Abstract
Human metapneumovirus (hMPV) is a recently discovered pathogen in respiratory tract infection. The published literature suggests milder illness severity in hMPV compared with respiratory syncytial virus (RSV) infection. In two consecutive seasons, 637 nasopharyngeal aspirates from pediatric patients were tested by hMPV polymerase chain reaction, and risk factors and clinical and laboratory items were analyzed. The hMPV patients were compared with hMPV-negative but RSV-positive patients by matched pair analysis. HMPV was detected in 17.9% of all samples. In total, 88 hMPV-infected patients with complete datasets were considered. More than half of all hMPV patients were older than 12 months, 45.5% had at least one risk factor for a severe course of viral respiratory tract infection, and 27.3% were born prematurely, 15.9% with a birth weight <1,500 g. At least one other virus was also detected in 39 patients (44.3%; RSV in 29.5%). Coinfection did not result in greater severity of illness. On matched pair analysis (hMPV-positive/RSV-negative vs. hMPV-negative/RSV-positive), the epidemiological and clinical features of hMPV infection were similar to those of RSV infection, as in the hMPV group higher proportions of patients with hypoxemia on admission (33% vs. 21%) and of intensive care treatment (20.8% vs. 10.4%) were observed. More hMPV patients showed lobar infiltrates in radiological chest examination. In 60% of all hMPV infections, the attending physicians prescribed antimicrobial chemotherapy. We conclude that in hospitalized children, hMPV infection is as serious as RSV infection and therefore deserves the same attention. Virologic diagnosis from respiratory secretions is mandatory because clinical, laboratory, and radiological signs cannot sufficiently discriminate between viral and bacterial respiratory tract infection in infants and children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human metapneumovirus (hMPV) was discovered by van den Hoogen et al. in 2001 [36]. As a member of the paramyxovirus family, hMPV is closely related to the pneumovirus respiratory syncytial virus (RSV). hMPV is an important viral pathogen in pediatric and adult viral respiratory tract infection (VRTI) [4, 37, 39], responsible for acute wheezing triggered by VRTI [15, 31], bronchiolitis, and pneumonia predominantly in infants and children in the first 36 months of life. hMPV seems to share important clinical features and risk factors with RSV [1, 24, 26]. The hMPV-related hospitalization rate and the risk of severe complications (pneumonia, acute respiratory failure, apnea-bradycardia syndrome) seem to be higher in prematurely born infants with chronic lung disease of prematurity [35], children with hemodynamically relevant congenital heart disease, children with neuromuscular diseases, and patients with profound immunosuppression after blood stem cell or organ transplantation [14, 28]. Nosocomial hMPV infections due to direct or indirect transmission are difficult to identify because no reliable bedside antigen test is available, and no generally accepted incubation period is known [4]. Here we report on 88 hospitalized pediatric patients with hMPV infections. From these patients, nasopharyngeal aspirates were sampled for the early detection of respiratory infections, a procedure performed routinely in all admitted patients with clinical signs of respiratory infection during the winter months.
The study covers two consecutive seasons of a prospective surveillance study, DSM RSV Paed 2002–2004 (http://www.dsmrsvpaed.de). Based on our observation of two consecutive hMPV seasons, we suggest that the clinical course of hMPV infections in hospitalized children is not milder than that of RSV infections.
Materials and methods
Inclusion criteria and surveillance methods
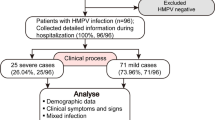
Nasopharyngeal aspirates were routinely collected from all admitted patients with clinical signs of respiratory tract infection in two consecutive seasons (2002/2003 and 2003/2004). The prospective surveillance period covers 7 months each year (1 October–30 April). All inpatients treated for at least 24 h at the Children’s Hospital Medical Center, University of Bonn, Germany, with a virologically confirmed hMPV infection were included irrespective of age and other underlying illness or comorbidity. The attending physicians and nurses in charge were trained in the collection of essential information because prospective surveillance of RSV infections with the same methods has been done at our institution since 1997. A standardized set of 85 clinical and laboratory items was extracted from the files, controlled by a pediatric infective disease consultant, and entered into a Microsoft Excel alias of the DSM RSV Paed database. All radiological diagnoses were confirmed by a pediatric radiologist blinded to the clinical data. The study protocol of the DSM RSV Paed surveillance study was approved by the local study review board and ethics committee.
