Abstract
The aim of the aborted trial was to determine whether the short early dexamethasone (DX) given after the birth improves the early outcome. We also reviewed the evidence (meta-analysis) to determine whether the duration of early DX treatment influences the early outcome, particularly in terms of bronchopulmonary dysplasia (BPD). The participants of the randomised multicentre, double-blinded placebo-controlled trial had a birth weight 500–999 g, gestation ≤31.0 weeks, and respiratory failure by the age of 4 h. The infants received either four doses of DX (0.25 mg/kg at 12 h intervals) or placebo. The meta-analysis was performed to determine the beneficial and adverse effects of early short (<96 h duration) versus early prolonged (>96 h) DX treatment. The trial was discontinued after 109 infants had been enrolled. There was a non-significant improvement in the outcome (survival without BPD, severe intracranial haemorrhage or periventricular leukomalacia; RR 1.27; 95% CI 0.87–1.85). The risks for gastrointestinal perforation and hyperglycaemia tended to increase. A total of 15 trials were included in the meta-analysis: 10 involved prolonged (i.e. >96 h; 1594 infants) and five short interventions (1069 infants). Early prolonged DX decreased the RR for BPD to 0.72 (95% CI 0.61–0.87), whereas early short DX course did not significantly decrease the risk (RR 0.82; 95% CI 0.64–1.05). Gastrointestinal haemorrhages and perforations were significantly increased only in the early prolonged DX group. Conclusion:The dosage and duration of early corticosteroid given to small premature infants influences the risk of the side-effects and the early outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the advances in treatment of extremely low birth weight (ELBW) infants, bronchopulmonary dysplasia (BPD) continues to be a serious problem associated with re-hospitalisation and adverse neurodevelopmental outcome [31,41]. The degree of prematurity, the severity of respiratory distress syndrome (RDS), the need for mechanical ventilation, pre- and postnatal infections and persistent ductus arteriosus (PDA) are factors associated with BPD [4, 26,35]. According to randomised studies, dexamethasone (DX) treatment decreases the duration of mechanical ventilation and supplemental oxygen in very premature infants with respiratory failure [7, 13, 14, 15, 16, 19, 28, 30, 34, 40, 44,45]. Early DX treatment started before 3 days of age has been shown to decrease the incidence of BPD in small preterm infants [7, 10, 15, 28,45]. Meta-analyses of early (<96 h), moderately early (<7–14 days) and delayed corticosteroids show a decreased risk for BPD regardless of the onset of DX [14, 15,16]. Despite the favourable effect on BPD, DX given to premature infants has been associated with side-effects including hyperglycaemia, hypertension, cardiac hypertrophy, infections, suppression of cortisol secretion, gastrointestinal bleeding and perforation, impaired somatic and brain growth, and periventricular leukomalacia (PVL) [10, 14, 15, 16, 20, 23, 43,45]. Follow-up studies revealed more developmental problems such as impairment of neuromotor function and an increased risk of cerebral palsy, among children in the DX arm [24, 33,46]. The crucial issue is to find out whether the favourable respiratory outcome can be achieved without serious side-effects.
Based on a non-randomised pilot study done at Oulu University Hospital, we performed a randomised, placebo-controlled multicentre study on short early DX treatment beginning 6 h after birth and lasting for 2 days. The plan was to evaluate whether the treatment increases the survival without BPD, PVL, or severe intracranial haemorrhage (ICH). The study enrollment was discontinued early (February 2001), after serious adverse effects had been reported by others. In order to analyse the possible influence of the duration of very early DX on the outcome, a meta-analysis was performed.
Subjects and methods
Study population
The study was a randomised, placebo-controlled trial involving six neonatal units. The study protocol was approved by the ethics committees of all the participating hospitals. Written informed consent was obtained from the parents before enrolment. The entry criteria for the eligible infants were: birth weight 500–999 g, gestation ≤31.0 weeks, need for mechanical ventilation and supplemental oxygen by the age of 4 h and no life-threatening congenital anomalies or known chromosomal anomaly.
