Abstract
Human rhinoviruses (HRVs) are a common cause of lower respiratory tract infections (LRTIs) and are associated with chronic respiratory morbidity. Our aim was to determine whether HRV species A or C were associated with chronic respiratory morbidity and increased health care utilisation in prematurely born infants. A number of 153 infants with a median gestational age of 34 (range 23–35) weeks were prospectively followed. Nasopharyngeal aspirates were collected whenever the infants had LRTIs regardless of hospitalisation status. Parents completed a respiratory diary card and health questionnaire about their infant when they were 11 and 12 months corrected age, respectively. The health-related cost of care during infancy was calculated from the medical records using the National Health Service (NHS) reference costing scheme and the British National Formulary for children. There were 32 infants that developed 40 HRV LRTIs; samples were available from 23 of the 32 infants for subtyping. Nine infants had HRV-A LRTIs, 13 HRV-C LRTIs, and one infant had a HRV-B LRTI. Exclusion of infants who also had RSV LRTIs revealed that the infants who had a HRV-C LRTI were more likely to wheeze (p < 0.0005) and use respiratory medications (p < 0.0005) and had more days of wheeze (p = 0.01) and used an inhaler (p = 0.02) than the no LRTI group. In addition, the respiratory cost of care was greater for the HRV-C LRTI than the no LRTI group (p < 0.0005). Conclusion: Our results suggest HRV-C is associated with chronic respiratory morbidity during infancy in prematurely born infants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human rhinoviruses (HRVs) are a common cause of lower respiratory tract infections (LRTIs) in both term [20] and prematurely [16] born infants. HRVs had been classified into two genetic groups (HRV-A and HRV-B) comprising over 100 serotypes, with HRV-A being more prevalent than HRV-B. A further HRV group (HRV-C) has subsequently been identified by genomic nucleotide sequencing [14]. HRV-C group viruses have been reported to have a similar prevalence to HRV-A in hospitalised [8, 10], ambulatory [1] and asymptomatic patients [8, 10]. There are, however, conflicting data as to whether HRV-A or HRV-C causes more severe acute disease [12, 17, 18, 21]. HRV LRTIs have been associated with chronic respiratory morbidity in infants born at term or prematurely [3, 9, 11]. In addition, in our prospective cohort study [7], we demonstrated that HRV LRTIs in prematurely born infants were associated with increased health-related cost of care during infancy [6]. The aim of this study was to assess in our prospective cohort study [7] whether HRV group A or C viruses were associated with chronic respiratory morbidity and increased healthcare utilisation in prematurely born infants.
Materials and methods
Infants born less than 36 weeks of gestation were eligible for entry into a prospective cohort study [7] if they were born prior to the onset of the RSV season in 2008 or 2009. The RSV season was defined as from October 1st to March 31st as per UK experience [4]. Consecutive infants, whose parents gave informed written consent, were recruited soon after birth. The study was approved by the Research Ethics Committee of King's College Hospital NHS Foundation Trust. The lung function results at 36 weeks PMA from the cohort have been previously reported [7]. In addition, the healthcare utilisation results of the cohort related to whether or not the infant had a rhinovirus LRTI have also been published [6].
Following neonatal unit discharge, infants were followed prospectively until 1 year of corrected age. The parents were asked to contact the research team when their infant was symptomatic with signs consistent with a LRTI, i.e. cough, wheeze and/or shortness of breath. In addition, parents were telephoned every 2 weeks by a researcher to ascertain whether their infant had been or was symptomatic. A researcher visited the home on every occasion that an infant had a LRTI and a nasopharyngeal aspirate (NPA) was obtained. An NPA was obtained on each occasion an infant was admitted to hospital with a LRTI. Real-time reverse transcriptase polymerase chain reaction (PCR) had been performed on the NPAs for 11 viruses (HRV, RSV A and B, human metapneumovirus, influenza A and B, parainfluenza 1–3, enterovirus and parechovirus), and adenovirus and bocavirus were tested by real-time PCR as previously described [2, 6].