Definitions
Premature birth was considered to be birth before 37 weeks of gestation. The clinical pictures and courses (bronchitis, bronchiolitis, central pneumonia, lobar pneumonia, respiratory failure, etc.) were summarized by one of the attending physicians to a clinical diagnosis comprising the whole episode of the RSV or hMPV infection.
In the DSM RSV Paed study, the term bronchiolitis is strictly limited to infants (<12 months) with severe, sometimes silent, airway obstruction, tachydyspnea, hypoxemia, and hyperinflation without pneumonic infiltrates on chest radiography. A lower respiratory tract infection was documented as pneumonia only if chest radiography, interpreted by a pediatric radiologist, had confirmed the clinical diagnosis. In cases of perihilar and peribronchial infiltrates, the diagnosis of central pneumonia was made. In contrast, lobar infiltrates with or without pleural effusion were documented as “other pneumonia.” Vital parameters such as respiratory rate and oxygen saturation were compared with age-related normal values to identify the patients with tachypnea or hypoxemia [12]. Apnea was defined as lack of breathing activity for at least 20 s (plus a decrease in oxygen saturation as measured by pulse oximetry on the ward). Hypoxemia referred to an oxygen saturation <94% (by pulse oximetry), which was the criterion to supplement oxygen; in addition, oxygen was given to all patients with severe tachydyspnea. Acute otitis media was diagnosed only with severe local findings (effusion, inflammation, and bulging of the tympanic membrane in a child with earache).
Virological methods
The nasopharyngeal aspirates were collected in a suctioning trap after a nasal washing with 2–5 ml of isotonic NaCl. This procedure was performed by two skilled nurses and yielded an aspirated volume of 2–3 ml. The diagnostic procedure was performed at the discretion of the attending physician, who followed a written guideline for the management of RSV infections. This guideline states that at least one nasopharyngeal aspirate sample should be collected in each patient with clinical signs of VRTI during the surveillance period.
All hMPV cases documented in the database were virologically confirmed by reverse transcriptase polymerase chain reaction (rt-PCR) as described previously [31, 32, 38]. RSV and influenza A/B infections were confirmed by an antigen ELISA (immunochromatography test; Becton Dickinson Directigen, Becton Dickinson, Sparks, MD, USA) [30] and cell culture inoculation using MS (permanent monkey-derived cell line) and MDCK cells. The cell cultures were examined daily or every other day for cytopathic effects for at least 14 days or, especially for detection of influenza virus, at the end of the incubation period were retested for viral antigen; rt-PCR-based methods for detecting RSV genomic sequences in respiratory specimens [1] were not applied during the study period.
Clinical severity grading, index, and mortality
In 1993 McIntosh and coworkers [21] proposed a practical and reasonable grading of illness severity in RSV-infected patients, which has been transposed to hMPV in this study (1= mechanical ventilation due to hMPV; 2= supplemental oxygen, no mechanical ventilation; 3= only supportive care, no oxygen required). Patients with nasopharyngeal continuous positive airway pressure support were allocated to grade 2. In addition, grade 4 was established for patients who acquired the hMPV infection while being on mechanical ventilation if this supportive intervention had not been hMPV-related. The clinical severity index (CLSI) illustrates the severity of RSV-(hMPV-) related disease in a distinct population, i.e., all patients in one season or patients in different subgroups. The CLSI describes the proportion of patients who require at least supplemental oxygen in relation to grade 3 episodes as [grade 3/(grade 2 + grade 1)] and for all figures as proportion in percent. A CLSI <1 indicates a group of patients of whom more than 50% required at least oxygen supplementation. The lower the clinical severity index, the higher the mean clinical severity.
