar. In accordance with this hypothesis, cases of neonatal haemangiomatosis associated with placental haemangioma (chorangioma) have been reported [29].
-
3.
Hypoxia-induced proliferation—Tissue hypoxia may be a stimulus for vascular proliferation. Conditions associated with hypoxia (preterm delivery, placental anomalies, low birth weight, increased maternal age) are prevalent cofactors for the formation of IH. In posterior fossa-haemangioma-arterial-cardiac-eye anomaly (PHACE) syndrome, absence or stenosis of cerebral vessels correlates with ipsilateral segmental haemangiomas [17, 35]. Induction and proliferation of EPC are mediated by HIF-1α.
Clinical course and complications
IH exhibit a characteristic non-linear growth pattern: they usually manifest during the first or second week of life, but not later than 12 weeks of age [49]; precursor lesions are observed at birth in up to 65 % [57]. The majority of growth occurs before 12 weeks of age [57]; growth velocity decreases thereafter and usually comes to a halt between 4 and 6 months. Segmental haemangiomas and large focal haemangiomas (mainly deep, particularly in the parotid area) can exhibit an extended growth phase during the second and third year of life [8].
Eighty-five to 90 % of IH undergo spontaneous involution between the 2nd and 6th (and sometimes up to the 10th) year of life [15, 42]. Between 5 and 10 % of all infantile haemangiomas develop complications requiring therapy, with some studies quoting as high as 20 % [24]. Typical complications are ulceration, functional impairment (sometimes life threatening) and (imminent or actual) disfigurement.
-
1.
Ulceration—Ulceration is the most common complication of IH, affecting up to 10 % of all IH, and 15–25 % of patients with IH referred for specialist care [10, 27]. Predilection sites are the lower lip, neck and the anogenital area. Large, segmental and superficial IH are more often affected than small, focal or deep IH. Ulceration occurs at a median age of 4 months during the proliferation phase and can cause bleeding and/or infection, but pain is the most significant associated complaint.
-
2.
Functional impairment—Periocular haemangiomas can compromise vision and cause amblyopia, astigmatism or strabism. Nasal haemangiomas can impair breathing. Airway involvement, often heralded by IH around the “beard area”, can lead to life-threatening subglottic obstruction. Lip haemangiomas can affect oral feeding. Bulky neck haemangiomas can affect neck mobility and lead to positional torticollis. Children with multiple cutaneous IH (defined as either >5 [34] or ≥10 [59] IH) are at increased risk for hepatic haemangiomas and should thus be screened by ultrasound. In rare cases, multifocal hepatic IH and large IH can induce high-output congestive heart failure [53] and/or thyroid dysfunction [58].
-
3.
Disfigurement—Disfigurement can result from haemangiomas located in the central areas of the face, particularly nose, lips, forehead and cheek, and on the ears. Nasal and lip haemangiomas are known to exhibit slow and frequently incomplete regression. IH of the parotid area, often large, tend to persist longer than other IH. Around 50 % of IH will end up with sequelae such as scars, redundant/anetodermic skin or telangiectasia [5, 12].
Complimentary investigations and differential diagnosis
IH is usually diagnosed clinically. For large subcutaneous haemangiomas, especially when located in the parotid, supraglottic or paratrachealregion, ultrasound with Doppler sonography is recommended and MRI may be required for some IH. The latter should also be performed in the presence of multiple cutaneous IH to rule out hepatic haemangiomas. Echocardiography is advisable in children with large haemangiomas due to their increased risk to develop high-output cardiac failure. These children and those with intrahepatic haemangiomas should also be screened for hypothyroidism (TSH). Segmental haemangioma of the face/neck region is frequently associated with cerebrovascular and cardiac anomalies (with or without other features of the PHACE syndrome) and should be screened by MRI angiography of the head and neck and by echocardiography, ideally prior to the start of propranolol therapy.
Most infantile haemangiomas can be distinguished easily from vascular malformations according to three essential criteria (Table 1). The differential diagnosis of other vascular tumours presenting in infancy has recently been reviewed [13, 30].
