Abstract
Piritramide is indicated for treatment of postoperative pain and analgosedation in the intensive care unit (ICU) setting. In an open prospective study the pharmacokinetics of piritramide were investigated in four groups: newborns (NB, age: 1–28 days) (n=8), infants 1 (IF1, age: 2–4 months) (n=7), infants 2 (IF2, age: 5–12 months) (n=14) and young children (YC, age: 2–4 years) (n=10). The recommended paediatric dose range for therapy of postoperative pain is 50–200 μg/kg. Piritramide was administered intravenously as a single dose by bolus injection of 50 μg/kg. Blood samples were collected at 0, 15, 45, 90 min and 3, 6, 9, 12 h after application, and urine samples were collected before application and during the following intervals: 1–2, 2–6, 6–12 h. Piritramide was measured in blood and urine by HPLC-ESI-MS. The following pharmacokinetic parameters: maximum plasma concentration (Cmax), distribution half-life \({\left( {t_{{{\text{1}} \mathord{\left/ {\vphantom {{\text{1}} {2{\text{ $ \alpha $ }}}}} \right. \kern-\nulldelimiterspace} {2{\text{ $ \alpha $ }}}}} } \right)},\), elimination half-life\({\left( {t_{{{\text{1}} \mathord{\left/ {\vphantom {{\text{1}} {2{\text{ $ \beta $ }}}}} \right. \kern-\nulldelimiterspace} {2{\text{ $ \beta $ }}}}} } \right)},\), total clearance (Clt) and median volume of distribution at equilibrium (Vdss) were calculated using a non-compartment and a two-compartment model for the disposition of piritramide (TOPFIT and NONMEM-pharmacokinetic analysis). Newborns (NB) showed the highest maximum plasma concentrations (median±SD) Cmax (79±240 μg/l) compared to the other three groups (IF1 36±367, IF2 12±81 and YC 16±9 μg/l) without statistical significance. The median elimination half-lives \({\left( {t_{{{\text{1}} \mathord{\left/ {\vphantom {{\text{1}} {{\text{2 $ \beta $ }}}}} \right. \kern-\nulldelimiterspace} {{\text{2 $ \beta $ }}}}} } \right)}\) were 702±720 min in NB, 157±102 min in IF1, 160±68 min in IF2 and 166±143 min in YC. For \(t_{{{\text{1}} \mathord{\left/ {\vphantom {{\text{1}} {{\text{2 $ \beta $ }}}}} \right. \kern-\nulldelimiterspace} {{\text{2 $ \beta $ }}}}} \) the difference between NB and the other three groups (IF1, IF2 and YC) was statistically significant (Mann-Whitney-U, P<0.05). Clt was 15.9±16.7, 46.6±76.9, 235.5±454.1 and 338±168.1 ml/min in NB, IF1, IF2 and YC respectively. The total clearance increased exponentially with an elimination half-life of 702 min from 15.9 ml/min in NB to 46.6 ml/min in IF2. Differences between the NB/IF1 groups and IF2/YC groups were significantly significant (NB vs. IF2, NB vs. YC, IF1 vs. IF2 and IF1 vs. YC). Vdss was 2.0±4.93, 1.7±2.5, 7.0±5.2 and 6.7±2.2 l/kg in NB, IF1, IF2 and YC respectively. In comparison to group IF1 the Vdss was significantly larger in groups IF2 and YC (Mann-Whitney U, P<0.05). Newborns showed a high initial concentration and a distinct prolongation of the elimination half-life of piritramide compared to infants, young children and adults. Therefore, dosage needed to treat postoperative pain should be reduced, and the repetitive doses should be geared to the analgesic effects. In infants and young children the elimination of piritramide is increased compared to adults; therefore the duration of the effects of piritramide will be shortened, and dose intervals ought to be reduced. Subsequent clinical trials for detailed dose adjustment of piritramide in paediatric patients comparing pharmacokinetics and effectiveness are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Piritramide (PIR) has been well established for 30 years in postoperative pain management in Europe [34] and is indicated primarily for the treatment of postoperative pain and analgosedation in the ICU setting [13, 27, 28]. It is a 4-amino piperidine derivative (2,2,-diphenyl-4-[1-(4-carbamoyl-4-piperidino)-piperidine]-butyro-nitrile) with an agonistic effect on μ-opioid receptors and is roughly equipotent to morphine and commonly used in paediatric patients. In therapeutic doses, hemodynamic and emetic adverse effects are marginal, and compared to morphine, the incidence and extent of side effects such as respiratory suppression and itching are significantly reduced [4, 5, 13, 14, 27, 28].