Randomisation and intervention
Randomisation and preparation of the coded vials was performed blindly by the study investigators in the pharmacy of the coordinating centre. The infants were stratified into two weight groups (500–749 g and 750–999 g). They received from identical vials either four doses of DX (0.25 mg/kg) each at 12 h intervals or normal saline as placebo. The first dose was given before the age of 6 h. Open-label postnatal DX was allowed when deemed necessary according to the attending physician, but its use was discouraged. Any use of glucocorticoid therapy during hospitalisation was recorded. Intention to treat analysis was performed.
Outcome
The primary outcome was survival at 36 weeks post conception without ICH, grade 3–4 [25], PVL or BPD. PVL was defined as the presence of periventricular echodensities after the 1st week or periventricular cysts on a cranial ultrasound [9]. BPD was diagnosed as requirement for supplemental oxygen consistent with signs of respiratory disease at 36 weeks’ gestation.
Secondary outcomes were clinical status and growth at 7 and 28 days and at 36 weeks post conception, the number of days on assisted ventilation and supplemental oxygen, and treatment with open steroids. Nosocomial infection was defined as a positive blood or CSF culture or a strong suspicion with clinical deterioration and increased infection markers (C-reactive protein, leucocytes) after day 3 of life. Hyperglycaemia and hypo- or hypertension requiring therapy according to criteria in each centre, retinopathy of prematurity (ROP) [1], patent ductus arteriosus (PDA) detected by cardiac ultrasound and requiring medical or surgical therapy, and gastrointestinal bleeding, and gastrointestinal perforations were recorded. Necrotising enterocolitis (NEC) was defined according to the criteria of Bell et al. [5].
Cranial ultrasound examination was conducted on all infants between the days 4 to 8, at 36 weeks of gestation, and otherwise as necessary. Ophthalmic examination was performed 4 to 7 weeks after birth or at 32 weeks’ postmenstrual age. Ophthalmic examination was repeated until retinas were mature, and the highest stage of retinopathy was reported. To analyse the growth, z scores (standard deviation score; (measurement of growth parameter minus population mean)/population standard deviation) were computed for weight, length, and head circumference using a reference for intrauterine growth [27].
Meta-analysis
The relevant articles were identified by searching for randomised, controlled studies from the MEDLINE and Cochrane databases, including the Controlled Trials Register. The search terms were: steroids and preterm infants or dexamethasone and BPD, or CLD and dexamethasone limited to children aged <23 months. We searched for additional studies manually from the proceedings of American Pediatric Society/Society of Pediatric Research during years 2000–2003 and articles recently published in Pediatrics, The Journal of Pediatrics, European Journal of Pediatrics, Acta Pediatrica, Archives of Disease of Children and Pediatric Pulmonology, and from the reference lists of the publications.
We evaluated the randomised, placebo-controlled, blinded trials on premature infants with birth weight ≤2000 g given DX treatment or placebo initiated within 3 days after birth. The purpose of our study was to determine whether the use of very early DX therapy is beneficial in preventing BPD and death in premature infants and, on the other hand, whether the duration of DX therapy influences the outcome or the adverse effects.
The primary outcomes were mortality and BPD. BPD was defined as need for oxygen at 36 weeks post conceptional age. The secondary outcomes included ICH, PDA, PVL, and ROP. Finally, the incidence of reported complications was analysed. These included gastrointestinal complications, NEC, infection defined as typical signs and positive blood culture, and hyperglycaemia requiring insulin treatment. We were not able to verify the incidence of hypertension or hypotension because these outcomes were not reported in most studies, and the criteria of hypertension and the indications for antihypertensive treatment varied widely or were not identified.
The selection of articles for analyses and data extraction were performed by one author (O.P.) and independently verified by another investigator (M.H.). When further information was needed, the principal investigators of included studies were contacted. When the data were not available, the study was excluded from the meta-analysis of secondary outcomes.
