Abstract
Importance
It is unclear if systemic steroids decrease the risk of Bronchopulmonary Dysplasia (BPD) while increasing the risk of neurodevelopmental impairment (NDI).
Objective
Conduct a systematic review of randomized controlled trials of systemic steroids to evaluate the risk of BPD, mortality, and NDI in premature infants ≤30 weeks.
Data sources
MEDLINE, EBSCOhost, Web of Science, Cochrane Library, Embase, and CINAHL.
Study selection
Randomized clinical trials of Dexamethasone (DEX) or Hydrocortisone (HC) to prevent BPD in premature infants ≤ 30 weeks.
Data extraction and synthesis
Data were extracted using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. Random-effects meta-analyses and multivariable meta-regression were conducted.
Main outcomes and measures
Primary outcomes were BPD, mortality, and NDI. Secondary outcomes were hypertension, hyperglycemia, sepsis, intestinal perforation, necrotizing enterocolitis (NEC), and retinopathy of prematurity (ROP). The a priori hypothesis was that steroids would reduce the risk of BPD without increasing NDI.
Results
There were 6377 preterm infants in the 44 (32 DEX, 13 HC) selected studies. DEX significantly reduced the risk of BPD, RR = 0.66, (95% CI, 0.56–0.78). The most effective DEX regimen was medium cumulative dose (2 to 3 mg/kg), RR = 0.43 (95% CI, 0.29–0.65); day of initiation <8 days: RR = 0.68, (95% CI, 0.59–0.79); and treatment for ≥14 days: RR = 0.67 (95% CI, 0.55–0.80). HC did not significantly decrease the risk of BPD, RR = 0.98, (95% CI, 0.87–1.10). Neither DEX, (RR = 0.92, 95% CI, 0.78–1.09) nor HC (RR = 0.83, 95% CI, 0.68–1.01) decrease the risk of mortality. The risk of CP was not increased by either DEX (RR = 1.09, 95% CI, 0.55–2.17) or HC (RR = 1.18, 95% CI, 0.75–1.87). There were no significant differences between steroids and placebo for MDI/PDI scores. Multivariable meta-regression models showed that DEX significantly reduced the risk of BPD without increased risk of CP. DEX increased the risk of hypertension and hyperglycemia. Studies showed high heterogeneity, differing treatment regimen, missing data and different rates of follow-up.
Conclusion and relevance
DEX, but not HC, significantly decreased the risk of BPD. Neither steroid showed an increased risk of NDI or mortality.
Similar content being viewed by others
Introduction
Bronchopulmonary dysplasia (BPD) continues to be a persistent morbidity in extremely premature infants. The incidence of BPD, in preterm infants (<28 weeks’ gestation) born at centers that are part of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network, increased from 44.7% (2008 to 2012) [1] to 49.8% (2013 to 2018) [2]. A 2018 study reported BPD in 69.8% of infants who were <27 weeks’ gestation [3].
A diagnosis of BPD increases the risk of short-term adverse effects such as pulmonary hypertension [4], decreases right ventricular function [5], and increases the risk of death before discharge [6]. Of concern is the increasing tracheostomy [7] and home ventilation in infants with BPD [8, 9].
In infants with BPD compared to those without BPD, there is decreased lung function and exercise tolerance, increased risk of hospital admissions for respiratory infections in the first year, poor growth and neurodevelopmental deficits (cerebral palsy, decreased vision, speech, cognition, learning and behavioral problems) [10,11,12]. At 7–8 years of age, premature infants are at increased risk of abnormal lung function if receving oxygen (<29%) at 36 weeks and increased risk of adverse lung function and neurodevelopment if receiving oxygen (>30%) or positive pressure at 36 weeks [13]. Late death or serious respiratory morbidity increases from 10% to 77% in infants without BPD compared to those with Grade 3 BPD [14].
Postnatal steroids have been used for more than 40 years to prevent or treat BPD [15]. The American Academy of Pediatrics (AAP) 2002 statement recommended against routine use of steroids to prevent BPD due to concerns for neurodevelopmental impairment [16]. The use of dexamethasone (DEX), to prevent BPD, decreased from 25% (1996-1998) to 8% (2004), while the use of hydrocortisone (HC) increased [17]. This corresponded to a significant increase of BPD from 19% to 25% within the years 1997 and 2006 [18]. Recent reviews differ regarding the efficacy of steroids to prevent BPD [19, 20].
