Abstract
Generalized arterial calcification of infancy (GACI, MIM#208000) is a rare autosomal recessive disorder characterized by extensive calcifications in the media of large- and medium-sized muscular arteries. Most affected children die in early infancy because of cardiac failure. GACI is linked to mutations in the ENPP1 gene, which encodes for an enzyme that generates inorganic pyrophosphate (PPi), a potent inhibitor of hydroxyapatite crystal formation. Treatment with bisphosphonates, which are synthetic PPi analogues, has been proposed as a means of reducing arterial calcifications in GACI patients, but no formalized treatment approach exists. We report on the long-term survival of a severe case of GACI linked to a novel homozygous missense mutation c.583T/C in the ENPP1 gene, diagnosed prenatally, and treated with bisphosphonates. Intravenous disodium pamidronate (three infusions at days 8, 15, and 18 of 0.25, 0.50, and 0.50 mg/kg, respectively) was changed to oral disodium etidronate (starting dose of 20 mg/kg daily, 50 mg die) at 3 weeks of age. Although the etidronate dose was maintained at 50 mg daily in our patient (corresponding to a progressive decrease from 20 to 5 mg/kg daily), the progressive resolution of arterial calcifications seen by 3 months of age was maintained until 2 years of age. Throughout the 2-year follow-up, our patient developed mild hypophosphatemia, due to renal phosphate wasting, without clinical, biochemical, or radiological sign of rickets. Conclusion: High-dose bisphosphonate therapy may not be necessary for an extended period of time in children with GACI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Generalized arterial calcification of infancy (GACI, MIM#208000) is a rare autosomal recessive disorder characterized by extensive calcifications in the media of large- and medium-sized muscular arteries and myointimal proliferation leading to arterial stenoses [14]. Diagnosis is often made prenatally or in early infancy, with variable clinical presentations including heart failure, hypertension, failure to thrive, or convulsions [7]. Usually this disease has a rapid and fatal course due to vascular occlusion, leading to myocardial infarction or congestive heart failure associated with arterial hypertension [14]. Most affected children die in early infancy with an estimated 85% of cases dying within the first 6 months of life [7].
GACI is linked to mutations in the ENPP1 gene, which encodes for ectonucleotide pyrophosphatase/phosphodiesterase 1 (NPP1), an enzyme that generates inorganic pyrophosphate (PPi), a potent inhibitor of hydroxyapatite crystal formation [21]. In patients with GACI, the NPP1 enzyme is deficient [19], leading to low serum and urinary PPi levels [22]. No genotype–phenotype correlation has been documented in GACI [19], and a broad phenotypic variation has been reported in siblings, who inherited identical mutations [5, 6]. Loss-of-function ENPP1 mutations have also been associated with autosomal recessive hypophosphatemic rickets [10]. Treatment with bisphosphonates, which are synthetic PPi analogues, given at a dose known to cause a mineralization defect in bone, has been proposed to reduce arterial calcifications in GACI patients [17]. However, no formalized treatment approach exists for patients affected by GACI, and the efficiency of the therapy remains unclear. We report on the long-term survival of an infant with severe GACI diagnosed prenatally and treated with pamidronate and then etidronate.
Methods
Clinical evaluation
Height and weight measurements were converted to age- and sex-specific z scores on the basis of reference data published by the Centers for Disease Control and Prevention [15].
Biochemical measurements
Serum creatinine, total calcium, phosphate, and alkaline phosphatase, and urinary calcium, phosphate, and creatinine were measured using colorimetric methods (Monarch®; Instrumentation Laboratories Inc., Lexington, MA, USA).
Dual-energy X-ray absorptiometry
Areal bone mineral density (aBMD) was measured by dual-energy X-ray absorptiometry (DXA) performed at the lumbar spine (L2–L4) using a Lunar Prodigy device (GE Healthcare). These results were converted to age- and sex-specific z scores using normal reference data obtained from a cross-sectional study performed at the CHU Sainte-Justine [1].