Management
Indications for treatment
Since IH are essentially benign tumours and tend to regress spontaneously as of the second year of life, treatment is not required in the majority of cases. On the other hand, all obstructive and ulcerated IH require immediate therapy (Table 2). Large haemangiomas may also require therapy in case of imminent high-output cardiac failure.
Small haemangiomas close to the eye may or may not become obstructive depending on their individual growth dynamics. The same holds true for haemangiomas with a “risk of disfigurement”, e.g. all IH located on the face, or larger IH located in the peri-mammary area in girls. In these cases, “watchful waiting” or “active non-intervention” is an option, provided that close monitoring is adopted (Table 3). Monitoring intervals need to be adjusted to the age of the child, which is inversely related to the growth velocity of IH. The recommended interval for follow-up of haemangiomas “at risk” with respect to age can thus be expressed by the following formula:
It is recommended to take photographs in order to document the state of the haemangioma at each follow-up visit.
Treatment options
With the advent of oral propranolol therapy for IH, many “classical” therapeutic options have become obsolete. This refers to topical, systemic and surgical procedures sometimes used for decades.
Topical treatment options (Table 4)
The most promising potentially useful topical treatment option is the use of topical beta blockers. Several case reports and case series have claimed efficacy of propranolol or timolol; however, so far, only one randomized controlled trial (RCT) comparing timolol (n = 15 patients) and placebo (n = 17) has been published [11]. No commercial topical preparation for use in infantile haemangiomas is available yet. Currently, used topical preparations of beta blockers are not standardized. Serum drug levels or potential signs of systemic resorption (bradycardia, hypotension) have not been studied systemically so far in infants. Transcutaneous resorption can lead to unexpected systemic effects; this risk is enhanced when timolol is used close to the eye where transconjunctival absorption is an issue [46]. The risk of transcutaneous absorption is also enhanced in intertriginous and/or ulcerated areas. While oral drugs undergo a “first-pass effect”, i.e. hepatic detoxification, which leads to a significant decrease of serum levels, topically applied drugs circumvent this effect, which can result in increased serum levels. As long as safety and efficacy have not been studied more systematically, topical beta blockers cannot be recommended as a standard of therapy. They have, on the other hand, the potential to become the first-line agent for the treatment of small and superficial IH located in “problematic” regions, thus obviating systemic treatment in a significant minority of children with IH.
Surgical treatment options (Table 5)
For many years, surgical treatment options were considered essential for the treatment of complicated IH. There are no prospective studies comparing laser surgery or cryosurgery and propranolol; retrospective studies however indicate that oral propranolol is more effective than both [37]. Cryosurgery might be indicated in selected cases, but the evidence to support this is purely empirical. Conventional surgery is indicated for the rare haemangioma resistant to propranolol therapy and may occasionally be indicated in an emergency situation but still has an important role in reconstruction post-resolution to excise redundant skin and residual scarring. Pulsed dye laser (595 nm) and intense pulsed light (IPL) can be used for the treatment of persistent telangiectasias; in some cases, additional treatment with the Nd:YAG laser may be required.
Systemic treatment options (Table 6)
Retrospective studies comparing oral propranolol and corticosteroid treatment of IH have shown that propranolol therapy was more effective, resulting in fewer surgical interventions and demonstrating better tolerance, with minimal adverse effects [52]. Two small RCTs comparing propranolol and prednisolone therapy have been published so far; both involved 10 or less patients per study arm. In the first study [43], propranolol had a more rapid therapeutic effect compared to prednisolone and the combination of both did not result in higher efficacy. In the other study [6], both medications were equally effective: while prednisolone had a faster response rate, propranolol was significantly better tolerated.
Oral corticosteroids could still be considered for patients with complicated IH who do not respond to propranolol or those who exhibit primary contraindications or develop side effects. A combination of low dose corticosteroids and propranolol has been proposed as a potentially safer approach to treatment for segmental haemangiomas in patients with PHACES syndrome with cerebral vascular involvement [22]. There is however a theoretical concern that the combination of oral corticosteroids and propranolol might increase the risk of hypoglycaemia, particularly following discontinuation of corticosteroids, because adrenal suppression may inhibit counter-regulatory cortisol release [9].