Dexamethasone did not significantly decrease BPD but facilitated extubation in DART (Dexamethasone: A Randomized Trial, low dose DEX, 0.89 mg/kg cumulative dose over 10 days vs. placebo). The trial was discontinued after recruiting 70 instead of planned 814 infants [21]. Despite these issues the DART protocol is commonly used to prevent BPD [22]. Regardless, the 2022 American Academy of Pediatrics (AAP) policy statement recommends against routine use of steroids to prevent BPD due to concerns of risks [23]. So the question is, do systemic steroids decrease the risk of BPD in premature infants while increasing the risk of neurodevelopmental impairment (NDI)? Hence, we conducted a systematic review, meta-analysis, and multivariable meta-regression of randomized clinical trials of systemic steroids (DEX vs placebo and HC vs placebo), in premature infants born at <30 weeks’ gestation, to evaluate three outcomes: BPD, mortality, and NDI.
Methods
A systematic review, with meta-analyses and multivariable meta-regressions, was conducted using PRISMA 2020 [24] to evaluate the impact of postnatal systemic steroids on BPD, mortality, and NDI on premature infants. The full protocol, methodology, abbreviations, eFigures, eTables, eReferences are in Supplement 1. A PICOTS framework (infants born <30 weeks’ gestation, systemic steroids, placebo, BPD, mortality, randomized controlled trials, NICU, follow-up: ≥12 months) was used to conduct literature searches from MEDLINE: National Library of Medicine, EBSCOhost, Web of Science, Cochrane Library, PubMed, Embase, and CINAHL. Date of last search was December 12, 2023; detailed literature searches by database are shown in eTable 1.
Search criteria were limited to randomized clinical trials and Cochrane reviews. Excluded were studies that were not within the PICOTS framework, did not report BPD or chronic lung disease, not randomized trials, and not available in English. Identified articles were entered into EndNote X9 (Clarivate Analytics), which removed duplicates. Three reviewers (RZ, RL, and SB) independently screened each record. Disagreements were resolved by consensus.
Primary outcomes were BPD (defined as need for supplemental oxygen and/or invasive or non-invasive respiratory support at day 28 or at 36 weeks postmenstrual age, PMA), mortality before discharge, and NDI at follow-up. Secondary outcomes were hyperglycemia (HBG), hypertension (HBP), sepsis, intestinal perforation (IP), necrotizing enterocolitis (NEC), and retinopathy of prematurity (ROP). Covariates included birth gestational age in weeks, birthweight (g), sex, race, type of steroid, day of drug initiation, cumulative dose (DEX) or cumulative equivalent dose (HC), drug duration (days), steroid exposure in the placebo group, year and location of recruitment. Neurodevelopmental impairment at follow-up included diagnosis of cerebral palsy (CP), and mean scores <70 for Mental and Psychomotor Developmental Indices (MDI and PDI) of the Bayley II Scales of Infant Development.
Effect sizes for BPD, mortality, and other binary variables were calculated as risk ratios (RR) with 95% confidence intervals (CI). Effect measures for continuous variables were reported as standardized mean differences (SMD) with 95% CI. Continuous measures for drug regimens (dosage, treatment initiation, and duration) were categorized based on prior research [20, 25,26,27]. Cut points based on the distribution of drug regimens were explored (eFigure 1). The Cumulative Equivalent Dose (CED) for HC was calculated for comparable doses of DEX based on an anti-inflammatory potency ratio of 1:25 for the two steroids [28, 29]. Risk of bias was evaluated using the Revised Cochran risk-of-bias Excel tool (V9) for randomized trials (RoB 2) [30]. Certainty of evidence was conducted using the online tool GRADEpro [31]. Publication bias was assessed with funnel plots.
Statistical analyses
Statistical analyses were conducted in R 4.1.3 software using the metafor package [32, 33]. The goal of the analysis was to generalize results and assess heterogeneity (high; I 2 > 50%). Given that studies were from multiple populations, we focused on random-effects models [34, 35]. These models assume treatment effects are not the same across studies and adjusts for both within- and between-study variance.
To explore treatment differences and subgroup drug effects among covariates and factors, a series of meta-analyses with forest plots were conducted to evaluate the risk of BPD, mortality, and NDI. Multivariable, random-effects models with bubble plots were used to assess pooled effect sizes. Heterogeneity measures included Cochran’s Q, Higgins and Thompson’s I2 and H2. Where a zero event was reported, an adjustment of 0.5 was added to any one outcome category. The Sidik-Jonkman (SJ) method and Knapp-Hartung adjustments (knha) were used to avoid false positives from low powered studies.