For the purposes of this study, the nucleic acid from the stored clinical specimens was subtyped for HRV A, B and C. RNA extraction was carried out using the QIAamp Viral RNA Mini Kit (Qiagen) according to the manufacturer's instructions. cDNA synthesis and PCR amplification of the VP4/VP2 region for typing was carried out as previously described [13]. PCR products were cleaned with microClean (Microzone) before cycle sequencing with BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) on the automated sequencer 3130xl Genetic Analyser (Applied Biosystems). Sequence analysis was carried out using the software BioEdit (version 7.0.9) and MEGA5 (version 5.03). Sequencing was performed in one direction using the forward primer. More than 10 % divergence in VP4/VP2 is required to identify a different HRV type [15, 19], this is well above the contribution of any potential polymerase error.
Parents completed a diary card for 1 month when their infant was 11 months corrected age, which asked them to state on each day whether their infant coughed, wheezed or used any respiratory-related medications (e.g. inhalers, oral steroids, antibiotics). Parents also filled in a respiratory health-related questionnaire about their infant when their infant was 1 year of corrected age. Similar questions were incorporated into the questionnaire as were used during the telephone interviews, in particular, regarding the use of medications. Follow-up costs after neonatal discharge were calculated using the NHS reference costing scheme (2007–2008) [5] and the British National Formulary for Children (2008). The respiratory costs were also reported, and these were defined as costs related to an LRTI episode regardless of whether this was incurred in hospital or the community, e.g. a general practitioner (GP) attendance.
Analysis
Infants who had LRTIs for which no virus was detected were excluded from the analysis. If an infant had an LRTI with a virus other than HRV-A or HRV-C, but had not had an HRV-A or HRV-C LRTI, they also were excluded from the analysis.
The remaining infants were divided into three groups:
-
(i)
Infants who never had a symptomatic LRTI—no LRTI group (n = 74)
-
(ii)
Infants who had a LRTI with HRV-A detected from the NPA—HRV-A LRTI group (n = 9)
-
(iii)
Infants who had a LRTI with HRV-C detected from the NPA—HRV-C LRTI group (n = 13)
Data were tested for normality using the Shapiro–Wilk test and found not to be normally distributed. Differences were assessed for statistical significance using the Fisher's exact test or Kruskal–Wallis test with a post hoc ANOVA test. Statistical analysis was performed using IBM SPSS Statistics (version 19, New York, USA).
Results
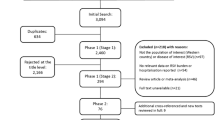
A number of 153 infants with a median gestational age of 34 (range 23–35) weeks and a birth weight of 1,890 (range 534–3610) g were prospectively followed, and 32 infants developed 40 HRV LRTIs (Fig. 1). Of the 40 HRV LRTIs, there were 29 samples from 23 infants with sufficient nucleic acid for subtyping (Table 1). There were no significant differences in the demographics of the infants who did and did not have subtyping of their HRV LRTIs. Nine infants had a HRV-A LRTI [one infant had two different HRV-A serotype LRTIs (serotypes 80 and 56); the LRTIs were 3 months apart], one infant had a HRV-B LRTI (serotype 42), and 13 infants had a HRV-C LRTI [one infant had two different HRV-C serotype LRTIs (serotypes C40 and C12); the LRTIs were 3 months apart]. One infant had a HRV LRTI which was not possible to type and in three samples subtyping failed. Six of the infants in the HRV-A group and three of the infants in the HRV-C group also had RSV LRTIs (either as dual infections or as a separate LRTI). Other viral LRTIs in the HRV-A group were RSV A (n = 5), RSV B (n = 3), adenovirus (n = 5), human metapneumovirus (n = 1), influenza B (n = 2), parainfluenza 1 (n = 1), parainfluenza 3 (n = 1), enterovirus (n = 3), parechovirus (n = 2); and for the HRV-C group they were RSV A (n = 2), RSV B (n = 1), adenovirus (n = 4), human metapneumovirus (n = 2), influenza A (n = 1), influenza B (n = 1), parainfluenza 1 (n = 2), parainfluenza 3 (n = 2), enterovirus (n = 7), parechovirus (n = 1), bocavirus (n = 2). Nine infants in the HRV-A group and 12 in the HRV-C group had a dual or triple infection; their results did not differ significantly from those with a single infection (data not shown).