Statistical analysis
Randomised trial
Sample size justification was based on data from the Vermont-Oxford Neonatal Network database and on data from an unpublished Finnish pilot study performed at Oulu University Hospital. According to prospective analyses of the non-randomised data from Oulu, Finland, early DX would increase the percentage of good outcomes from 40% to 65%. The present study was powered to show evidence of the improvement of the outcome from 45% to 65% among all infants. Given the errors (α=0.05 and β=0.2), 96 patients in each group would be required. Baseline data for the infants enrolled in the study were compared by unpaired t -tests for continuous variables and by chi-squared tests for categorical data. In outcome analysis, we calculated the relative risks (RR) with 95% confidence intervals (CI) and also compared the differences by chi-squared tests for categorical data. The single continuous variables were compared by unpaired t -tests between the two groups and the repeated measurements were analysed by the repeated measurements of ANOVA and by using summary measures [22]. Statistical analyses were performed using SPSS 11.5 for Windows (SPSS Inc, Chicago).
Meta-analysis
The pooled risk ratios for event reduction with 95% CI for the outcome were calculated using the random effect model (Mantel-Haenszel). The testing of within-study and between-studies variability was included in the analysis. The data were analysed using Comprehensive Meta-Analysis, version 1.0.25 (BioStat).
Results
Randomised trial
Patient population
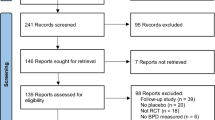
During the study period from June 1998 to February 2001, altogether 109 infants were enrolled, 53 in the DX group and 56 in the placebo group. The Safety Monitoring Committee recommended the continuation of the trial after recruitment of 96 infants as no difference in early adverse events between the study groups was seen. However, the Steering Committee decided to discontinue the trial after enrolment of 109 patients because of the published studies reporting late neurodevelopmental problems in infants who had received postnatal DX [24, 33, 46]. The baseline characteristics of the study groups are shown in Table 1. There were no significant differences in the gestational ages or the incidence of vaginal deliveries between DX and placebo groups, when analysed in stratified weight groups (500–749 g and 750–999 g).
Primary outcome
The frequency of good outcomes (survival without BPD, ICH grade 3 to 4, or PVL) tended to be higher in the DX group (Table 2). Given the present risk ratio of 1.27, the result would not have reached the significance with recruitment of 96 infants in each arm (95% CI 0.95–1.68). The RR for death or BPD at the age of 36 gestational weeks was 0.78 (95% CI 0.54 to 1.13) in the DX group, and 0.61 (95% CI 0.33 to 1.11) in the subgroup of DX infants with a birth weight from 750 to 999 g. There were no detectable trends in mortality, severe ICH or PVL.
Secondary outcomes
The rates of ROP, PDA or neonatal infections did not differ between the DX and placebo groups (Table 3). In infants receiving indomethacin for PDA, the incidence of any of the gastrointestinal complications (haemorrhage, perforation, or NEC) was 15% in the DX group versus 9% in the placebo group (RR 1.7, 95% CI 0.59 to 4.84, P =0.4).
The mean arterial blood pressures were increased ( P =0.015) in the DX group during the 1st week (Fig. 1). However, the number of infants receiving medication to increase blood pressure and the duration of this medication were similar in both groups (Table 3). The DX group tended to require more insulin therapy (49% vs. 39%, P =0.25), and the duration of insulin therapy was longer in that group (mean 4.8 days vs. 2.3 days, P =0.045).
At enrolment, the mean ventilation index (VI = mean airway pressure × FiO2) was similar in both groups (5.3 vs. 5.1, P =0.7). After the study period on day 3 (1.2 vs. 3.1, P =0.036) and day 7 (1.5 vs. 2.4, P =0.039), VI was lower in the DX group. Of infants in both study groups, 91% received surfactant shortly after the birth (mean 4 h). Only 42% of infants in the DX group versus 69% in the placebo group ( P =0.006) received more than one dose of surfactant. The other respiratory outcomes did not differ between the groups.