Mixed-effects, multivariable meta-regression models explored all possible predictor combinations, including interactions, using multimodel inference. Effect sizes were reported as relative risk which were adjusted (RRadj) for all other factors in the multivariable model. Correlations were conducted to evaluate multi-collinearity. To avoid overfitting, predictors were selected using Akaike’s information criterion corrected for small samples (AICc). Sensitivity analyses were conducted to exclude studies with potential confounders. Outliers and studies with undue influence were assessed with Cook’s distance, externally standardized residuals, and DFFITS values. Permutation tests using 10,000 iterations were conducted to validate the robustness of the final models.
Results
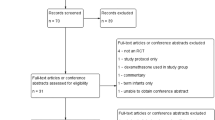
Literature search identified 373 articles (Fig. 1). After removing duplicates, the remaining 233 records were screened, 140 of 233 articles were fully reviewed and 44 studies met the inclusion criteria, eTable 2. Reasons for exclusion were tracked using the PICOTS criteria (eTable 3). Of the 44 selected studies, 31 studies compared DEX to placebo (published between 1972 and 2006) and 13 compared HC to placebo (published between 1999 and 2022). These 44 studies included 6377 premature infants from 15 countries. There were 3642 infants (57.1%) in DEX studies and 2735 infants (42.9%) in HC studies. Group comparisons by steroid are shown in eTable 4a, b.
*Figure shows the original randomized clinical trials included in the review. The follow-up studies are in eTable 2, Supplement.
Risk of Bias
An elevated risk of bias was observed in all studies (eFigure 2). Studies shown in forest plots indicate high risk (“hr”) for 17 studies (14 DEX and 3 HC). In addition, steroid exposure (“sp”) occurred in the placebo group, prior to BPD diagnosis, in 12 out of 31 (38.7%) DEX studies and 8 out of 13 (61.5%) HC studies.
Systemic steroids vs placebo: primary outcomes (Table 1)
Compared to placebo, systemic steroids significantly decreased the risk of BPD (RR = 0.75, 95% CI 0.66–0.85, RRadj = 0.76, 95% CI 0.58–0.99, P = 0.021). Steroids did not significantly decrease the risk of mortality. Importantly, steroids did not significantly increase the risk of CP (RR = 1.19, 95% CI 0.77–1.84, RRadj = 0.86, 95% CI 0.21–3.54, P = 0.785).
Forest plots from various meta-analyses are shown in eFigure 3(1-46) with results summarized in eTable 11.
Dexamethasone subgroup analyses: primary outcomes
Compared to placebo, DEX significantly decreased the risk of BPD (RR = 0.66, 95% CI 0.56– 0.78), eFigure 3(4). Forest plots for DEX subgroup analyses are shown in eFigure 3(15–30). Dexamethasone decreased the risk of BPD whether BPD was diagnosed at 36 weeks post-menstrual age (RR = 0.65, 95% CI 0.53–0.80 or at day 28, RR = 0.69, 95% CI 0.50–0.95), eFigure 3(15).
Meta-analyses for DEX regimen are shown in Fig. 2A–C. The strongest effects for reducing BPD occurred with the medium cumulative dose of DEX (2–3 mg/kg) (RR = 0.43, 95% CI 0.29–0.65), (Fig. 2A), when DEX was initiated at <8 days (RR = 0.68, 95% CI 0.59–0.79) or between 8 to 14 days (RR = 0.73, 95% CI 0.55–0.97), (Fig. 2B) and when DEX was administered for 14 days or more (RR = 0.67, 95% CI 0.55–0.80), (Fig. 2C). Dexamethasone decreased the risk of BPD across other factors including the country of recruitment, studies with no steroid exposure in the placebo group, and year of recruitment (eFigure 3(20-22).
a Dexamethasone cumulative dose and risk of BPD. This Forest Plot shows the results of a meta-analysis of 31 studies with subgroups by dose. All cumulative doses of DEX, Low (<2 mg/kg), Medium (2–3 mg/kg), and High (>3 mg/kg) significantly decreased the risk of BPD, although the risk of BPD was lowest with the Medium Cumulative Dose (2–3 mg/kg); RR = 0.43, 95% CI (0.29, 0.65). *Citations are in Supplement, eTable 2. b Day of Initiation of DEX and the risk of BPD. This Forest Plot shows the results of a meta-analysis of 31 studies comparing start day of DEX compared to placebo. While all initiation times significantly reduced the risk of BPD, early initiation of DEX (<8 days old) appeared to have the strongest effect, RR = 0.68, 95% CI (0.59, 0.79). * Citations are in Supplement, eTable 2. c Duration of DEX treatment and the risk of BPD. This Forest Plot that shows the results of a meta-analysis of 31 studies of the duration of treatment with DEX compared to placebo for its effects on the risk of BPD. While all duration times showed reduced risk of BPD, the strongest effect was with a longer duration of DEX (>14 days), RR = 0.67, 95% CI (0.55, 0.80). * Citations are in Supplement, eTable 2.