There were no statistically significant differences in the demographic data between the three groups, although both HRV groups tended to be lighter at birth (p = 0.056) and a greater proportion of them tended to have received surfactant (p = 0.050) or Palivizumab (p = 0.051) than the no LRTI group (Table 2). Three (33 %) of the HRV-A LRTI group had hospital admissions (one for a RSV LRTI, one for a HRV-A/enterovirus dual LRTI, and one had multiple admissions for viral LRTIs including RSV and HRV-A). Three (23 %) of the HRV-C LRTI group had hospital admissions (one for an adenovirus/human bocavirus dual LRTI, one for a RSV/human metapneumovirus/HRV [unsubtyped] triple LRTI, and one for a nonrespiratory cause). Nine (12 %) infants of the no LRTI group had hospital admissions, all for nonrespiratory causes.
Analysis of the diary card data demonstrated that the HRV-A LRTI group had significantly more days of antibiotic (p = 0.021) and inhaler (p < 0.0005) use than the no LRTI group (Table 3). Exclusion of infants who had an RSV LRTI from the analysis, however, demonstrated no significant differences between the HRV-A LRTI group and the no LRTI group (Table 4), but there were only three infants who had an HRV-A LRTI who did not also have an RSV LRTI. Compared to the no LRTI group, infants in the HRV-C LRTI group tended to have more days of cough (p = 0.06) and wheeze (p = 0.06) (Table 4). Exclusion of the infants who had an RSV LRTI from the analyses revealed that the HRV-C LRTI group had significantly more days of wheeze (p = 0.01) and more days of inhaler use (p = 0.024) than the no LRTI group (Table 4).
Analysis of the respiratory health-related questionnaire demonstrated that compared to the no LRTI group, infants in the HRV-A LRTI group were more likely to wheeze (p < 0.0005), use respiratory medications (p < 0.0005), bronchodilators (p = 0.027), antibiotics (p = 0.005) or a preventer (p = 0.017) (Table 3). Exclusion of infants who had an RSV LRTI from the analysis, however, demonstrated no significant differences between the HRV-A LRTI group and the no LRTI group (Table 4). Compared to the no LRTI group, infants in the HRV-C LRTI group were more likely to wheeze (p < 0.0005), use respiratory medications (p < 0.0005), bronchodilators (p < 0.0005), antibiotics (p = 0.008) or a preventer (p = 0.002) (Table 3).
Exclusion of infants who had an RSV LRTI from the analyses revealed that, compared to the no LRTI group, the ten remaining infants in the HRV-C LRTI group were more likely to wheeze (p < 0.0005), use respiratory medications (p = 0.001), bronchodilators (p < 0.0005), antibiotics (p = 0.002) or a preventer (p < 0.0005) (Table 4).
The respiratory cost of care was significantly greater for both the HRV-A (p = 0.003) and the HRV-C (p < 0.0005) LRTI groups compared to the no LRTI group. When the infants who had a RSV LRTI were excluded from the analysis, however, the respiratory cost of care compared to the no LRTI group was only significantly greater for the HRV-C LRTI group (p < 0.0005; Table 5). The total cost of care compared to the no LRTI group was significantly greater only for the HRV-C LRTI group (p = 0.017), but the difference was not statistically significant when infants who had an RSV LRTI were excluded from the analysis (Table 5).
Discussion
We have demonstrated that infants who had a HRV-C LRTI compared to infants who did not have an LRTI were significantly more likely to wheeze and use respiratory medications and had a greater respiratory cost of care. Initial analysis demonstrated HRV-A LRTIs were associated apparently with similar effects, but the significant differences compared to the no LRTI group disappeared once the infants who also had RSV LRTIs were excluded from the analysis, but there were only three infants who had an HRV-A LRTI and not an RSV LRTI. The significant differences between the HRV-C and no LRTI groups remained after excluding infants who had an RSV LRTI. Indeed, exclusion of the infants who also had an RSV LRTI revealed HRV-C LRTIs were associated with significantly more days of wheeze and use of an inhaler. Our results, thus suggest HRV-C is associated with increased chronic respiratory morbidity during infancy in prematurely born infants.