The open glucocorticoid therapy was common in both groups (66% in the DX group vs. 73%, Table 4), and the total dosage of glucocorticoid did not differ between the groups. The increase in weight, length, and head circumference from birth to 36 weeks of gestational age was similar in both groups (Table 5), although the z-scores for weight at birth were higher in the DX group than in the placebo group.
Meta-analysis
Selection of studies
Using our keywords, we found 2160 articles. We selected the placebo-controlled randomised trials studying the efficacy of DX treatment starting before 3 days of age. With the inclusion criteria of early (≤72 h of age) DX trials, we found 15 articles. Early prolonged (>96 h duration) DX treatment was used in ten trials, and early short (<96 h duration) DX treatment in five studies, in which the present report was included (Table 6 and Table 7). In the early short DX trials, the dosage varied from a single dose to six doses at 12 h intervals, and the total dose of DX varied from 0.2 to 1.5 mg/kg. In the early prolonged DX studies, the duration of treatment varied from 5 to 28 days and the total dosage from 0.9 to 14.0 mg/kg.
Neonatal mortality and morbidity
There was no significant difference in neonatal mortality either in the meta-analysis or in any individual study (Table 8). Early short DX treatment tended to reduce the risk of BPD at the age of 36 gestational weeks, but the difference was not statistically significant (Fig. 2). Early prolonged DX therapy had no effect on neonatal mortality (Table 9), but it significantly reduced the incidence of BPD at 36 weeks (Fig. 3).
Meta-analysis of the RR of BPD at the age of 36 gestational weeks for the randomised controlled trials concerning early short DX treatment starting within 3 days of life in preterm infants. The duration of early short DX treatment is less than 96 h in the included studies. BPD is defined as the need for oxygen therapy at the age of 36 post conceptional weeks. Test of heterogeneity between studies ( P =0.713)
Meta-analysis of the RR of BPD at the age of 36 gestational weeks for the randomised controlled trials concerning early prolonged DX treatment starting within 3 days of life in preterm infants. The duration of DX treatment is more than 96 h in the included studies. BPD is defined as the need for oxygen therapy at the age of 36 post conceptional weeks. Test of heterogeneity between studies ( P =0.066)
According to the meta-analysis, the risk of PDA was significantly reduced in the DX group in both early short and prolonged DX therapy (Table 8 and Table 9). The risk of severe ROP was reduced in the early prolonged DX group, but there was no difference in the rate of total ROP. DX increased the need for insulin for hyperglycaemia regardless of the duration of DX therapy.
The early prolonged DX therapy was significantly associated with gastrointestinal bleeding and perforations. All other secondary outcomes were similar between the study groups (Table 8 and Table 9).
Discussion
In our study comparing short early DX to placebo given to very high-risk ELBW infants, we demonstrated a non-significant improvement in the good outcome defined as survival without BPD, severe ICH, or PVL. The study was under-powered because only about 50% of the planned patients had been enrolled before the termination of enrolment due to concern about the late adverse effects [24, 33,46]. The severity of early respiratory failure was milder in the DX arm in our study, as also seen in the previously published reports of postnatal DX treatment [14, 15, 16, 18, 21, 28, 30,42]. Despite the evidence of improvement of the cardio-respiratory measurements, the observed acute increase in mean blood pressure did not decrease the use of dopamine or dobutamine as would have been desirable. The data shown here are not inconsistent with the current recommendation against the use of systemic DX for the prevention or treatment of chronic lung disease in infants with very low birth weight [2].
No significant difference in early growth was found between the study groups either in the present trial or in other trials of early DX therapy [10, 29, 30,35]. Although the pharmacological dose of DX strongly suppresses growth, there is remarkable postnatal growth retardation among the infants born very premature, regardless of the use of steroids [11]. The influence of DX on early growth and the risk of late metabolic consequences remain unknown [17,39].
A bias and notable concern related to virtually all the DX trials reported is caused by the fact that both study arms were contaminated by open steroid use. This further complicates the interpretation of both beneficial and harmful effects of steroids.