Compared to placebo, DEX did not affect the risk of mortality (RR = 0.92, 95% CI 0.78–1.09), eFigure 3(5). The subgroup analyses of DEX showed that the risk of mortality was not associated with timing of BPD diagnosis, dosing regimen (cumulative dose, duration of treatment, day of initiation), country of recruitment, steroid contamination in the placebo group, or the year of recruitment (eFigure 3(23–26, 28–30).
A total of 919 infants, from 10 DEX studies, were followed for NDI (Table 2). Compared to placebo, DEX did not significantly increase the risk of CP (RR = 1.09, 95% CI 0.55–2.17). There was no significant difference between DEX vs placebo for MDI scores (SMD = 0.13, 95% CI −0.32–0.58) or for PDI scores (SMD = 0.05, 95% CI −0.32–0.42). All the mean scores were above the 70-point threshold for NDI.
Hydrocortisone subgroup analyses: primary outcomes
Compared to placebo, HC did not decrease the risk of BPD (RR = 0.98, 95% CI 0.87–1.10), eFigure 3(4). BPD was diagnosed at 36 weeks PMA in all HC studies. Compared to placebo, HC did not decrease the risk of BPD risk regardless of CED, duration of treatment, day of initiation, risk of bias, country of recruitment, steroid exposure vs no steroid exposure in placebo group, or for year of recruitment, eFigures 3(31–38). Overall, HC did not significantly decrease the risk of mortality (RR = 0.83, 95% CI 0.68–1.01), eFigure 3(5). The two subcategories that showed significant reduced risk of mortality with HC were international recruitment (RR = 0.77, 95% CI 0.62–0.95) and recruitment after 2010 (RR = 0.80, 95% CI 0.65–0.99) (eFigure 3(44 and 46).
A total of 1372 infants, from 5 HC studies, were followed for NDI (Table 2). Compared to placebo, HC did not increase the risk of CP (RR 1.18, 95% CI, 0.75 to 1.87). There was no significant difference between HC vs placebo for MDI scores (SMD = −0.01, 95% CI −0.47–0.45) or PDI scores (SMD = −0.05, 95% CI −0.30–0.20).
Multivariable meta-regression models and Primary Outcomes
Details of the multivariable meta-regression models are shown in eTable 5–8. eTable 5 shows two models, one for predicting BPD and one for mortality. Certain factors (exposures) increased or reduced the relative risk of each outcome, while adjusting for other factors in the models. For example, in eTable 5, birth gestational age (weeks) significantly reduced the relative risk of BPD by 10% (P = 0.017) but was not a significant risk factor for mortality. Similarly, DEX reduced the relative risk of BPD by 24% as compared to HC (P = 0.021) but was not significant for mortality. eTable 6 shows the DEX subgroup multivariable meta-regression model for BPD. A significant finding was observed for the medium cumulative dose of DEX (2–3 mg/kg), which reduced the relative risk of BPD in the steroid group compared to placebo by 41%, P = 0.004 (given other factors in the model).
The association of steroids and mortality were further explored using multimodel.inference program with results shown in eTable 7. Two models are displayed, one that included all studies and one for studies without steroid exposure in the placebo group. The first model (shown on the left of eTable 7) revealed that steroid exposure in the placebo group, as compared with no exposure, significantly increased the relative risk of mortality (RRadj = 1.44, 95% CI 1.04–2.00). The second model (shown on the right of eTable 7) included a subgroup of studies without steroid contamination and showed that risk of mortality was twice as likely for International vs. U.S. recruited participants.
The multivariable model for cerebral palsy, shown in eTable 8, suggested that birth gestational age (weeks) may increase the risk of CP but it was not significant after 10,000 iterations (95% CI 0.97–3.05). This model showed that no steroid regimen (treatment start day, cumulative dose, treatment duration) was significant for increasing the risk of CP, which was further demonstrated by the horizontal line (slope of unity) in the bubble plots (Fig. 3).