ENPP1 mutational analysis
After informed consent was obtained from the parents, DNA from the patient and her parents was analysed at Dr Rutsch's lab at Münster University Children's Hospital, Münster, Germany. All 25 exons of ENPP1 and their flanking splice sites were amplified from genomic DNA and sequenced conventionally as previously described [2].
Case report
A 32-week gestation female fetus was diagnosed with pericardial effusion and diffuse arterial calcification involving the aorta and the pulmonary, iliac, and carotid arteries on prenatal ultrasonographic examination and fetal MRI. Her parents were healthy and nonconsanguineous. The mother had one first trimester miscarriage, and family history was unremarkable for bone metabolic diseases.
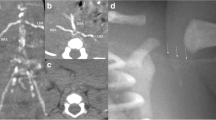
The baby was born by vaginal delivery at 36 4/7 weeks gestation with a birth weight of 2,750 g (SDS 0.9) and a birth length of 44 cm (SDS −1.7). Apgar scores were 9 and 10 at 5 and 10 min. At birth, no femoral pulse was palpable. Her blood pressure values varied widely at birth and were first noted in the normal range at day 6 (80/42 mmHg). Secondary to pericardial effusion, she developed mild respiratory distress in the first hours of life and required pericardial fluid drainage on day 3 and respiratory support for 6 days. On day 7, radiological evaluation confirmed diffuse arterial calcifications without involvement of cerebral vasculature (Fig. 1).
ENPP1 sequence analysis revealed the presence of the novel missense mutation c.583T/C on both alleles leading to the amino acid substitution p.C195R. Both parents were shown to be heterozygous for the mutation. This mutation is thought to be most likely pathogenic because the cysteine residue at position 195 is highly conserved across species.
Evolution
Because of the disease severity and initial enteral feeding intolerance, intravenous disodium pamidronate (three infusions of 0.25, 0.50, and 0.50 mg/kg at days 8, 15, and 18, respectively) was firstly initiated. Following the first administration, mild asymptomatic hypocalcemia (minimum serum levels of calcium, 1.74 mmol/l) was corrected within 48 h with calcium supplementation, and no acute-phase inflammatory reaction was noted. This treatment was followed by oral disodium etidronate (starting dose of 20 mg/kg daily, 50 mg die) at 3 weeks of age when oral feeding was well tolerated. The child was discharged from the hospital at 4 weeks of age. Owing to digestive intolerance, the etidronate dose was kept at 50 mg daily without adjustment for weight during the 2-year follow-up (corresponding to a progressive decrease from 20 to 5 mg/kg daily).
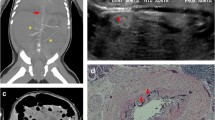
Follow-up included three monthly clinical evaluation and assessment of mineral homeostasis, six monthly DXA and cardiac ultrasonography, and annual X-rays of the wrists and knees. Using a low-dose radiation pediatric thoracoabdominal computer tomography (CT) protocol (1.5 mSv of total radiation dose per scan), CT studies were performed every 6 months during the first year of life to better assess the changes in calcification and to ensure the safety of the patient under the rapid decrease in etidronate treatment regimen over the first year (Table 1).
On physical examination, the femoral pulses became palpable at 2 months of age and were considered normal at 3 months. On CT scans, arterial calcifications decreased by 6 months and completely disappeared by 1 year of age (Fig. 2). Mild hyperechogenicity of the ascending aortic and main pulmonary arterial walls without cardiac involvement was still present on the last cardiac ultrasonography at 2 years of age.
Throughout the 2-year follow-up, our patient developed mild hypophosphatemia due to renal phosphate wasting, without clinical, biochemical, or radiological sign of rickets (Table 1). Areal bone mineral densitometry of the lumbar spine remained in the normal range (Table 1). We plan to maintain etidronate at the same dose and to discontinue this therapy at 3 years of age.