The relationship of exogenous glucocorticoid with the birth process is likely to be critical in view of efficacy and safety. We approached this issue by a randomised trial and a meta-analysis of the early neonatal DX trials with the hypothesis that a short course would be beneficial and have minimal side-effects. The previously published controlled trials and meta-analyses have shown that despite the beneficial effects of postnatal DX treatment on the incidence of BPD [10, 14, 15, 16, 28,45], the treatment had acute side-effects including hyperglycaemia, hypertension, intestinal haemorrhage and perforation [7, 10, 13, 14, 15, 16, 32, 36,44]. Since the dosage and duration of DX treatment in prevention of BPD have varied widely in controlled trials, the meta-analysis focused on the duration of early DX treatment.
According to previous meta-analyses, early postnatal DX treatment had no effect on neonatal mortality despite the statistically significant decrease in the risk of BPD [15]. In these meta-analyses, the duration of DX treatment varied from 1 day to 4 weeks and was started within 2 h to 15 days from birth [14, 15,16]. In our meta-analysis we divided the trials starting within 3 days from birth on the basis of duration of DX into short (<96 h) and prolonged (>96 h) therapies. This enabled us to further evaluate the benefits and risks of the duration of early DX treatment. According to the present analysis, the early short course of DX therapy had no statistically significant positive effect on the risk of BPD, and no trend in postnatal mortality at the latest reported age. On the other hand, the early prolonged DX therapy had a significant beneficial effect by reducing the incidence of BPD, but no significant effect on mortality. Both early and prolonged DX therapy promoted spontaneous closure of PDA, confirming the earlier results [15].
An increased risk of infections has been associated with DX therapy in preterm infants [16]. However, our meta-analysis did not show this trend (Tables 8 and Table 9). The risk of hyperglycaemia was significantly increased in the DX group, regardless of the duration of therapy.
DX treatment increases the risk of gastrointestinal perforations [14, 15,16]. According to the present meta-analysis, this tendency was significant only among the infants on early prolonged DX therapy. In contrast, the previous meta-analysis on early DX reported no increase in the NEC rate [15]. This difference between the gastrointestinal bleedings and perforations versus NEC may be explained by the pathogenesis of these diseases. NEC is an inflammatory disease, which could explain why anti-inflammatory DX does not increase the risk [3,12]. In contrast, gastrointestinal perforation is likely to have a non-inflammatory pathogenesis. The interference of steroids with rapid proliferation of gastrointestinal tissue could serve as a factor leading to intestinal perforation [6,47]. DX appears to increase the susceptibility to focal injury in the watershed areas in the intestine and the periventricular area of the brain. However, the mechanism remains elusive.
There are concerns about neurodevelopmental complications in very preterm infants due to DX therapy in the perinatal period. We evaluated the neonatal intracerebral insults that serve as risk factors for abnormal neurodevelopment. The difference in risk of severe or any grade of ICH was not confirmed in present meta-analysis. Antenatal DX has been shown to protect against ICH [8]. Only three individual trials (including the present one) on early short DX therapy had data available for the evaluation of the risk of PVL [10,32]. There was a tendency towards an increased risk of PVL, but the difference was not significant (Table 8). Halliday et al. [15] pooled all the early DX trials together into a meta-analysis and found a similar risk of PVL (RR 1.37; 95% CI 0.91 to 2.05). The present results are consistent with the possibility that even a short course of DX after very premature birth may insult the brain of a very premature infant.
We conclude that a short course of DX acutely improves the respiratory adaptation of ELBW infants shortly after birth. However, a course of DX longer than 4 days (5 to 28 days) may be required for substantial protection against BPD at 36 weeks gestation. Lengthening of the duration of DX may also increase the risk of intestinal complications. Routine administration of corticosteroid to ELBW infants is not indicated. Controlled studies are required to evaluate the critical roles of corticosteroids after very premature birth.