These Bubble plots are from the Mixed Effects Multivariable Regression models for CP: cumulative dose or cumulative equivalent dose (CED), start day and duration of steroids (15 studies, 2291 infants). The Meta-Regression Prediction line is a straight line indicating that there is no significant association between CP and steroids for treatment start day, cumulative dose/cumulative equivalent dose (mg/kg) or treatment duration. Some studies are outliers with sample sizes as indicated by smaller bubbles.
Secondary outcomes
Dexamethasone significantly increased the risk of hyperglycemia (RR = 1.29, 95% CI 1.08–1.55) and systemic hypertension (RR = 2.26, 95% CI 1.38–3.69) but HC did not (eFigure 3(8-9). Neither steroid significantly increased the risk of intestinal perforation, NEC, ROP, or sepsis, (eFigure 3(7,10-13).
Certainty of evidence (CoE)
For DEX, (eTable 9a) the certainty of evidence (CoE) was high for BPD when diagnosed at 36 weeks PMA but was very low when diagnosed at day 28. CoE was very low for mortality and low for CP. For HC, (eTable 9b) the CoE was moderate for both BPD and mortality, and very low for CP. Publication bias was suspected of both DEX and HC studies (eFigure 4).
Discussion
In this study, DEX significantly reduced the risk of BPD, with the greatest BPD risk reduction of 57% with the medium cumulative dose (2–3 mg/kg) and 33% with treatment duration of 14 days or more. Multivariable meta-regression revealed that medium cumulative dose of DEX (2–3 mg/kg) reduced BPD risk by 41% when compared to low cumulative dose (<2 mg/kg) and that longer treatment was more effective.
In our study, DEX initiated at both <8 days and 8–14 days significantly decreased BPD. Similar to our results, Doyle et al. showed that DEX significantly decreased the risk of BPD when initiated early (first six days of life) [36] or late (>7 days) [37]. In the Zeng et al. study, early initiated high dose of DEX (>3 mg/kg, >3 days) decreased BPD [38]. In the Hay et al. study, the DEX regimens of early initiated, moderate dose (2–4 mg/kg) and late initiated, high dose (>4 mg/kg) decreased BPD [39]. In the van de Loo et al. study, DEX initiated ≥7 days of life decreased BPD [40].
Hydrocortisone did not decrease the risk of BPD in our study. We speculate that, as the dose of HC was low (CED < 2 mg/kg) in 12 out of 13 studies, the anti-inflammatory effect from low dose HC treatment may be insufficient to decrease BPD. The studies by Doyle et al. and van de Loo also found that HC did not decrease the risk of BPD [36, 37, 40].
Our results showed neither steroid decreased mortality. In contrast, Doyle et al. showed that early HC decreased mortality [36], and late initiation [37] of DEX reduced BPD or mortality. Hay et al found that early and late steroids did not decrease mortality [39].
Dexamethasone increased the risk of hyperglycemia and hypertension. However, neither steroid increased the risk of NEC, ROP, sepsis, or intestinal perforation. Other studies have reported that early use of steroids increased the risk of intestinal perforation [19, 36, 39]. Hay et al. found that late initiated, medium dose of DEX increased the risk of sepsis [39].
Our rigorous multivariable regression model (adjusted for infant sex, birth gestational age, drug regimen) demonstrated that neither steroid significantly increased the risk of NDI when compared to placebo. In the van de Loo study, late initiated (>7 days) did not increase the risk of NDI [40]. In contrast, Doyle et al. found that early initiation of DEX increased the risk of CP, whereas late DEX did not [36, 37]. Ramaswamy et al. [20] found that steroids did not increase the risk of CP (secondary outcome), Zeng et al. [38] suggested that high dose DEX might increase the risk of CP, whereas Hay et al. [39] found that early low dose DEX increased CP while late DEX did not.
Two potential issues were observed on closer inspection of the three studies [36, 37, 39] reporting an increased risk of CP at follow-up. First, each reported results using the total number of infants recruited into the trials, not just those who were evaluated at follow-up. This may erroneously increase the sample size per study and influence the weighting scheme. Second, fixed-effect models were conducted. These models give more weight to larger studies by ignoring between study variances. In contrast, we have reported results of infants who were evaluated for NDI, resulting in smaller sample sizes with less influence on the weight scheme. In addition, we used random effects for all models, which is more conservative and generally preferred as the data from randomized controlled studies were from multiple populations [34, 35]. This method considers between study variance during the weighting process thus appropriately addressing within and between study heterogeneity resulting in a more balanced weight to the evidence.