Matching procedures
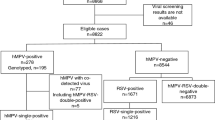
Out of the total collective of 88 hMPV-positive patients, 48 individuals without evidence of RSV (negative results obtained from antigen test and culture) were selected and compared with 48 hMPV-negative, RSV-positive patients selected from the DSM RSV Paed study population. It cannot be completely excluded that in a small number of patients, a method with higher sensitivity (in addition to antigen testing and cell culture), such as PCR, would have detected some additional RSV infections. We were unable to find a comparable RSV-positive, hMPV-negative patient for a 15-year-old girl with Noonan syndrome, Evans syndrome, and severe neurological impairment who was on oral immunosuppressive therapy with cyclophosphamide. In 48 patients we found a comparable RSV-positive, hMPV-negative patient with applicable values for age at diagnosis, birth weight, gestational age at birth, gender, season, and underlying condition (such as chronic lung disease of prematurity). Table 1 shows information about both groups in comparison.
Statistical analysis
SPSS 11.0 (SPSS, Chicago, IL, USA) was used with the χ2 test or Fisher’s exact test for discontinuous variables, and Student’s t-test or the Mann-Whitney U-test was used for continuous variables. Tests of significance were two-sided, and p<0.05 was considered statistically significant. In the sequel all p-values refer to the respective significance level of distribution differences between RSV-positive and hMPV-positive unless otherwise specified.
Results
In total, 637 respiratory samples were retrospectively investigated by hMPV rt-PCR. Five samples were collected in 2001–2002, 300 in 2002–2003, and 332 in 2003–2004. The targeted hMPV genomic sequence was detected in 114 out of 632 (2002–2004) specimens (18%). The proportion of hMPV-positive samples by season was 13.7% (41/300) for the first season and 22.0% (73/332) for the second season. The corresponding numbers for RSV-positive specimens were n=80 (26.7%) and n=101 (30.4%) in the periods 2002–2003 and 2003–2004, respectively. Thus, the entire proportion of specimens positive for either hMPV or RSV was 40.3% (2002–2003) and 52.4% (2003–2004). Related to 100 admissions, a cumulative incidence of 2.3 for 2002–2003 and 3.7 for 2003–2004 was observed for all hMPV infections during the two consecutive surveillance periods. Referring to 1,000 inpatient days, a cumulative incidence density of 4.0 for 2002–2003 and 6.1 for 2003–2004 was observed for all hMPV infections during the two consecutive surveillance periods. Including five cases from the season 2001–2002, a total of 88 inpatients who acquired their hMPV infections from October to April (no repeats were counted) were analyzed. In the following, the 88 hMPV-infected patients are referred to as the total collective.
Out of the total collective, 49 (55.7%) patients were male. The mean age at diagnosis was 967±1,310 days (median 471 days, range 32–6,506 days). According to the age categories of the DSM RSV Paed study, the following distribution was found: newborns (≤4 weeks), n=0 (0.0%); 5 weeks to 5 months, n=16 (18.2%); 6–12 months, n=20 (22.7%); 13–24 months, n=21 (23.9%); >24 months, n=31 (35.2%). More than one-half of all hMPV episodes were detected in patients older than 12 months, and more than one-third were older than 24 months. This is probably the result of nasopharyngeal aspirate testing in all patients with clinical signs of respiratory infection with no age restriction.
In 49 of 88 patients from the total collective (55.7%), hMPV was the only detected pathogen detected. Of those, 48 patients with hMPV single infection are herein referred to as hMPV-positive (see matching procedure and Table 1).
Time course of the epidemic
The time courses (October to April) for the investigated hMPV and RSV seasons are shown in Fig. 1. During the season 2002–2003, a continuously persisting hMPV activity was seen (number of episodes per months), with a small peak in December. In the second season (2003–2004), the hMPV-season peaked in January (18 hospitalized patients). The corresponding course of the RSV season 2002–2003 displayed a peak in December (20 hospitalized patients). In the following winter, the season started in December and peaked in February (21 patients; Fig. 1).
Time course of the hMPV and RSV season in the winter months 2002–2003 and 2003–2004 (October to April). During 2002–2003 a continuously persisting hMPV activity was seen (number of episodes per months) without a definite peak. In 2003–2004 the hMPV season started in November (earlier than that for RSV) and peaked in January (18 hospitalized patients). The corresponding course of the RSV season 2002–2003 showed a peak in December (20 hospitalized patients). In the following winter, the season started in December and peaked in February (21 patients)
Risk factors
Referring to the risk factors that have been described for RSV [1, 24, 26], 45.5% (n=40) of the total collective had at least one risk factor for a severe course of VRTI: 27.3% (n=24) were born prematurely, 15.9% (n=14) had a birth weight <1,500 g and 8% <1,000 g, and 5.7% were born at gestational age <28 weeks. Six patients (6.8%) suffered from chronic lung disease of prematurity, including 4.5% (n=4) who had been treated with oxygen or diuretics during the 6 months preceding the hMPV infection.