Sirolimus, an inhibitor of the mammalian target of rapamycin (mTOR), has been demonstrated to have potent anti-angiogenic activity both in vitro and in kaposiform haemangioendothelioma. Case reports indicate that it might be helpful in complicated IH as well [38], but RCTs demonstrating safety and efficacy are needed before any recommendations for the role of sirolimus in IH can be given.
Oral propranolol
For the treatment of hypertension and other indications, e.g. hypertrophic cardiomyopathy, hyperthyroidism or migraine, propranolol had been used in infants and children at doses up to 6–8 mg/kg/day since 1964 before the discovery of its effects on haemangioma [20]. Since the serendipitous discovery of the effect of propranolol on proliferating IH in 2008, nearly 500 case reports and case series have been published involving more than 2000 patients. So far, two RCTs [32, 40] have been published. As demonstrated in these RCTs, in large case series [28, 56] and in a meta-analysis of 1264 reported cases [44], the response rate of oral propranolol at a dose of 2–3 mg/kg/day and after a mean of 6 months of therapy is 96–98 %; side effects are reversible and in the majority of cases benign (Table 7). Sleep disturbance, somnolence and irritability can be observed in 15–25 % of all infants. The degree of lipophily of beta blockers correlates with their ability to cross the blood-brain barrier [45]. As propranolol is a highly lipophilic betablocker, there are theoretical concerns regarding potentially relevant neurodevelopmental or cognitive side effects of propranolol [39].
Due to its impressive efficacy and safety profile, propranolol has become the first-line therapy for IH worldwide and in a remarkably short course of time. This is certainly attributable in part to the fact that unlike “new” drugs, propranolol, which was introduced to the market as an anti-hypertensive agent 40 years ago, is readily available as a generic drug. Its widespread use without a formal RCT or a formal “market launch” has lead to some variability and uncertainty among practicing paediatricians, dermatologists and general practitioners concerning pretreatment investigations, dosing and monitoring.
Pretreatment evaluation and contraindications
Prescribers should be experienced in evaluating young children. Treatment should be initiated only in clinical settings equipped and qualified for the safe and immediate management of any adverse event, in particular cardiovascular events. Before the start of propranolol therapy, infants need to be checked for contraindications to therapy (Table 8). A pretreatment ECG is required when history and/or clinical examination (bradycardia [see Table 8], arrhythmia) raise any concern about rhythm anomalies. In these cases, consultation with a paediatric cardiologist is mandatory.
Since all other possible cardiac contraindications can be detected by history, physical examination, a routine echocardiography, is not essential unless there is a specific clinical indication [7, 18]. Obviously, baseline values are required for heart rate and blood pressure. Baseline glucose levels are only required in preterm or small for date infants, in infants with failure to thrive and those with a history of hypoglycaemic episodes.
Initiation of therapy and dosage
Dose escalation is recommended with propranolol as the individual cardiovascular response of infants cannot be anticipated; the initial cardiac response to β-blockade can be pronounced [18].
In order to ascertain optimal cardiovascular monitoring during the initiation of propranolol therapy, hospital admission is recommended at least for the most sensitive population of infants:
-
1.
All infants ≤2 months of age (age corrected for prematurity) should be admitted for monitoring HR and BP at baseline and after 1 and 2 h [4, 18, 56]. No consensus could be reached whether these precautions should also be extended to older infants (up to the age of 3 months) as well. Older infants might as well be admitted to a day clinic where HR and BP are controlled at baseline and after 1 and 2 h following every dose increase [4, 56].
-
2.
Babies weighing less than 3.5 kg should be admitted and monitored during dose escalation. Premature babies in particular are at increased risk for bradycardia and hypotension when treated with propranolol [19].
-
3.
All infants at immediate risk of life-threatening (subglottic) haemangioma [18].
-
4.
Children with significant comorbidity affecting the cardiovascular system, the respiratory system or the blood glucose maintenance [18].
-
5.
Children with inadequate social support [18].
US authors, based on a small retrospective study [41], concluded that hospitalization might not be necessary at all for the induction of propranolol therapy. Indications and duration of hospital admissions depend on national regulations and practice habits. US recommendations are certainly due to a much stricter admission policy in general.