Certain studies [1, 18], but not all, have reported that HRV-C compared to HRV-A results in more severe initial illness. In this study, three infants in both the HRV-A and HRV-C groups were admitted to hospital, but only one infant was admitted solely for an HRV-A LRTI and none for a HRV-C LRTI, thus we cannot comment in this prematurely born population whether HRV-A or HRV-C is associated with more severe acute disease. During the same time period, only one infant had an HRV-B LRTI, suggesting HRV-B infections are less common than HRV-A or HRV-C infections in prematurely born infants, as has been found in infants and children hospitalised with LRTIs and controls without respiratory symptoms [8, 10].
Our study has a number of strengths and some limitations. We assessed respiratory morbidity using a variety of techniques, telephone calls, questionnaires and diary cards. Certain similar questions were used during the telephone calls and on the questionnaire. The questionnaire gave information about the infant's health throughout infancy, the diary card more detailed information for 1 month. All assessments were applied to all three groups. Strengths include that NPAs were collected whenever the infants had an LRTI, regardless of whether this was in hospital or in the community. In addition, we tested the NPAs for a wide range of respiratory viruses and hence were able to demonstrate that the apparent association of HRV-A LRTIs with chronic respiratory morbidity might be explained by the infants having also had RSV LRTIs. A limitation is that the nucleic acid was subtyped only after other investigations had been undertaken and this meant there was insufficient nucleic acid for subtyping in nine of the 32 infants who had HRV LRTIs. The infants with and without subtyping of their HRV LRTIs, however, did not differ in their demographics, thus we feel our results are generalisable. The infants with HRVA and HRVC infections had a variety of other viral LRTIs which may have increased their respiratory morbidity. We did not undertake analysis excluding other respiratory viral LRTIs as, except for RSV LRTI, there is very little information on the long-term outcomes of prematurely born infants following those infections. After excluding infants who also had an RSV LRTI, which is known to increase chronic respiratory morbidity, we highlighted that HRV-C LRTIs were associated with an adverse outcome.
In conclusion, we have demonstrated that HRV-C LRTIs were associated with chronic respiratory morbidity during infancy in prematurely born infants, whether there is a similar adverse outcome in infants born at term merits testing.
References
Arden K, Chang A, Lambert S, Nissen M, Sloots T, Mackay I (2010) Newly identified respiratory viruses in children with asthma exacerbation not requiring admission to hospital. J Med Virol 82:1458–1461
Auburn H, Zuckerman M, Broughton S, Greenough A, Smith M (2011) Detection of nine respiratory RNA viruses using three multiplex RT-PCR assays incorporating a novel RNA internal control transcript. J Virol Methods 176:9–13
Chidekel AS, Rosen CL, Bazzy AR (1997) Rhinovirus infection associated with serious lower respiratory illness in patients with bronchopulmonary dysplasia. Pediatr Infect Dis J 16:43–47
Clark SJ, Beresford MW, Subhedar NV, Shaw NJ (2000) Respiratory syncytial virus infection in high risk infants and the potential impact of prophylaxis in a United Kingdom cohort. Arch Dis Child 83:313–316
Department of Health (2009) NHS reference costs 2007–08. NSRC04 NHS trust and PCT Department of Health. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_098951.xls
Drysdale S, Alcazar M, Wilson T, Smith M, Zuckerman M, Broughton S, Rafferty GF, Peacock JL, Johnston SL, Greenough A (2013) Rhinovirus infection and healthcare utilisation in prematurely born infants. Eur Respir J 42:1029–1036
Drysdale S, Wilson T, Alcazar M, Broughton S, Zuckerman M, Smith M, Rafferty GF, Johnston SL, Greenough A (2011) Lung function prior to viral lower respiratory tract infections in prematurely born infants. Thorax 66:468–473
Fry AM, Lu X, Olsen SJ, Chittaganpitch M, Sawatwong P, Chantra S, Baggett HC, Erdman D (2011) Human rhinovirus infections in rural Thailand: epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS One 6:e17780