Twenty patients (22.7%) had been mechanically ventilated before and independently of the acute hMPV infection. In 6.8% (n=6), a hemodynamically relevant congenital heart disease had been documented. There were 4.5% (n=4) patients with asthma bronchiale [31].
In 3.4% (n=3), the hMPV infection occurred in pediatric cancer patients, one of whom was still receiving intensive cytotoxic treatment. One patient (1.1%) had cystic fibrosis.
Clinical symptoms and diagnoses
In most patients, the hMPV infection was confirmed during the first week of the respiratory illness (median at day 7 after the onset of symptoms). Table 2 indicates the symptoms in the two matched groups. At least one episode of apnea was documented in 10.2% of all patients. Regarding only the children born prematurely (total collective n=24, hMPV-positive n=12, RSV-positive n=13), apnea occurred in 29.2%, 41.7%, and 30.8%. All seizures reported (in the total collective, n=6, 6.8%) were either febrile convulsions or seizures in patients with known epilepsy. No significant differences were confirmed between the two matched groups. The final diagnoses in the hMPV-positive group were obstructive bronchitis in 47.9%; upper respiratory tract infection in 20.8%; other pneumonia in 10.4%; croup or acute otitis media in 8.3%; central pneumonia, febrile seizure, apnea-bradycardia syndrome or exacerbation of asthma in 6.3%; and serositis, urinary tract infection, and encephalitis [32] in 1.9%. In the RSV-positive patients, 43.8% were diagnosed as obstructive bronchitis; 20.8% as central pneumonia; 16.7% as upper respiratory tract infection; 14.6% as acute otitis media; 4.2% as other pneumonia, croup, or apnea; and 2.1% as exacerbation of asthma [31]. Laboratory data are summarized in Table 3 and radiological findings in Fig. 2.
Distribution of the radiographic findings on chest x-ray obtained during the acute hMPV infection. A minimum of one chest x-ray was performed per patient during the acute hMPV infection. Neither the higher percentage of central pneumonias in RSV-positive (p=0.063) nor the predominance of other pneumonias in hMPV-positive (p=0.241) showed statistical significance. [hMPV+ patients infected only with hMPV, RSV+ RSV-positive, hMPV-negative (matched) controls, NA no abnormalities]
Therapy
Various systemic and inhalative treatment approaches were used in the hMPV-infected patients studied at the discretion of the attending physicians (Fig. 3).
Proportion of clinical episodes in which inhalative and systemic treatment approaches were used. [hMPV+ patients infected only with hMPV, RSV+ RSV-positive, hMPV-negative (matched) controls, cort inhal budesonide inhalation, adrenaline epinephrine inhalation, cort syst intravenous or oral prednisone]
In 58.3% of all hMPV-positive episodes, empirical antibacterial chemotherapy was administered.
Complications
In 4.5% (n=4), at least one episode of arrhythmia was described (febrile sinus tachycardia excluded). One patient in the total collective (1.1%) had to be intubated and mechanically ventilated. This patient was initially positive for RSV and negative for hMPV and tested positive for hMPV 7 days later, most probably indicating a nosocomial infection. In the matched RSV-positive group, one of 48 patients (2.1%) had to be intubated and mechanically ventilated. A relevant proportion of the patients were treated in the intensive care unit [total collective n=16 (18.2%), hMPV-positive n=10 (20.8%), RSV-positive n=5 (10.4%)]. In the hospital, apnea-bradycardia syndrome in prematurely born infants was detected in 16.7% (n=4) of the total collective, 25.0% (n=3) of the hMPV-positive patients, and 15.4% (n=2) of the RSV-positive patients.
Severity of illness
The distribution of the illness severity grades and the clinical severity indices (Fig. 4) showed no significant differences between the groups of hMPV-positive patients and RSV-positive patients (p=0.76). Of the 88 hMPV-infected patients, none was intubated directly as a result of hMPV infection (grade 1), 42% received supplemental oxygen (grade 2), 52% needed only supportive care without oxygen (grade 3), and 6% were mechanically ventilated because of other reasons at the time of the infection (grade 4).