Abbreviations
- BPD :
-
bronchopulmonary dysplasia
- CI :
-
confidence interval
- DX :
-
dexamethasone
- ELBW :
-
extremely low birth weight (<1000 g)
- ICH :
-
intracranial haemorrhage
- NEC :
-
necrotising enterocolitis
- PDA :
-
patent ductus arteriosus
- PVL :
-
periventricular leukomalacia
- RDS :
-
respiratory distress syndrome
- ROP :
-
retinopathy of prematurity
- RR :
-
relative risk
References
Anonymous (1984) An international classification of retinopathy of prematurity. Pediatrics 74: 127–133
American Academy of Pediatrics, Committee of Fetus and Newborn, Canadian Paediatric Society, Fetus and Newborn Committee (2002) Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics 109: 330–338
Ballance WA, Dahms BB, Shenker N, Kliegman RM (1990) Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr 117: S6–S13
Bancalari E, Claure N, Sosenko IR (2003) Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol 8: 63–71
Bell MJ, Ternberg, JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T (1978) Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 187: 1–7
Brownlee KG, Ng PC, Henderson MJ, Smith M, Green JH, Dear PR (1992) Catabolic effect of dexamethasone in the preterm baby. Arch Dis Child 67:1–4
Collaborative Dexamethasone Trial Group (1991) Dexamethasone therapy in neonatal chronic lung disease: an international placebo-controlled trial. Pediatrics 88: 421–427
Crowley P (2004) Prophylactic corticosteroids for preterm birth (Cochrane Review). In: The Cochrane Library, Issue I, Wiley, Chichester
De Vries LS, Eken P, Dubowitz LM (1992) The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res 49: 1–6
Garland JS, Alex CP, Pauly TH, Whitehead VL, Brand J, Winston JF, Samuels DP, McAuliffe TL (1999) A three-day course of dexamethasone therapy to prevent chronic lung disease in ventilated neonates: a randomized trial. Pediatrics 104: 91–99
Hack M, Schluchter M, Cartar L, Rahman M, Cuttler L, Borawski E (2003) Growth of very low birth weight infants to age 20 years. Pediatrics 112: e30
Halac E, Halac J, Begue EF, Casanas JM, Indiveri DR, Petit JF, Figueroa MJ, Olmas JM, Rodriguez LA, Obregon RJ (1990) Prenatal and postnatal corticosteroid therapy to prevent neonatal necrotizing enterocolitis: a controlled trial. J Pediatr 117: 132–138
Halliday HL, Patterson CC, Halahakoon CW (2001) A multicenter, randomized open study of early corticosteroid treatment (OSECT) in preterm infants with respiratory illness: comparison of early and late treatment and of dexamethasone and inhaled budesonide. Pediatrics 107: 232–240
Halliday HL, Ehrenkranz RA, Doyle LW (2004) Delayed (>3 weeks) postnatal corticosteroids for chronic lung disease in preterm infants (Cochrane Review). In: The Cochrane Library. Issue I, Wiley, Chichester
Halliday HL, Ehrenkranz RA, Doyle LW (2004) Early postnatal (<96 hours) corticosteroids for preventing chronic lung disease in preterm infants (Cochrane Review). In: The Cochrane Library, Issue I, Wiley, Chichester
Halliday HL, Ehrenkranz RA, Doyle LW (2004) Moderately early (7–14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants (Cochrane Review). In: The Cochrane Library, Issue I, Wiley, Chichester
Kajantie E, Eriksson J, Barker DJ, Forsen T, Osmond C, Wood PJ, Andersson S, Dunkel L, Phillips DI (2003) Birth size, gestational age and adrenal function in adult life: studies of dexamethasone suppression and ACTH1–24 stimulation. Eur J Endocrinol 149: 569–575
Kopelman AE, Moise AA, Holbert D, Hegemier SE (1999) A single very early dexamethasone dose improves respiratory and cardiovascular adaptation in preterm infants. J Pediatr 135: 345–350
Kothadia JM, O’Shea TM, Roberts D, Auringer ST, Weaver RG III, Dillard RG (1999) Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants. Pediatrics 104: 22–27