The recommended paediatric dose range is 50–200 μg/kg, and until now the pharmacokinetics of piritramide have only been investigated in adults [1, 13]. The recommended dose of piritramide for the management of postoperative pain is 200–300 μg/kg intramuscularly in adults. For premedication, an intramuscular dose of 200–300 μg/kg is recommended [1].
After intravenous bolus application in adult surgical patients (22–53 years) Kietzmann et al. found an elimination half-life of 8.0 h. The volume of distribution and clearance of piritramide was 4.7 l/kg and 7.8 ml/min per kg respectively [32].
At this point there are no data available regarding the pharmacokinetics and pharmacodynamics of piritramide in newborns, infants and young children. Although the metabolism and elimination of the drug is not well known in any age group including adults, the drug is considered to depend almost exclusively on hepatic metabolism, while renal elimination is negligible [4].
Therefore the aim of this study was to investigate the population pharmacokinetics of piritramide after intravenous bolus administration in newborns, infants and young children during therapy of postoperative pain using sparse plasma samples of small volume according to the guidelines of the European Society for Developmental Pharmacology [3].
Materials and methods
Study population
After approval by the ethics review board of the University of Cologne the study was carried out as an open, prospective clinical trial from January 2000 to November 2002. During this period 41 paediatric patients (two were excluded from the study due to inadequate blood and urine sampling), who required piritramide for the treatment of postoperative pain and analgosedation, were included in our study after written informed consent by the parents. Patients were excluded if they suffered from bile duct disorders, pancreatitis, obstructive gastrointestinal diseases, respiratory disorders or congenital deformities.
Clinical data were collected at the same time as blood and urine samples for further pharmacokinetic analysis.
The paediatric patients were divided into four groups according to the study protocol: newborns (NB, age: 1–28 days, n=8), infants1 (IF1, age: 2–4 months, n=7), infants2 (IF2, age: 5–12 months,n=14) and young children (YC, age: 2–4 years, n=10). Sixteen were female and 23 male. Median age, weight and dosage with SD of each group are listed in Table 1.
Dosage regimen
Piritramide was administered as a single bolus injection of 50 μg/kg, and analgesic effectivness was assessed by using the Hartwig score (mechanical ventilation, [20]) or CHIPP-scale [9] (“Children’s and Infant’s Postoperative Pain Scale”), no mechanical ventilation. Repetitive bolus injections of 15 μg/kg at intervals of 7 min were permitted after the initial bolus injection in the case of an inadequate sedation score or other clinical necessity.
If needed, additional analgosedation during the 12-h study period was achieved using another opioid e.g. morphine (50–100 μg/kg i.v.). This procedure assured an undisturbed pharmacokinetic analysis of the first piritramide-bolus application.
Frequently midazolam was used on a continuous infusion base if further sedation was intended e.g. to tolerate mechanical ventilation.
Renal elimination of unchanged piritramide was determined using four urine collection samples. A baseline before piritramide application and three samples during the intervals 0–2 h, 2–6 h and 6–12 h after piritramide application were analysed with the serum samples, which were collected at 0, 15, 45, 90 min and 3, 6, 9, 12 h after application. The majority of the patients had a Foley catheter placed; in some cases the urine was collected in disposible urine bags with adhesive seal around the genitals.
Common clinical parameters such as blood pressure, heart rate and oxygen saturation and laboratory tests were documented in addition to the described study data.
Assay of piritramide
Piritramide was measured in serum and urine by a modification of the HPLC-ESI-MS (high-pressure liquid chromatography electrospray ionization mass spectrometry) method of Martens-Lobenhofer et al. [36].
Blood samples (S-Monovette, total volume 1.1 ml) from patients were gently shaken and centrifuged at 4°C for 10 min at 3,000g (Minifuge GL, Heraeus-Christ, Osterode). The supernatant was transferred to microvials and frozen at −80°C until analysis.
For LC-MS-MS analysis the samples were thawed, vortexed (vortex, Bender and Hobein, Zurich, Switzerland) for 15 s and centrifuged for 5 min at 15,800 g and 4°C (Eppendorf centrifuge 5402). Protein precipitation was achieved by admixing 100 μl serum (Eppendorf micropipette) with 300 μl acetonitril in plastic microvials. Clomipramine solution (50 μl, 0.1% formiatic solution of 0.5 mg in 1,000 ml water/methanol, 80:20, v/v) was added as internal standard. Subsequently the vials were vortexed for 30 s and centrifuged for 10 min at 15,800g and 4°C (Eppendorf centrifuge 5402). The supernatant was transferred into disposable glass microvials (volume 100 μl), and 5 μl of the sample was injected into the HPLC-ESI-MS system.