Mild, clinically asymptomatic reductions (by about 5–8 mmHg) of mean systolic and diastolic BP, respectively, are a common finding during the first 3 days of propranolol therapy in infants, while follow-up studies have shown that there is no further reduction of BP during the remainder of the 6-months treatment period [26, 54].
In older childen, therapy can be initiated on an outpatient basis. Dosage should be increased once a week (starting with 1.0 mg/kg/day) until the final dose of 2.0 (or 3.0) mg/kg/day is reached. Baseline values for heart rate and blood pressure should be assessed before and 1 and 2 h after each dose increase; the same is recommended after interruption of therapy.
For inpatients, therapy should be started at a dose of 1.0 mg/kg/day, with cardiovascular assessment before and 1 and 2 h after the prescribed dose is administered. If well tolerated, the propranolol dose can be increased to 2 mg/kg/day the next day if the child remains in hospital. For outpatients, the dose is increased once weekly. In case of bradycardia or hypotension (see Table 8 for age-related reference values), dose increases should be postponed or reduced.
Hypoglycaemia and hypoglycaemic seizures are among the most serious risk factors of propranolol therapy [9, 18, 33, 54]. Routine screening of serum glucose is however not indicated because the timing of reported hypoglycaemic events was variable and unpredictable [18, 54]. Care should be taken to prevent hypoglycaemia by strictly administering propranolol during or after routine feeding. Propranolol therapy should be interrupted in case of poor oral intake, vomiting, pronounced diarrhoea or during episodes of obstructive bronchitis/bronchiolitis.
The standard dose in the majority of studies reported to date is 2 mg/kg/day [3, 4, 7, 26, 28, 32, 44, 50, 54, 56] with a range of dosing between 0.5 and 3.0 mg/kg. As demonstrated in the multicenter study [40], 3 mg/kg/day is superior to 1 mg/kg/day, but studies comparing 2 and 3 mg/kg/day have not been reported yet. Since both regimes, 2 and 3 mg/kg/day [40], have documented efficacy and safety, the recommended dose of propranolol is 2–3 mg/kg/day.
Another dose-related issue is the dosing interval. Again, in the majority of studies, three equal doses were applied [4, 7, 18, 26, 28, 32, 44, 50, 54, 56] as opposed to two in the multicenter study [40]. Mean serum levels are about 12 % higher (67.1 vs. 60.1 ng/ml, range 24–127 and 26–140 ng/ml, respectively) with two instead of three doses [14]. For the purpose of daily life practicability, two doses (given at least 9 h apart) are preferential.
Duration of therapy and ongoing monitoring
Six months is considered the standard duration of therapy [4, 7, 18, 28, 32, 40, 44, 50]. Our experience suggests that in some children treatment might be necessary for a longer time, i.e. up to 12 months or even longer. While the relapse rate is around 17–20 % after 6 months of propranolol therapy [2, 3], courses of 12 months of treatment have been associated with a significantly lower rate of relapse (5 %) [21]. It is recommended to check the heart rate and to adjust the dose to the increasing body weight every 4 weeks (Table 9). Interruptions of therapy are mandatory during episodes of obstructive bronchitis for as long as symptoms persist and/or beta-mimetic inhalations are required. Serious event such as cardio-respiratory arrest following bronchiolitis in one infant treated with propranolol has been described [55]. Propranolol treatment should be interrupted in case of poor oral intake and intercurrent obstructive bronchitis.
At the end of therapy, some authors suggest tapering the dose for 2 weeks before discontinuation [4], this is however not universally accepted [18]. It is advisable to take photographs of the haemangioma and perform follow-up examinations 3 and 6 months after discontinuation of therapy in order to check for relapse.
Special precautions in children with PHACE syndrome
Many if not most segmental haemangiomas of the face and/or neck are disfiguring; some also cause occlusion of the eye or life-threatening paratracheal compression; they also frequently tend to ulcerate. Children with PHACE syndrome are at risk of having associated cardiovascular anomalies. Long-segment narrowing of major cerebral or cervical vessels without collateral circulation represents an indication of increased risk of stroke [47]. It is therefore strongly recommended to observe several precautions in children with segmental haemangioma before propranolol treatment is started:
-
1.
Pretreatment evaluation—Preferentially, MRI angiography and echocardiography should be performed before initiation of propranolol therapy [18, 47].