LeFlore JL, Salhab WA, Broyles RS, Engle WD (2002) Association of antenatal and postnatal dexamethasone exposure with outcomes in extremely low birth weight neonates. Pediatrics 110: 275–279
Lin YJ, Yeh TF, Hsieh WS, Chi YC, Lin HC, Lin CH (1999) Prevention of chronic lung disease in preterm infants by early postnatal dexamethasone therapy. Pediatr Pulmonol 27: 21–26
Matthews JN, Altman DG, Campbell MJ, Royston P (1990) Analysis of serial measurements in medical research. BMJ 300: 230–235
Murphy BP, Inder TE, Huppi PS, Warfield S, Zientara GP, Kikinis R, Jolesz FA, Volpe JJ (2001) Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics 107: 217–221
O’Shea TM, Kothadia JM, Klinepeter KL, Goldstein DJ, Jackson BG, Weaver RG III, Dillard RG (1999) Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants: outcome of study participants at 1-year adjusted age. Pediatrics 104: 15–21
Papile LA, Burstein J, Burstein R, Koffler H (1978) Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 92: 529–534
Parker RA, Lindstrom DP, Cotton RB (1992) Improved survival accounts for most, but not all, of the increase in bronchopulmonary dysplasia. Pediatrics 90: 663–668
Pihkala J, Hakala T, Voutilainen P, Raivio K (1989) Characteristic of recent fetal growth curves in Finland. Duodecim 105: 1540–1546
Rastogi A, Akintorin SM, Bez ML, Morales P, Pildes RS (1996) A controlled trial of dexamethasone to prevent bronchopulmonary dysplasia in surfactant-treated infants. Pediatrics 98: 204–210
Romagnoli C, Zecca E, Vento G, Maggio L, Papacci P, Tortorolo G (1999) Effect on growth of two different dexamethasone courses for preterm infants at risk of chronic lung disease. A randomized trial. Pharmacology 59: 266–274
Sanders RJ, Cox C, Phelps DL, Sinkin RA (1994) Two doses of early intravenous dexamethasone for the prevention of bronchopulmonary dysplasia in babies with respiratory distress syndrome. Pediatr Res 36: 122–128
Shennan AT, Dunn S, Ohlsson A, Lennox K, Hoskins EM (1988) Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 82: 527–532
Shinwell ES, Karplus M, Zmora E, Reich D, Rothschild A, Blazer S, Bader D, Yurman S, Dolfin T, Kuint J, Milbauer B, Kohelet D, Goldberg M, Armon Y, Davidson S, Sirota L, Amitai M, Zaretsky A, Barak M, Gottfried S (1996) Failure of early postnatal dexamethasone to prevent chronic lung disease in infants with respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed 74: F33–F37
Shinwell ES, Karplus M, Reich D, Weintraub Z, Blazer S, Bader D, Yurman S, Dolfin T, Kogan A, Dollberg S, Arbel E, Goldberg M, Gur I, Naor N, Sirota L, Mogilner S, Zaritsky A, Barak M, Gottfried E (2000) Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Arch Dis Child Fetal Neonatal Ed 83: F177–F181
Sinkin RA, Dweck HS, Horgan MJ, Gallaher KJ, Cox C, Maniscalco WM, Chess PR, D’Angio CT, Guillet R, Kendig JW, Ryan RM, Phelps DL (2000) Early dexamethasone-attempting to prevent chronic lung disease. Pediatrics 105: 542–548
Speer CP (2003) Inflammation and bronchopulmonary dysplasia. Semin Neonatol 8: 29–38
Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S, Donovan EF, Oh W, Bauer CR, Saha S, Poole WK, Stoll BJ (2001) Adverse effects of early dexamethasone in extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med 344: 95–101
Suske G, Oestreich K, Varnholt V, Lasch P, Kachel W (1996) Influence of early postnatal dexamethasone therapy on ventilator dependency in surfactant-substituted preterm infants. Acta Paediatr 85: 713–718
Tapia JL, Ramirez R, Cifuentes J, Fabres J, Hubner ME, Bancalari A, Mercado ME, Standen J, Escobar M (1998) The effect of early dexamethasone administration on bronchopulmonary dysplasia in preterm infants with respiratory distress syndrome. J Pediatr 132: 48–52