The corresponding results for the RSV-positive group were 2% grade 1, 38% grade 2, 56% grade 3, and 4% grade 4. The prematurely born infants of the hMPV-positive subgroup deserve foremost attention because these children showed the lowest CLSI (0.5), corresponding to the highest illness severity.
Discussion
In contrast to smaller surveys [2, 3, 37], this study did not find a lower illness severity when comparing hMPV-infected patients with thoroughly matched RSV-positive patients without hMPV coinfection [16]. Indeed, there was a trend to a higher proportion of patients with hypoxemia on admission and intensive care treatment in the hMPV-positive group. We also identified apnea as a relevant symptom and complication in patients with hMPV infection, in particular in more than 40% of all hMPV-positive prematurely born infants. This information is important for emergency physicians so they can admit high-risk patients for adequate inpatient monitoring during the acute illness [10].
According to Bosis et al. [3], who investigated 35 hMPV-infected children attending a pediatric outpatient service, 5.7% had to be admitted to the hospital, and 50% featured at least one risk factor linked to higher rehospitalization rates in infants with VRTI or to a complicated clinical course [24].
Furthermore, data concerning the incubation period of hMPV in humans are still lacking. Kuiken et al. [17] observed a viral excretion peak at day 4 postinfection and no residual virus at day 10 in experimentally infected macaques. Interestingly, in our study hMPV-RNA was isolated from nasopharyngeal aspirate specimens of two patients 3 and 4 weeks after the first detection. Although the time of positivity has not been systematically investigated for hMPV-PCR after acute hMPV infection, prolonged viral shedding after cessation of clinical symptoms has not been described in hMPV [4].
Thus, hMPV is identified almost exclusively in patients with acute respiratory infection [2, 37]. This contrasts with the situation in RSV-infected children, who may shed the virus and remain antigen-positive for up to 6 weeks (data from the DSM RSV Paed database), and up to 15% of asymptomatic infants may be RSV-antigen-positive in the winter months. Although the sensitivity of PCR methods to detect RSV-RNA is higher[1, 26], the cost and duration of this method impede its routine use in clinical practice. In addition, the positive predictive value of a positive PCR is unclear, as virus RNA may persist as detectable by PCR after symptoms have been gone for a long time.
We compared only symptomatic patients with acute respiratory illness leading to hospitalization, a situation in which the immunochromatography test used in this study for detecting RSV antigen has displayed sufficient sensitivity and specificity (both >90%). We did not investigate serum samples from the time point of admission for antibodies against hMPV or RSV to exclude children with reinfection. Standardized antibody detection methods for anti-hMPV immunoglobulin are missing or do not differentiate maternally-derived antibodies in infants [18]. Most children at the age of 24 months have experienced at least one RSV infection [1], but the mounted immune response often fails to persist or is not protective against the actual circulating RSV serotype [26]. The children investigated here were ill enough to become hospitalized irrespective of their preexisting antibody status.
The cumulative proportion of hMPV-positive specimens in this study (17.9%) complies with the results of two recent German investigations. In addition to the single center report of Viazov et al. [38] (17.4%), König et al. detected hMPV in 18% (15 of 85) of patients admitted to intensive care during a prospective multicenter prevalence study for respiratory pathogens in four German hospitals [16].
Of those children, 60% were coinfected with RSV. In other countries, hMPV has been detected in 3.9% [25] to 25.6% [20] of all symptomatic children. Due to the characteristic hMPV seasonality [4, 23, 37], the proportion of hMPV positive specimens may be higher in late winter and early spring [5]. Seasonal differences in the burden of disease (i.e., number of hospitalized patients) and in mean illness severity impede tentative conclusions from the analysis of a single hMPV season [9]. In contrast to other studies [4, 39], we noted that the hMPV epidemic did not peak later than the corresponding RSV activities (Fig. 1).