Discussion
In this article, we report a severe case of GACI diagnosed prenatally and successfully treated with pamidronate and then etidronate, which was kept at the same dose of 50 mg daily from the neonatal period to 2 years of age. The efficacy of bisphosphonates as compared to the spontaneous evolution of the disease is difficult to evaluate. Long-term survival has been reported in patients receiving no specific antimineralization therapy [8, 11, 24, 26], suggesting that spontaneous resolution of calcifications may also occur. In a retrospective study, bisphosphonate therapy was associated with survival in 11 of 17 treated patients, compared to 8 of 26 untreated patients [20]. However, no information about the severity of the disease in these two groups of patients was available. Patients with GACI who survived the first 6 months had a better prognosis [20]. Presentation in utero, as seen in our patient, may represent a more severe form of GACI, associated with a higher incidence of aortic calcification and mortality [25]. However, the diagnosis of faint calcifications of the aorta presenting as increased echogenicity of the vessel wall may be missed in prenatal ultrasound studies. Also, fetuses might die in utero before the diagnosis can be established.
Bisphosphonates are well-established drugs for the treatment of bone diseases associated with excessive bone resorption. However, as synthetic PPi analogues, these compounds were first shown to prevent the experimentally induced calcification of many soft tissues, including blood vessels, as well as to inhibit mineralization of ectopic bone and normal calcified tissues such as bone and cartilage [23]. The earliest clinical application of etidronate included its use in fibrodysplasia ossificans progressiva [3]. Although usually not recommended for use in children owing to the risk of rickets, etidronate by its ability to inhibit mineralization and its moderate antiresorptive effects on bone seems to be the best choice of treatment for GACI. Of the 21 children reported in the literature with GACI, who were treated with bisphosphonates, 13 children received etidronate at a usual dose of 20 mg/kg daily [5, 17, 20, 27, 28] as was done in our patient. Other bisphosphonate compounds with more bone antiresorptive activity (such as risedronate or pamidronate) were also effective [5, 17]. The mechanisms by which bisphosphonates influence GACI are uncertain. In vitro studies have shown that bisphosphonates accumulate within vessel walls suggesting that these drugs may have a direct effect on calcification [31]. Bisphosphonates might also influence arterial smooth muscle differentiation and phenotype plasticity [9]. Duration and doses of bisphosphonate treatment remain unclear. Based on the literature, calcifications disappear between 4 months and 2 years of therapy and do not reappear after biphosphonate treatment has been stopped [7, 12, 29]. In our patient, although etidronate dose was maintained at 50 mg daily, the progressive resolution of arterial calcifications seen by 3 months of age was maintained until 2 years of age. Higher phosphate levels, probably due to low glomerular filtration rate and subsequent retention of phosphate, are found in healthy newborns and infants [4, 13]. These higher levels may lead to an increased risk of arterial calcification in young patients with NPP1 deficiency during the first months of life, which could subsequently decline with age thereafter. Consequently, the full bisphosphonate dose may not be necessary for a long period of time.
Possible adverse effects of etidronate, such as rickets, may be dose dependent and were not observed in our patient during the 2-year follow-up. Short- to long-term use of intravenous bisphosphonates in children with osteogenesis imperfecta seems to be safe and well tolerated [18]. Data on oral use of etidronate are more limited. The appearance of rickets has been described in children affected with GACI [29] and pulmonary alveolar microlithiasis [16] treated with etidronate. Although long-term treatment with etidronate did not lead to other adverse effects, the long-term safety remains to be determined in children. Our patient developed mild hypophosphatemia without rickets, due to renal phosphate wasting, as previously described in children with GACI not treated with biphosphonates [10]. Loss-of-function ENPP1 mutations have also been associated with autosomal recessive hypophosphatemic rickets [10]. The phosphaturia observed in patients with ENPP1 mutations may involve fibroblast growth factor 23 (FGF23), a key regulator of phosphate reabsorption [30]. Indeed, it has been reported that patients with ENPP1 mutations show FGF23 levels above the normal range or inappropriately high in the context of low phosphate serum levels [10]. This suggests that NPP1 could negatively regulate the synthesis and/or secretion of FGF23. Interestingly, hypophosphatemia may lead to a counteractive effect on the phenotype of GACI [21] suggesting an as yet elusive mechanism that balances arterial calcification with bone mineralization. This factor may as well have contributed to the favorable clinical course in our patient.