Guilbert TW, Singh AM, Danov Z, Evans MD, Jackson DJ, Burton R, Roberg KA, Anderson EL, Pappas TE, Gangnon R, Gern JE, Lemanske RF Jr (2011) Decreased lung function after preschool wheezing rhinovirus illnesses in children at risk to develop asthma. J Allergy Clin Immunol 128:532–538
Iwane MK, Prill MM, Lu X, Miller EK, Edwards KM, Hall CB, Griffin MR, Staat MA, Anderson LJ, Williams JV, Weinberg GA, Ali A, Szilagyi PG, Zhu Y, Erdman DD (2011) Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis 204:1702–1710
Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF Jr (2008) Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 178:667–672
Jin Y, Yuan X-H, Xie Z-P, Gao HC, Song JR, Zhang RF, Xu ZQ, Zheng LS, Hou YD, Duan ZJ (2009) Prevalence and clinical characterization of a newly identified human rhinovirus C species in children with acute respiratory tract infections. J Clin Microbiol 47:2895–2900
Lauinger IL, Bible JM, Halligan EP, Aarons EJ, MacMahon E, Tong CY (2012) Lineages, sub-lineages and variants of enterovirus 68 in recent outbreaks. PLoS One 7:e36005
Lee W-M, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, Jakiela B, Lemanske RF Jr, Shult PA, Gern JE (2007) A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One 7:e966
McIntyre CL, Knowles NJ, Simmonds P (2013) Proposals for the classification of human rhinovirus species A, B and C into genotypically assigned types. J Gen Virol 94(Pt 8):1791–1806, epub ahead of print
Miller EK, Bugna J, Libster R, Shepherd BE, Scalzo PM, Acosta PL, Hijano D, Reynoso N, Batalle JP, Coviello S, Klein MI, Bauer G, Benitez A, Kleeberger SR, Polack FP (2012) Human rhinoviruses in severe respiratory disease in very low birth weight infants. Pediatrics 129:e60–e67
Miller EK, Edwards KM, Weinberg GA, Iwane MK, Griffin MR, Hall CB, Zhu Y, Szilagyi PG, Morin LL, Heil LH, Lu X, Williams JV, Network NVS (2009) A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol 123:98–104
Miller EK, Khuri-Bulos N, Williams JV, Shehabi AA, Faouri S, Al Jundi I, Chen Q, Heil L, Mohamed Y, Morin LL, Ali A, Halasa NB (2009) Human rhinovirus C associated with wheezing in hospitalised children in the Middle East. J Clin Virol 46:85–89
Simmonds P, McIntyre C, Savolainen-Kopra C, Tapparel C, Mackay IM, Hovi T (2010) Proposals for the classification of human rhinovirus species C into genotypically assigned types. J Gen Virol 91:2409–2419
van der Zalm MM, Uiterwaal CSPM, Wilbrink B, Koopman M, Verheij TJM, van der Ent CK (2011) The influence of neonatal lung function on rhinovirus-associated wheeze. Am J Respir Crit Care Med 183:262–267
Xiang Z, Gonzalez R, Xie Z, Xiao Y, Liu J, Chen L, Liu C, Zhang J, Ren L, Vernet G, Paranhos-Baccalà G, Shen K, Jin Q, Wang J (2010) Human rhinovirus C infections mirror those of human rhinovirus A in children with community-acquired pneumonia. J Clin Virol 49:94–99
Acknowledgments
Sources of support
Dr. Simon Drysdale was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas' NHS Foundation Trust/King's College London and the research nurses (MA, TW) by Abbott Laboratories. The work on HRV typing was funded by the British Medical Association H C Roscoe grant awarded to CYWT and which supported Dr. Ina Lauinger as research associate. SLJ is supported by the Asthma UK Clinical Chair CH11SJ and ERC FP7 advanced grant 233015. SLJ and AG are MRC and Asthma UK Centre in Allergic Mechanisms of Asthma Investigators, supported by MRC Centre grant G1000758. AG and SLF are NIHR Senior Investigators.
Disclosure statement
MA and TW (research nurses) were funded by Abbott Laboratories who market Palivizumab. AG has held grants and received honoraria for giving lectures from Abbott Laboratories and MedImmune, the latter company manufactures Palivizumab. For the remaining authors, no conflict of interest was declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Willem Proesmans
Rights and permissions
About this article
Cite this article
Drysdale, S.B., Alcazar, M., Wilson, T. et al. Respiratory outcome of prematurely born infants following human rhinovirus A and C infections. Eur J Pediatr 173, 913–919 (2014). https://doi.org/10.1007/s00431-014-2262-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-014-2262-1