Our multivariable meta-regression modeling did not show an increased risk for CP, regardless of variables, indicating no significant association with steroid use. Onland et al’s. [41] random-effects model demonstrated that DEX decreased the risk of BPD without an increased risk of NDI. In contrast, the fixed-effect meta-regression by Doyle et al. [42] showed that steroids increased the risk of death or CP. We recommend more research to understand whether systemic steroids, when used to decrease the risk of BPD, increase the risk of CP.
Strengths and limitations
Our study is unique compared to previous meta-analyses [20, 38, 39] because the effects of systemic steroids on NDI was a primary outcome. In addition, the results of our study, are from validated, multivariable random effects models that identified both within and between study differences and allowed for explorations of dose response.
Our study has several limitations. Heterogeneity was high, and we were unable to fully address this issue, even with multivariable meta-regression. While population characteristics and treatment regimens differed across studies, these were partially addressed using random-effects models that included adjustments and 10,000 iterations. Moreover, there were unreported or missing data for mortality, secondary outcomes, and NDI, that were not imputed. For DEX, the certainty of evidence was very low for mortality and low for CP and for HC the certainty of evidence was moderate for mortality and very low for CP. It should be noted that infants in the studies we analyzed were on mechanical ventilation. However, in the current era, a significant percent of premature infants treated with non-invasive respiratory support develop BPD [43]. As new studies continue to report significant adverse short and long term health impact from BPD [7,8,9, 13, 44], a well designed randomized controlled trial is needed to research the question whether DEX (cumulative dose of 2–3 mg/kg, initiated after day 8 and administered for >14 days) can decrease the risk of BPD, without increasing the risk of NDI, in high-risk premature infants on invasive and non-invasive respiratory support.
Conclusions
Results from our study showed that a medium cumulative dose (2–3 mg/kg) of DEX, administered for 14 days or more, significantly reduced the risk of BPD. Hydrocortisone did not decrease the risk of BPD. Neither steroid showed a significant effect on mortality. Importantly, neither DEX nor HC increased the risk of NDI. More research is urgently needed to further study the benefits and risks of the use of systemic steroids to decrease BPD.
References
Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–51. https://doi.org/10.1001/jama.2015.10244.
Bell EF, Hintz SR, Hansen NI, Bann CM, Wyckoff MH, DeMauro SB, et al. Mortality, in-hospital morbidity, care practices, and 2-year outcomes for extremely preterm infants in the US, 2013-2018. JAMA. 2022;327:248–63. https://doi.org/10.1001/jama.2021.23580.
Jensen EA, Edwards EM, Greenberg LT, Soll RF, Ehret DEY, Horbar JD. Severity of bronchopulmonary dysplasia among very preterm infants in the United States. Pediatrics. 2021;148. https://doi.org/10.1542/peds.2020-030007.
Hwang JS, Rehan VK. Recent advances in bronchopulmonary dysplasia: pathophysiology, prevention, and treatment. Lung. 2018;196:129–38. https://doi.org/10.1007/s00408-018-0084-z.
Levy PT, Dioneda B, Holland MR, Sekarski TJ, Lee CK, Mathur A, et al. Right ventricular function in preterm and term neonates: reference values for right ventricle areas and fractional area of change. J Am Soc Echocardiogr. 2015;28:559–69. https://doi.org/10.1016/j.echo.2015.01.024.
Bui CB, Pang MA, Sehgal A, Theda C, Lao JC, Berger PJ, et al. Pulmonary hypertension associated with bronchopulmonary dysplasia in preterm infants. J Reprod Immunol. 2017;124:21–29. https://doi.org/10.1016/j.jri.2017.09.013.
Donda K, Agyemang CO, Adjetey NA, Agyekum A, Princewill N, Ayensu M, et al. Tracheostomy trends in preterm infants with bronchopulmonary dysplasia in the United States: 2008-2017. Pediatr Pulmonol. 2021;56:1008–17. https://doi.org/10.1002/ppul.25273.
Collaco JM, McGrath-Morrow SA. Respiratory phenotypes for preterm infants, children, and adults: bronchopulmonary dysplasia and more. Ann Am Thorac Soc. 2018;15:530–8. https://doi.org/10.1513/AnnalsATS.201709-756FR.
Manimtim WM, Agarwal A, Alexiou S, Levin JC, Aoyama B, Austin ED, et al. Respiratory outcomes for ventilator-dependent children with bronchopulmonary dysplasia. Pediatrics. 2023;151. https://doi.org/10.1542/peds.2022-060651.