Conclusion
In this child with GACI, although the etidronate dose was maintained at 50 mg daily (corresponding to a progressive decrease from 20 to 5 mg/kg daily), the progressive resolution of arterial calcifications seen by 3 months was maintained until 2 years of age without signs of mineralization defects of bone.
References
Alos N, Eugène D, Chabot G, Gougeon M, Dubois J (2006) Pediatric reference data for bone mineral density of the lumbar spine in infants and young children. J Bone Miner Res 21(suppl 1):S327
American Academy of Pediatrics (2000) Diagnostic imaging of child abuse. Pediatrics 105(6):1345–1348
Bassett CA, Donath A, Macagno F, Preisig R, Fleisch H, Francis MD (1969) Diphosphonates in the treatment of myositis ossificans. Lancet 2(7625):845
Brodehl J, Gellissen K, Weber HP (1982) Postnatal development of tubular phosphate reabsorption. Clin Nephrol 17(4):163–171
Cheng KS, Chen MR, Ruf N, Lin SP, Rutsch F (2005) Generalized arterial calcification of infancy: different clinical courses in two affected siblings. Am J Med Genet A 136(2):210–213. doi:10.1002/ajmg.a.30800
Ciana G, Trappan A, Bembi B, Benettoni A, Maso G, Zennaro F, Ruf N, Schnabel D, Rutsch F (2006) Generalized arterial calcification of infancy: two siblings with prolonged survival. Eur J Pediatr 165(4):258–263. doi:10.1007/s00431-005-0035-6
Glatz AC, Pawel BR, Hsu DT, Weinberg P, Chrisant MR (2006) Idiopathic infantile arterial calcification: two case reports, a review of the literature and a role for cardiac transplantation. Pediatr Transplant 10(2):225–233. doi:10.1111/j.1399-3046.2005.00414.x
Gleason MM, Weber HS, Cyran SE, Baylen BG, Myers JL (1994) Idiopathic infantile arterial calcinosis: intermediate-term survival and cardiac sequelae. Am Heart J 127(3):691–695
Johnson K, Polewski M, van Etten D, Terkeltaub R (2005) Chondrogenesis mediated by PPi depletion promotes spontaneous aortic calcification in NPP1−/− mice. Arterioscler Thromb Vasc Biol 25(4):686–691. doi:10.1161/01.ATV.0000154774.71187.f0
Lorenz-Depiereux B, Schnabel D, Tiosano D, Hausler G, Strom TM (2010) Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet 86(2):267–272. doi:10.1016/j.ajhg.2010.01.006
Marrott PK, Newcombe KD, Becroft DM, Friedlander DH (1984) Idiopathic infantile arterial calcification with survival to adult life. Pediatr Cardiol 5(2):119–122
Meradji M, de Villeneuve VH, Huber J, de Bruijn WC, Pearse RG (1978) Idiopathic infantile arterial calcification in siblings: radiologic diagnosis and successful treatment. J Pediatr 92(3):401–405
Mitchell DM, Juppner H (2010) Regulation of calcium homeostasis and bone metabolism in the fetus and neonate. Curr Opin Endocrinol Diabetes Obes 17(1):25–30. doi:10.1097/MED.0b013e328334f041
Moran JJ (1975) Idiopathic arterial calcification of infancy: a clinicopathologic study. Pathol Annu 10:393–417
Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL (2002) Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109(1):45–60
Ozcelik U, Yalcin E, Ariyurek M, Ersoz DD, Cinel G, Gulhan B, Kiper N (2010) Long-term results of disodium etidronate treatment in pulmonary alveolar microlithiasis. Pediatr Pulmonol 45(5):514–517. doi:10.1002/ppul.21209
Ramjan KA, Roscioli T, Rutsch F, Sillence D, Munns CF (2009) Generalized arterial calcification of infancy: treatment with bisphosphonates. Nat Clin Pract Endocrinol Metab 5(3):167–172. doi:10.1038/ncpendmet1067
Rauch F, Glorieux FH (2004) Osteogenesis imperfecta. Lancet 363(9418):1377–1385. doi:10.1016/S0140-6736(04)16051-0