-
2.
Initiation of therapy—Patients should be admitted for the initiation of therapy. It is recommended to start at 0.5 mg/kg/day and titrate the dose slowly (in 0.5 mg/kg intervals).
-
3.
Dose interval—Three times per day dosing is at present considered advisable to minimize critical changes in blood pressure [18, 47].
-
4.
Close follow-up including neurological consultation is recommended.
Future developments
As propranolol crosses the blood-brain barrier, long-term follow-up studies are needed in particular with respect to neuro-development [31, 39]. There is no evidence to date of any long-term issues from its use in cardiology. Preliminary studies indicate that hydrophilic beta blockers such as nadolol [51] and atenolol [1] may be at least as effective for IH as propranolol. Due to their hydrophily, central nervous side effects are expected to be less likely. If confirmed in randomized controlled studies, these and perhaps other beta blockers might become a reasonable alternative to propranolol. The early use of topical beta blockers, such as propranolol or timolol, is potentially the way forward for superficial haemangiomas, but further studies are needed to evaluate their efficacy and safety.
Abbreviations
- EMA:
-
European Medicines Agency
- EPC:
-
Endothelial progenitor cells
- FDA:
-
Food and Drug Administration (USA)
- GLUT1:
-
Glucose transporter 1
- HemSC:
-
Haemangioma stem cells
- HIF-1:
-
Hypoxia-inducible factor
- IH:
-
Infantile haemangioma
- Nd:YAG:
-
Neodym-dotted Yttrium-aluminum-granate laser
- PHACE:
-
Posterior fossa-haemangioma-arterial-cardiac-eye anomaly
- RCT:
-
Randomized controlled trial
- VEGF(R):
-
Vascular endothelial growth factor (receptor)
References
Ábarzúa-Araya A, Navarrete-Dechent CP, Heusser F, Retamal J, Zegpi-Trueba MS (2014) Atenolol versus propranolol for the treatment of infantile hemangiomas: a randomized controlled study. J Am Acad Dermatol 70:1045–9
Ahogo C, Ezzedine K, Prey S, Colona V, Diallo A, Boralevi F, Taïeb A, Léauté-Labrèze C (2013) Factors associated with the relapse of infantile haemangiomas in children treated with propranolol. Brit J Dermatol 169:1252–1256
Bagazgoitia L, Hernández-Martín A, Torrelo A (2011) Recurrence of infantile hemangiomas treated with propranolol. Pediatr Dermatol 28:658–662
Bajaj Y, Kapoor K, Ifeacho S, Jephson CG, Albert DM, Harper JI, Hartley BE (2013) Great Ormond Street Hospital treatment guidelines for use of propranolol in infantile isolated subglottic haemangioma. J Laryngol Otol 127:295–298
Bauland CG, Luning TH, Smit JM, Zeebregts CJ, Spauwen PH (2011) Untreated hemangiomas: growth pattern and residual lesions. Plastic Reconstr Surg 127:1643–1648
Bauman NM, Shin JJ, Oh AK, Preciado DA, He J, Greene EA, Puttgen KB (2014) Propranolol vs prednisolone for symptomatic proliferating infantile hemangiomas: a randomized clinical trial. JAMA Otolaryngol, Head and Neck Surg 140:323–30
Blei F, McElhinney DB, Guarini A, Presti S (2014) Cardiac screening in infants with infantile hemangiomas before propranolol treatment. Pediatr Dermatol 31:465–467
Brandling-Bennett HA, Metry DW, Baselga E, Lucky AW, Adams DM, Cordisco MR, Frieden IJ (2008) Infantile hemangiomas with unusually prolonged growth phase: a case series. Arch Dermatol 144:1632–1637
Breur JM, de Graaf M, Breugem CC, Pasmans SG (2011) Hypoglycemia as a result of propranolol during treatment of infantile hemangioma: a case report. Pediatr Dermatol 28:169–171
Chamlin SL, Haggstrom AN, Drolet BA, Baselga E, Frieden IJ, Garzon MC, Horii KA, Lucky AW, Metry DW, Newell B, Nopper AJ, Mancini AJ (2007) Multicenter prospective study of ulcerated hemangiomas. J Pediatr 151:684–689