Cheong JLY, Doyle LW. An update on pulmonary and neurodevelopmental outcomes of bronchopulmonary dysplasia. Semin Perinatol. 2018;42:478–84. https://doi.org/10.1053/j.semperi.2018.09.013.
Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitefield MF. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289:1124–9. https://doi.org/10.1001/jama.289.9.1124.
Sriram S, Schreiber MD, Msall ME, Kuban KCK, Joseph RM, O’Shea TM, et al. Cognitive development and quality of life associated with BPD in 10-year-olds born preterm. Pediatrics. 2018;141:e20172719. https://doi.org/10.1542/peds.2017-2719.
Doyle LW, Ranganathan S, Mainzer RM, Cheong JLY. Victorian infant collaborative study G. Relationships of severity of bronchopulmonary dysplasia with adverse neurodevelopmental outcomes and poor respiratory function at 7-8 years of age. J Pediatr. 2024;269:114005. https://doi.org/10.1016/j.jpeds.2024.114005.
Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. an evidence-based approach. Am J Respir Crit Care Med. 2019;200:751–9. https://doi.org/10.1164/rccm.201812-2348OC.
Mammel MC, Green TP, Johnson DE, Thompson TR. Controlled trial of dexamethasone therapy in infants with bronchopulmonary dysplasia. Lancet. 1983;1:1356–8. https://doi.org/10.1016/s0140-6736(83)92139-6.
American Academy of Pediatrics. Committee on fetus and newborn. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109:330–8. https://doi.org/10.1542/peds.109.2.330.
Walsh MC, Yao Q, Horbar JD, Carpenter JH, Lee SK, Ohlsson A. Changes in the use of postnatal steroids for bronchopulmonary dysplasia in 3 large neonatal networks. Pediatrics. 2006;118:e1328–35. https://doi.org/10.1542/peds.2006-0359.
Yoder BA, Harrison M, Clark RH. Time-related changes in steroid use and bronchopulmonary dysplasia in preterm infants. Pediatrics. 2009;124:673–9. https://doi.org/10.1542/peds.2008-2793.
Abiramalatha T, Ramaswamy VV, Bandyopadhyay T, Somanath SH, Shaik NB, Pullattayil AK, et al. Interventions to prevent bronchopulmonary dysplasia in preterm neonates: an umbrella review of systematic reviews and meta-analyses. JAMA Pediatr. 2022;176:502–16. https://doi.org/10.1001/jamapediatrics.2021.6619.
Ramaswamy VV, Bandyopadhyay T, Nanda D, Bandiya P, Ahmed J, Garg A, et al. Assessment of postnatal corticosteroids for the prevention of bronchopulmonary dysplasia in preterm neonates: a systematic review and network meta-analysis. JAMA Pediatr. 2021;175:e206826. https://doi.org/10.1001/jamapediatrics.2020.6826.
Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: a multicenter, international, randomized, controlled trial. Pediatrics. 2006;117:75–83. https://doi.org/10.1542/peds.2004-2843.
Job S, Clarke P. Current UK practices in steroid treatment of chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 2015;100:F371. https://doi.org/10.1136/archdischild-2014-308060.
Cummings JJ, Pramanik AK. Postnatal corticosteroids to prevent or treat chronic lung disease following preterm birth. Pediatrics. 2022;149:e2022057530. https://doi.org/10.1542/peds.2022-057530.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. https://doi.org/10.1136/bmj.n160.
Onland W, Cools F, Kroon A, Ramful D, El Moussawi F, Nicaise C, et al. Effect of hydrocortisone therapy initiated 7 to 14 days after birth on mortality or bronchopulmonary dysplasia among very preterm infants receiving mechanical ventilation: a randomized clinical trial. JAMA. 2019;321:354–363. https://doi.org/10.1001/jama.2018.21443.
Doyle LW, Cheong JL, Ehrenkranz RA, Halliday HL. Late (>7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2017;10:CD001145. https://doi.org/10.1002/14651858.CD001145.pub4.
Baud O, Biran V, Trousson C, Leroy E, Mohamed D, Alberti C. Two-year outcomes after prophylactic hydrocortisone in extremely preterm neonates. EAPS Congress 2016. Eur J Pediatr. 2016;175:1393–880. https://doi.org/10.1007/s00431-016-2785-8.