A comparison with 397 prospectively documented RSV infections in hospitalized children (DSM RSV Paed surveillance at Children’s Hospital, University of Bonn, 1999–2003) confirmed that the median and mean age of hMPV-infected hospitalized patients was higher, and no newborn infants (age ≤4 weeks) were found to be hMPV-positive. It is unclear whether the low incidence of hMPV in newborns is a result of protection derived by maternal antibodies. When serum antibodies were measured in infants at the age of 4 months, 60% were seropositive for RSV and 43% for hMPV [7, 8]. As observed by Peiris et al. [27], clinically relevant radiological abnormalities were detected in the majority of patients in whom chest radiography was performed. Some of our hMPV patients with severe “obstructive bronchitis” might have been allocated to the diagnosis of bronchiolitis in other protocols [6, 10, 26].
Following the inclusion criteria of the respective studies, most patients investigated elsewhere were younger than 24 months at diagnosis [5, 37]. The proportion of hMPV-positive specimens is assumed to be higher in infants and children up to 36 months of age [27] compared with older children and adolescents. However, more than one-third of all cases would have been missed if nasopharyngeal aspirates were obtained only from children younger than 24 months.
Other groups documented higher proportions (34–38%) of patients with pneumonia [5, 27] but often did not confirm pneumonia by chest x-ray.
The proportion of patients with clinical “pneumonia” decreased from 38% to 9.4% when independent (blinded) radiologists reviewed the chest x-rays [27]. It seems prudent not to do chest x-rays in all hMPV-infected patients routinely because even in those with the clinical suspicion of pneumonia (indication for chest x-ray at our institution), 30–40% showed no abnormalities. As clearly proven in this study, hMPV infection may cause lobar infiltrates [6], documented as “other pneumonia.”
Treatment of viral lower respiratory tract infection in pediatric inpatients is symptomatic. Some preclinical data imply that ribavirin may be active against hMPV. Palivizumab, a humanized monoclonal antibody against the F-protein of RSV, does not display any antiviral activity against hMPV in vitro [40]. The use of corticosteroids and bronchodilatators is controversial in bronchiolitis, obstructive bronchitis, and pneumonia triggered by a viral infection because no benefit in terms of mortality, duration of symptoms, or median length of hospital stay has been confirmed by comprehensive studies [1, 10, 26]. In our study, patients with antibiotics had significantly higher median illness severity scores, but none of them fulfilled the internally defined laboratory criteria of a suspected bacterial infection (leukocytes <15.0×109/l, band forms >15%, and C-reactive protein >20 mg/l). There is no reason to assume that bacterial coinfection is more prevalent in hMPV-infected patients compared with the lower than 5% confirmed events in RSV-infected children [26]. The lack of a reliable and fast bedside test to rapidly confirm or exclude the viral etiology may have contributed to the unacceptably high proportion of children receiving empiric antibiotic treatment [19, 22, 34]. Laboratory signs of bacterial infection were not indicative for radiologically confirmed lobar or segmental pneumonia in hMPV-infected patients. The role of hMPV as a copathogen is still debated [2, 3, 16, 20].
In our study, it was impossible to discriminate hMPV-positive patients from patients with coinfection on clinical grounds. Other groups found coinfection rates in up to 70% (hMPV and RSV) in mechanically ventilated infants with bronchiolitis [13], a 10-fold increase in relative risk of admission to a pediatric intensive care unit for mechanical ventilation in hMPV/RSV-coinfected patients [33] and 60% coinfections in children requiring intensive care [16]. Although RSV has been linked to acute asthma exacerbation, and severe RSV infection in infancy significantly increases the risk of recurrent wheezing up to the age of 10 [1, 11, 26], the contribution of hMPV to these conditions is controversial [15, 27, 29, 31, 39].
In summary, our data underscore that hMPV infection is not milder but displays clinical signs and illness severity equal to RSV infection in hospitalized patients. In particular, prematurely born infants experience a more severe clinical course. From a clinical perspective and considering specific and nonspecific efforts of prevention, hMPV deserves as much attention as RSV.
Contributors
Anja Wilkesmann, Michael J. Lentze, Udo Bode, and Arne Simon were responsible for all patient-related medical procedures. Arne Simon is the principal investigator of the DSM RSV Paed study. Oliver Schildgen, Anna Maria Eis-Hübinger, Tilman Geikowski, and Thomas Glatzel were responsible for virological laboratory procedures.