Chan H, McKay C, Adams S, Wargon O (2013) RCT of timolol maleate gel for superficial infantile hemangiomas in 5–24 week-olds. Pediatrics 131:e1739–1747
Chen TS, Eichenfield LF, Friedlander SF (2013) Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics 131:99–108
Colmenero I, Hoeger PH (2014) Vascular tumours in infants. Part II: vascular tumours of intermediate dignity and malignant tumours. Brit J Dermatol 171:474–484
Del Frari L. Delarue A (2013) Data on file (Pierre Fabre). Presented at World Congress of Paediatric Dermatology, Madrid, 2013
Dickison P, Christou E, Wargon O (2011) A prospective study of infantile hemangiomas with a focus on incidence and risk factors. Pediatr Dermatol 28:663–669
Drolet BA, Swanson EA, Frieden IJ, Hemangioma Investigator Group (2008) Infantile hemangiomas: an emerging health issue linked to an increased rate of low birth weight infants. J Pediatr 153:712–5
Drolet BA, Frieden IJ (2010) Characteristics of infantile hemangiomas as clue to pathogenesis: does hypoxia connect the dots? Arch Dermatol 146:1295–1299
Drolet BA, Frommelt PC, Chamlin SI, Haggstrom A, Bauman NM, Chiu YE, Chun RH, Garzon MC, Holland KE, Liberman L, MacLellan-Tobert S, Mancini AJ, Metry D, Puttgen KB, Seefeldt M, Sidbury R, Ward KM, Blei F, Baselga E, Cassidy L, Darrow DH, Joachim S, Kwon EK, Martin K, Perkins J, Siegel DH, Boucek RJ, Frieden IJ (2013) Initiation and use of propranolol for infantile haemangioma: report of a consensus conference. Pediatrics 131:128–140
Filippi L, Cavallaro G, Bagnoli P, Dal Monte M, Fiorini P, Donzelli G, Tinelli F, Araimo G, Cristofori G, la Marca G, Della Bona ML, La Torre A, Fortunato P, Furlanetto S, Osnaghi S, Mosca F (2013) Oral propranolol for retinopathy of prematurity: risks, safety concerns, and perspectives. J Pediatr 163:1570–1577
Garin EH, Araya CE (2009) Treatment of systemic hypertension in children and adolescents. Curr Opin Pediatr 21:600–604
Giachetti A, Garcia-Monaco R, Sojo M, Scacchi MF, Cernadas C, Guerchicoff Lemcke M, Dovasio F (2014) Long-term treatment with oral propranolol reduces relapses of infantile hemangiomas. Pediatr Dermatol 31:14–20
Gnarra M, Solman L, Harper JI, Syed SB (2015) Propranolol and prednisolone combination for the treatment of segmental haemangioma in PHACES syndrome. Br J Dermatol. doi:10.1111/bjd.13588, Epub ahead of print
Greenberger S, Bischoff J (2013) Pathogenesis of infantile haemangioma. Brit J Dermatol 169:12–19
Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW, Newell B, Nopper AJ, Frieden IJ (2006) Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics 118:882–887
Hemangioma Investigator Group, Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW, Newell B, Nopper AJ, Frieden IJ (2007) Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatr 150:291–294
Hengst M, Oelert M, Hoeger PH (2015) Blood pressure monitoring during the induction and maintenance period of propranolol therapy for complicated infantile haemangiomas. A prospective study of 109 infants. Pediatr Dermatol 2015, in press
Hermans DJ, Boezeman JB, Van de Kerkhof PC, Rieu PN, Van der Vleuten CJ (2009) Differences between ulcerated and non-ulcerated hemangiomas, a retrospective study of 465 cases. Eur J Dermatol 19:152–156
Hermans DJ, Bauland CG, Zweegers J, van Beynum IM, van der Vleuten CJ (2013) Propranolol in a case series of 174 patients with complicated infantile haemangioma: indications, safety and future directions. Brit J Dermatol 168:837–843
Hoeger PH, Maerker JM, Kienast AK, Syed SB, Harper JI (2009) Neonatal haemangiomatosis associated with placental chorangiomas: report of three cases and review of the literature. Clin Exp Dermatol 34:e78–80