Ruf N, Uhlenberg B, Terkeltaub R, Nurnberg P, Rutsch F (2005) The mutational spectrum of ENPP1 as arising after the analysis of 23 unrelated patients with generalized arterial calcification of infancy (GACI). Hum Mutat 25(1):98. doi:10.1002/humu.9297
Rutsch F, Boyer P, Nitschke Y, Ruf N, Lorenz-Depierieux B, Wittkampf T, Weissen-Plenz G, Fischer RJ, Mughal Z, Gregory JW, Davies JH, Loirat C, Strom TM, Schnabel D, Nurnberg P, Terkeltaub R (2008) Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet 1(2):133–140. doi:10.1161/CIRCGENETICS.108.797704
Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Hohne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, Epplen JT, Knisely A, Superti-Furga A, McGill J, Filippone M, Sinaiko AR, Vallance H, Hinrichs B, Smith W, Ferre M, Terkeltaub R, Nurnberg P (2003) Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet 34(4):379–381. doi:10.1038/ng1221
Rutsch F, Schauerte P, Kalhoff H, Petrarulo M, August C, Diekmann L (2000) Low levels of urinary inorganic pyrophosphate indicating systemic pyrophosphate deficiency in a boy with idiopathic infantile arterial calcification. Acta Paediatr 89(10):1265–1269
Schenk R, Merz WA, Muhlbauer R, Russell RG, Fleisch H (1973) Effect of ethane-1-hydroxy-1,1-diphosphonate (EHDP) and dichloromethylene diphosphonate (Cl2MDP) on the calcification and resorption of cartilage and bone in the tibial epiphysis and metaphysis of rats. Calcif Tissue Res 11(3):196–214
Sholler GF, Yu JS, Bale PM, Hawker RE, Celermajer JM, Kozlowski K (1984) Generalized arterial calcification of infancy: three case reports, including spontaneous regression with long-term survival. J Pediatr 105(2):257–260
Stuart G, Wren C, Bain H (1990) Idiopathic infantile arterial calcification in two siblings: failure of treatment with diphosphonate. Br Heart J 64(2):156–159
Thomas P, Chandra M, Kahn E, McVicar M, Naidich J, LaCorte M (1990) Idiopathic arterial calcification of infancy: a case with prolonged survival. Pediatr Nephrol 4(3):233–235
Tran KH, Boechat MI (2006) Idiopathic infantile arterial calcification: imaging evaluation and the usefulness of MR angiography. Pediatr Radiol 36(3):247–253. doi:10.1007/s00247-005-0044-7
Van der Sluis IM, Boot AM, Vernooij M, Meradji M, Kroon AA (2006) Idiopathic infantile arterial calcification: clinical presentation, therapy and long-term follow-up. Eur J Pediatr 165(9):590–593. doi:10.1007/s00431-006-0146-8
Van Dyck M, Proesmans W, Van Hollebeke E, Marchal G, Moerman P (1989) Idiopathic infantile arterial calcification with cardiac, renal and central nervous system involvement. Eur J Pediatr 148(4):374–377
White KE, Evans WE, O'Riordan JLH et al (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26(3):345–348. doi:10.1038/81664
Ylitalo R, Kalliovalkama J, Wu X, Kankaanranta H, Salenius JP, Sisto T, Lahteenmaki T, Ylitalo P, Porsti I (1998) Accumulation of bisphosphonates in human artery and their effects on human and rat arterial function in vitro. Pharmacol Toxicol 83(3):125–131
Acknowledgments
TE was the recipient of a grant (MENTOR program) from the Canadian Institutes of Health Research. YN and FR are supported by a grant from the Interdisciplinary Center for Clinical Research Münster (IZKF).
Conflicts of interest
None of the authors have a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Edouard, T., Chabot, G., Miro, J. et al. Efficacy and safety of 2-year etidronate treatment in a child with generalized arterial calcification of infancy. Eur J Pediatr 170, 1585–1590 (2011). https://doi.org/10.1007/s00431-011-1572-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-011-1572-9