References
Black CP (2003) Systematic review of the biology and medical management of respiratory syncytial virus infection. Respir Care 48:209–231; discussion 231–203
Boivin G, De Serres G, Cote S, Gilca R, Abed Y, Rochette L, Bergeron MG, Dery P (2003) Human metapneumovirus infections in hospitalized children. Emerg Infect Dis 9:634–640
Bosis S, Esposito S, Niesters HG, Crovari P, Osterhaus AD, Principi N (2005) Impact of human metapneumovirus in childhood: comparison with respiratory syncytial virus and influenza viruses. J Med Virol 75:101–104
Crowe JE Jr (2004) Human metapneumovirus as a major cause of human respiratory tract disease. Pediatr Infect Dis J 23:S215–S221
Dollner H, Risnes K, Radtke A, Nordbo SA (2004) Outbreak of human metapneumovirus infection in Norwegian children. Pediatr Infect Dis J 23:436–440
Donnelly LF (2001) Practical issues concerning imaging of pulmonary infection in children. J Thorac Imaging 16:238–250
Ebihara T, Endo R, Kikuta H, Ishiguro N, Ishiko H, Hara M, Takahashi Y, Kobayashi K (2004) Human metapneumovirus infection in Japanese children. J Clin Microbiol 42:126–132
Ebihara T, Endo R, Kikuta H, Ishiguro N, Ishiko H, Kobayashi K (2004) Comparison of the seroprevalence of human metapneumovirus and human respiratory syncytial virus. J Med Virol 72:304–306
Esper F, Martinello RA, Boucher D, Weibel C, Ferguson D, Landry ML, Kahn JS (2004) A 1-year experience with human metapneumovirus in children aged <5 years. J Infect Dis 189:1388–1396
Fitzgerald DA, Kilham HA (2004) Bronchiolitis: assessment and evidence-based management. Med J Aust 180:399–404
Gern JE (2004) Viral respiratory infection and the link to asthma. Pediatr Infect Dis J 23:S78–S86
Goldstein B, Giroir B, Randolph A (2005) International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6:2–8
Greensill J, McNamara PS, Dove W, Flanagan B, Smyth RL, Hart CA (2003) Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg Infect Dis 9:372–375
Ison MG, Hayden FG (2002) Viral infections in immunocompromised patients: what’s new with respiratory viruses? Curr Opin Infect Dis 15:355–367
Jartti T, van den Hoogen B, Garofalo RP, Osterhaus AD, Ruuskanen O (2002) Metapneumovirus and acute wheezing in children. Lancet 360:1393–1394
Konig B, Konig W, Arnold R, Werchau H, Ihorst G, Forster J (2004) Prospective study of human metapneumovirus infection in children less than 3 years of age. J Clin Microbiol 42:4632–4635
Kuiken T, van den Hoogen BG, van Riel DA, Laman JD, van Amerongen G, Sprong L, Fouchier RA, Osterhaus AD (2004) Experimental human metapneumovirus infection of cynomolgus macaques (Macaca fascicularis) results in virus replication in ciliated epithelial cells and pneumocytes with associated lesions throughout the respiratory tract. Am J Pathol 164:1893–1900
Leung J, Esper F, Weibel C, Kahn JS (2005) Seroepidemiology of human metapneumovirus (hMPV) on the basis of a novel enzyme-linked immunosorbent assay utilizing hMPV fusion protein expressed in recombinant vesicular stomatitis virus. J Clin Microbiol 43:1213–1219
Low DE, Pichichero ME, Schaad UB (2004) Optimizing antibacterial therapy for community-acquired respiratory tract infections in children in an era of bacterial resistance. Clin Pediatr (Phila) 43:135–151
Maggi F, Pifferi M, Vatteroni M, Fornai C, Tempestini E, Anzilotti S, Lanini L, Andreoli E, Ragazzo V, Pistello M, Specter S, Bendinelli M (2003) Human metapneumovirus associated with respiratory tract infections in a 3-year study of nasal swabs from infants in Italy. J Clin Microbiol 41:2987–2991
McIntosh ED, De Silva LM, Oates RK (1993) Clinical severity of respiratory syncytial virus group A and B infection in Sydney, Australia. Pediatr Infect Dis J 12:815–819
McIntosh K (2002) Community-acquired pneumonia in children. N Engl J Med 346:429–437
McIntosh K, McAdam AJ (2004) Human metapneumovirus-an important new respiratory virus. N Engl J Med 350:431–433
Meissner HC (2003) Selected populations at increased risk from respiratory syncytial virus infection. Pediatr Infect Dis J 22:S40–S44; discussion S44–S45