Clauss C, Thomas S, Khodak I, Tack V, Akerman M, Hanna N, et al. Hydrocortisone and bronchopulmonary dysplasia: variables associated with response in premature infants. J Perinatol. 2020;40:1349–57. https://doi.org/10.1038/s41372-020-0680-7.
Htun ZT, Schulz EV, Desai RK, Marasch JL, McPherson CC, Mastrandrea LD. et al. Postnatal steroid management in preterm infants with evolving bronchopulmonary dysplasia. J Perinatol. 2021;41:1783–96. https://doi.org/10.1038/s41372-021-01083-w.
Sterne J, Savović J, Page M, Elbers R, Blencowe N, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:14898. https://doi.org/10.1136/bmj.14898.
GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2022. Available from gradepro.org. 2022.
Viechtbauer W. Conducting meta-analyses in R with metafor package. J Stat Softw. 2010;36:1–48.
Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta-analysis with R: a hands-on guide. 1st ed. Chapman & Hall/CRC Press; 2021.
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. https://doi.org/10.1002/jrsm.12.
Christiansen S, Iverson C, Flanagin A, Livingston EH, Fishcer L, Manno C, et al. AMA manual of style: A guide for authors and editors. American Medical Association manual of style. 11th edition. New York, NY: Oxford University Press; 2020.
Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL. Early (< 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2021;10:Cd001146. https://doi.org/10.1002/14651858.CD001146.pub6.
Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL. Late (≥ 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2021;11:Cd001145. https://doi.org/10.1002/14651858.CD001145.pub5.
Zeng LN, Tian JH, Song FJ, Li WR, Jiang LC, Gui G, et al. Corticosteroids for the prevention of bronchopulmonary dysplasia in preterm infants: a network meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2018;103:F506–11. https://doi.org/10.1136/archdischild-2017-313759.
Hay S, Ovelman C, Zupancic JA, Doyle LW, Onland W, Konstantinidis M, et al. Systemic corticosteroids for the prevention of bronchopulmonary dysplasia, a network meta-analysis. Cochrane Database Syst Rev. 2023;8:CD013730. https://doi.org/10.1002/14651858.CD013730.pub2.
van de Loo M, van Kaam A, Offringa M, Doyle LW, Cooper C, Onland W. Corticosteroids for the prevention and treatment of bronchopulmonary dysplasia: an overview of systemic reviews. Cochrane Database Syst Rev. 2024:CD013271. https://doi.org/10.1002/14651858.CD013271.pub2.
Onland W, Offringa M, Jaegere APD, Van Kaam AH. Finding the optimal postnatal dexamethasone regimen for preterm infants at risk of bronchopulmonary dysplasia: a systematic review of placebo-controlled trials. Pediatrics. 2009;123:367–77. https://doi.org/10.1542/peds.2008-0016.
Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. An update on the impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk of bronchopulmonary dysplasia. J Pediatr. 2014;165:1258–60. https://doi.org/10.1016/j.jpeds.2014.07.049.
Greenberg RG, McDonald SA, Laughon MM, Tanaka D, Jensen E, Van Meurs K, et al. Online clinical tool to estimate risk of bronchopulmonary dysplasia in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed. 2022. https://doi.org/10.1136/archdischild-2021-323573.
Shimotsuma T, Tomotaki S, Akita M, Araki R, Tomotaki H, Iwanaga K, et al. Severe Bronchopulmonary Dysplasia adversely affects brain growth in preterm infants. Neonatology. 2024. https://doi.org/10.1159/000538527.
Acknowledgements
The authors thank the contributions of Hayrettin Okut, PhD, who advised us on statistical aspects of model development, including programming in R.
Author information
Authors and Affiliations
Contributions
TSR: conceptualized, formulated the research methodology, wrote the original draft, reviewed and edited the manuscript, supervised/administered the whole project and guarantor for the project. REZ: conceptualized, formulated the research methodology, performed formal analysis, curated data and wrote the original draft, reviewed and edited the manuscript. RL: conceptualized, formulated the research methodology, wrote the original draft, reviewed and edited the manuscript. SAB: conceptualized, formulated the research methodology, wrote the original draft, reviewed and edited the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
TSR, REZ, RL, and SAB have no relevant conflicts to disclose. This study was not supported by funding from any source.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raghuveer, T.S., Zackula, R.E., Lakhotia, R. et al. Systemic steroids and bronchopulmonary dysplasia: a systematic review and meta-analysis. J Perinatol (2024). https://doi.org/10.1038/s41372-024-02097-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41372-024-02097-w
- Springer Nature America, Inc.