Hoeger PH, Colmenero I (2014) Vascular tumours in infants. Part I: benign vascular tumours other than haemangioma. Brit J Dermatol 171:466–473
Hoeger PH (2015) Propranolol for infantile haemangiomas: certain chances, potential risks. Br J Dermatol 172:3–4
Hogeling M, Adas S, Wargon O (2011) A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics 128:e259–e266
Holland KE, Frieden IJ, Frommelt PC, Mancini AJ, Wyatt D, Drolet BA (2010) Hypoglycemia in children taking propranolol for the treatment of infantile hemangioma. Arch Dermatol 146:775–777
Horii KA, Drolet BA, Frieden IJ, Baselga E, Chamlin SL, Haggstrom AN, Holland KE, Mancini AJ, McCuaig CC, Metry DW, Morel KD, Newell BD, Nopper AJ, Powell J, Garzon MC (2011) Hemangioma Investigator Group. Prospective study of the frequency of hepatic hemangiomas in infants with multiple cutaneous infantile hemangiomas. Pediatr Dermatol 28:245–253
Janmohamed SR, Madern GC, de Laat PCJ, Oranje AP (2015) Educational paper: pathogenesis of infantile haemangioma, an update 2014 (part I). Eur J Pediatr 174:97–103
Jinnin M, Medici D, Park L, Liu Y, Boscolo EB, Bischoff J, Boye E, Olsen BR (2008) Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med 14:1236–1246
Kagami S, Kuwano Y, Shibata S, Uwajima Y, Yamada D, Miyamoto A, Miyagawa T, Araki M, Takahashi K, Isomura S, Aozasa N, Masui Y, Yamamoto M, Inuzuka R, Katori T, Sato S (2013) Propranolol is more effective than pulsed dye laser and cryosurgery for infantile hemangiomas. Eur J Pediatr 172:1521–1526
Kaylani S, Theos AJ, Pressey JG (2013) Treatment of infantile hemangiomas with sirolimus in a patient with PHACE syndrome. Pediatr Dermatol 30:194–7
Langley A, Pope B (2015) Propranolol and central nervous system function: potential implications for pediatric patients with infantile hemangiomas. Br J Dermatol 172:13–23
Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, Phillips RJ, Caceres H, Lopez Gutierrez JC, Ballona R, Friedlander SF, Powell J, Perek D, Metz B, Barbarot S, Maruani A, Szalai ZZ, Krol A, Boccara O, Foelster-Holst R, Febrer Bosch MI, Su J, Buckova H, Torrelo A, Cambazard F, Grantzow R, Wargon O, Wyrzykowski D, Roessler J, Bernabeu-Wittel J, Valencia AM, Przewratil P, Glick S, Pope E, Birchall N, Benjamin L, Mancini AJ, Vabres P, Souteyrand P, Frieden IJ, Berul CI, Mehta CR, Prey S, Boralevi F, Morgan CC, Heritier S, Delarue A, Voisard JJ (2015) A randomized controlled trial of oral propranolol in infantile hemangioma. N Engl J Med 372:735–46
Liu LS, Sokoloff D, Antaya RJ (2013) Twenty-four-hour hospitalization for patients initiating systemic propranololtherapy for infantile hemangiomas—is it indicated? Pediatr Dermatol 30:554–560
Luu M, Frieden IJ (2013) Haemangioma: clinical course, complications and management. Brit J Dermatol 169:20–30
Malik MA, Menon P, Rao KL, Samujh R (2013) Effect of propranolol vs prednisolone vs propranolol with prednisolone in the management of infantile hemangioma: a randomized controlled study. J Pediatr Surg 48:2453–2459
Marqueling AL, Oza V, Frieden IJ, Puttgen KB (2013) Propranolol and infantile hemangiomas four years later: a systematic review. Pediatr Dermatol 30:182–191
McAinsh J, Cruickshank JM (1990) Beta-blockers and central nervous system side effects. Pharmacol Ther 46:163–97
McMahon P, Oza V, Frieden IJ (2012) Topical timolol for infantile hemangiomas: putting a note of caution in “cautiously optimistic”. Pediatr Dermatol 29:127–130
Metry D, Frieden IJ, Hess C, Siegel D, Maheshwari M, Baselga E, Chamlin S, Garzon M, Mancini AJ, Powell J, Drolet BA (2013) Propranolol use in PHACE syndrome with cervical and intracranial arterial anomalies: collective experience in 32 infants. Pediatr Dermatol 30:71–89