Mullins JA, Erdman DD, Weinberg GA, Edwards K, Hall CB, Walker FJ, Iwane M, Anderson LJ (2004) Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis 10:700–705
Ogra PL (2004) Respiratory syncytial virus: the virus, the disease and the immune response. Paediatr Respir Rev 5(Suppl A):S119–S126
Peiris JS, Tang WH, Chan KH, Khong PL, Guan Y, Lau YL, Chiu SS (2003) Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis 9:628–633
Pelletier G, Dery P, Abed Y, Boivin G (2002) Respiratory tract reinfections by the new human metapneumovirus in an immunocompromised child. Emerg Infect Dis 8:976–978
Rawlinson WD, Waliuzzaman Z, Carter IW, Belessis YC, Gilbert KM, Morton JR (2003) Asthma exacerbations in children associated with rhinovirus but not human metapneumovirus infection. J Infect Dis 187:1314–1318
Reina J, Gonzalez Gardenas M, Ruiz de Gopegui E, Padilla E, Ballesteros F, Mari M, Munar M (2004) Prospective evaluation of a dot-blot enzyme immunoassay (Directigen RSV) for the antigenic detection of respiratory syncytial virus from nasopharyngeal aspirates of paediatric patients. Clin Microbiol Infect 10:967–971
Schildgen O, Geikowski T, Glatzel T, Simon A, Wilkesmann A, Roggendorf M, Viazov S, Matz B (2004) New variant of the human metapneumovirus (HMPV) associated with an acute and severe exacerbation of asthma bronchiale. J Clin Virol 31:283–288
Schildgen O, Glatzel T, Geikowski T, Scheibner B, Matz B, Bindl L, Born M, Viazov S, Wilkesmann A, Knöpfle G, Roggendorf M, Simon A (2005) Human metapneumovirus RNA in encephalitis patient. Emerg Infect Dis 11:467–470
Semple MG, Cowell A, Dove W, Greensill J, McNamara PS, Halfhide C, Shears P, Smyth RL, Hart CA (2005) Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis 191:382–386
Toikka P, Irjala K, Juven T, Virkki R, Mertsola J, Leinonen M, Ruuskanen O (2000) Serum procalcitonin, C-reactive protein and interleukin-6 for distinguishing bacterial and viral pneumonia in children. Pediatr Infect Dis J 19:598–602
Ulloa-Gutierrez R, Skippen P, Synnes A, Seear M, Bastien N, Li Y, Forbes JC (2004) Life-threatening human metapneumovirus pneumonia requiring extracorporeal membrane oxygenation in a preterm infant. Pediatrics 114:e517–e519
van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD (2001) A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med 7:719–724
van den Hoogen BG, Osterhaus DM, Fouchier RA (2004) Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J 23:S25–S32
Viazov S, Ratjen F, Scheidhauer R, Fiedler M, Roggendorf M (2003) High prevalence of human metapneumovirus infection in young children and genetic heterogeneity of the viral isolates. J Clin Microbiol 41:3043–3045
Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, Wright PF, Crowe JE, Jr. (2004) Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med 350:443–450
Wyde PR, Chetty SN, Jewell AM, Boivin G, Piedra PA (2003) Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antiviral Res 60:51–59
Acknowledgements
The authors are grateful to Carola Maiworm and Sergei Viazov for critical comments on the manuscript and to Max Konstantin von Renesse for support with statistical analysis. This work was supported by a grant from the Else-Kröner-Fresenius-Stiftung (Bad Homburg, Germany; grant number A01/05//F00).
Conflict of interest statement
None of the investigators has any potential conflict of interest. All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and regional) and with the Helsinki Declaration of 1975, as revised in 2000. The DSM RSV Paed study is sponsored by a grant from Abbott, Wiesbaden, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
The first two authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wilkesmann, A., Schildgen, O., Eis-Hübinger, A.M. et al. Human metapneumovirus infections cause similar symptoms and clinical severity as respiratory syncytial virus infections. Eur J Pediatr 165, 467–475 (2006). https://doi.org/10.1007/s00431-006-0105-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-006-0105-4