Tenhola S, Martikainen A, Rahiala E, Herrgard E, Halonen P, Voutilainen R (2000) Serum lipid concentrations and growth characteristics in 12-year-old children born small for gestational age. Pediatr Res 48: 623–628
The Vermont Oxford Study Group (2001) Early postnatal dexamethasone therapy for the prevention of chronic lung disease. Pediatrics 108: 741–748
Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, Simon NP, Wilson DC, Broyles S, Bauer CR, Delaney-Black V, Yolton KA, Fleisher BE, Papile LA, Kaplan MD (2000) Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics 105: 1216–1226
Wang JY, Yeh TF, Lin YJ, Chen WY, Lin CH (1997) Early postnatal dexamethasone therapy may lessen lung inflammation in premature infants with respiratory distress syndrome on mechanical ventilation. Pediatr Pulmonol 23: 193–197
Wilson DM, Baldwin RB, Ariagno RL (1988) A randomized, placebo-controlled trial of effects of dexamethasone on hypothalamic-pituitary-adrenal axis in preterm infants. J Pediatr 113: 764–768
Yeh TF, Torre JA, Rastogi A, Anyebuno MA, Pildes R S (1990) Early postnatal dexamethasone therapy in premature infants with severe respiratory distress syndrome: a double-blind, controlled study. J Pediatr 117: 273–282
Yeh TF, Lin YJ, Hsieh WS, Lin HC, Lin CH, Chen JY, Kao HA, Chien CH (1997) Early postnatal dexamethasone therapy for the prevention of chronic lung disease in preterm infants with respiratory distress syndrome: a multicenter clinical trial. Pediatrics 100: e3
Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, Tsai CH (2004) Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med 350: 1304–1313
Yeung MY, Smyth JP (2002) Hormonal factors in the morbidities associated with extreme prematurity and the potential benefits of hormonal supplement. Biol Neonate 81: 1–15
Acknowledgements
The study was supported by grants from the Foundation for Pediatric Research, the Foundation of Alma and K. A. Snellman and the Sigrid Juselius Foundation (Finland). We thank the Department of Pharmacy, Oulu University Hospital, for performing the randomisation, coding and blinding of the study drugs. The attending physicians and participating centres were: D. Haumont (Coordinator; Steering Committee), I. Van Herreweghe, Department of Paediatrics University Hospital of St Pierre, Brussels, Belgium; E. Herting (Coordinator; Steering Committee), K. Harms, U. Gottschalk, Department of Paediatrics, University of Göttingen, Germany; S. B. Oetomo (Coordinator; Steering Committee), H. ter Horst, Department of Paediatrics, University Hospital of Groningen, The Netherlands; K. Heinonen (Coordinator; Steering Committee), P. Nykänen, Department of Paediatrics, Kuopio University Central Hospital, Finland; E. Anttila (Study Coordinator), M. Hallman (Chairman, Planning and Steering Committee), O. Peltoniemi (meta-analysis), M-L. Pokela, T. Saarela, Department of Paediatrics, University of Oulu University Central Hospital, Finland; P. Kero (Coordinator; Steering Committee), Department of Paediatrics, Turku University Central Hospital, Finland; J. Kokkonen (Chairman of the Safety Monitoring Committee), Department of Paediatrics, Oulu University Central Hospital, Finland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Eija Anttila and Outi Peltoniemi contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Anttila, E., Peltoniemi, O., Haumont, D. et al. Early neonatal dexamethasone treatment for prevention of bronchopulmonary dysplasia. Randomised trial and meta-analysis evaluating the duration of dexamethasone therapy. Eur J Pediatr 164, 472–481 (2005). https://doi.org/10.1007/s00431-005-1645-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-005-1645-8