Mihm MC, Nelson JS (2010) Hypothesis: the metastatic niche theory can elucidate infantile hemangioma development. J Cutan Pathol 37(suppl 1):83–87
Munden A, Butschek R, Tom WL, Marshall JS, Poeltler DM, Krohne SE, Alió AB, Ritter M, Friedlander DF, Catanzarite V, Mendoza A, Smith L, Friedlander M, Friedlander SF (2014) Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Brit J Dermatol 170:907–913
Parikh S, Darrow DH, Grimmer JF, Manning SC (2013) Propranolol use for infantile hemangiomas. American Society of Pediatric Otolaryngology Vascular Anomalies Task Force Practice Patterns. JAMA Otolaryngol Head Neck Surg 139:153–156
Pope E, Chakkittakandiyil A, Lara-Corrales I, Maki E, Weinstein M (2013) Br J Dermatol 168:222–4
Price CJ, Lattouf C, Baum B, McLeod MB, Schachner LA, Duarte AM, Connelly EA (2011) Propranolol vs. corticosteroids for infantile hemangiomas: a multicenter retrospective analysis. Arch Dermatol 147:1371–1376
Puttgen KB (2012) Multifocal infantile hepatic hemangiomas—imaging strategy and response to treatment after propranolol and steroids including review of the literature. Eur J Pediatr 171:1023–8
Puttgen KB, Summerer B, Schneider J, Cohen BA, Boss EF, Bauman NM (2013) Cardiovascular and blood glucose parameters in infants during propranolol initiation for treatment of symptomatic infantile hemangiomas. Ann Otol Rhinol Laryngol 122:550–554
Saidi W, Zaouali A, Saihi N, Mokni S, Denguezli M, Nouira R (2014) Arrêt cardiorespiratoire au décours d’une bronchiolite chez un nourisson traité par propranolol pour un hémangiome. Ann Dermatol Venereol 141:528–530
Solman L, Murabit A, Gnarra M, Harper JI, Syed SB, Glover M (2014) Propranolol for infantile haemangiomas; single centre experience of 250 cases and proposed therapeutic protocol. Arch Dis Child 99:1132–1136
Tollefson MM, Frieden IJ (2012) Early growth of infantile hemangiomas: what parents’ photographs tell us. Pediatrics 130:e314–20
Vergine G, Marsciani A, Pedini A, Brocchi S, Marsciani M, Desiderio E, Bertelli S, Vecchi V (2012) Efficacy of propranolol treatment in thyroid dysfunction associated with severe infantile hepatichaemangioma. Horm Res Paediatr 78:256–60
Vredenborg AD, Janmohamed SR, de Laat PCJ, Madern GC, Oranje AP (2013) Multiple cutaneous infantile haemangiomas and the risk of internal haemangioma. Brit J Dermatol 169:188–191
Acknowledgments
We thank Laboratoires Pierre Fabre Dermatologie, Inc., for sponsoring the meeting in Castres and for the technical support of the consensus process. Drs. Etienne André and Alain Delarue kindly acted as moderators of the discussion process.
Compliance with ethical standards
The manuscript complies to the ethical rules applicable to the European Journal of Pediatrics.
Conflict of interest
Pierre Fabre Inc., France, hosted the initial meeting of the Expert Group in Castres/France.
Author’s contributions
All coauthors attended the initial meeting and participated in the discussion of topics and in the design of the consensus paper. PHH wrote the initial draft of the manuscript which was then circulated to all coauthors. Comments and annotations of all coauthors were included in subsequent drafts. The final version of the manuscript was approved by all coauthors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Beat Steinmann
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 357 kb)
Rights and permissions
About this article
Cite this article
Hoeger, P.H., Harper, J.I., Baselga, E. et al. Treatment of infantile haemangiomas: recommendations of a European expert group. Eur J Pediatr 174, 855–865 (2015). https://doi.org/10.1007/s00431-015-2570-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-015-2570-0