The lower limit of quantification (LLOQ) of piritramide was 0.2 μg/l, and the limit of detection (LOD) was 0.1 μg/l. Linearity is demonstrated over a range of 0.2–320 μg/l. The intraday variability of the relative standard deviation (RSD) was 5.0% at 0.8 μg/l and 2.0% at 320 μg/l. The interday variability was 11.9% at 0.8 μg/l and 5.5% at 320 μg/l.
Drugs that may have been used during anaesthesia or during the perioperative period (theophylline, midazolam, phenobarbital, thiopental, pentobarbital, paracetamol, clonidine, morphine, fentanyl, furosemide, gentamicin, dopamine, cisatracurium, heparin) were tested for interference with the piritramide assay. None of the 14 tested drugs interfered in the assay.
Clomipramine was chosen as an internal standard for analytical reasons. In general clomipramine is unlikely to be part of the medication regime of paediatric patients. Regardless in each case we verified that clomipramine had never been administered to any of our patients at any point prior to the study period.
Pharmacokinetic analysis
Measured data were processed by a standard calculation program (Microsoft Excel 2002). Corresponding data sets were automatically imported into the pharmacokinetic program Topfit 2.0 [21], and in the first step of pharmacokinetic analysis, the measured concentrations of each group were analysed by non-compartmental methods. Cmax (maximum piritramide concentration) and the elimination half-life \({\left( {t_{{{\text{1}} \mathord{\left/ {\vphantom {{\text{1}} {2{\text{ $ \beta $ }}}}} \right. \kern-\nulldelimiterspace} {2{\text{ $ \beta $ }}}}} } \right)}\) were calculated. The urine data was used to calculate the urinary recovery of piritramide. Thereafter, the inidividual serum concentration–time data were fitted to an open two-compartment model.
The following pharmacokinetic parameters were investigated: maximum concentration (Cmax), fast distribution half-life \({\left( {t_{{1 \mathord{\left/ {\vphantom {1 {2{\text{ $ \alpha $ }}}}} \right. \kern-\nulldelimiterspace} {2{\text{ $ \alpha $ }}}}} } \right)},\), elimination half-life \({\left( {t_{{1 \mathord{\left/ {\vphantom {1 {2{\text{ $ \beta $ }}}}} \right. \kern-\nulldelimiterspace} {2{\text{ $ \beta $ }}}}} } \right)}\), volume of distribution at steady-state (Vdss) and total clearance (Clt). Half-lives corresponding to the exponents for the constants of disposition, volume of distribution, and clearance were calculated as follows:
-
\(t_{{1 \mathord{\left/ {\vphantom {1 {2{\text{ $ \alpha $ }}}}} \right. \kern-\nulldelimiterspace} {2{\text{ $ \alpha $ }}}}} \)= ln2/λ 1 (distribution,λ 1 is the rate constant corresponding to the intermediate distribution)
-
\(t_{{1 \mathord{\left/ {\vphantom {1 {2{\text{ $ \beta $ }}}}} \right. \kern-\nulldelimiterspace} {2{\text{ $ \beta $ }}}}} \)= ln2/λ z (elimination,λ z is the rate constant corresponding to the terminal elimination)
-
Vdss = V c+V c·k d12/k d21 (V c volume of distribution of the central compartment,k dij first order rate constants that describe mass transfer from compartment i to compartment j)
-
Clt = λ z·Vdss (total body clearance) [21].
In addition the software NONMEM V version 1.1 was used for compartmental population pharmacokinetic analysis [2]. An exponential error model was chosen for the interindividual variability of the kinetic parameters, and a proportional error model was used for the residual variability to describe the deviations between the model predictions and the plasma concentrations. Fitting was performed with the first-order conditional estimates algorithm, taking interactions between the parameters into account. According to the findings of Boullion et al. [4, 5], an initial attempt of data analysis was done using a three-compartment model. In comparison our investigation yielded a two-compartment model as the best fitting one based on subroutines ADVAN 5 TRAN 4. Inclusion in the population model was done if a decrease in the objective function of more than 3.84 was observed.
Statistical analysis
SPSS version 11.0.1 (SPSS, 2001) was used for statistical analysis. The non-parametric Mann-Whitney U-test was used to prove the differences between groups (NB vs. IF1; NB vs. IF2; NB vs. YC; IF1 vs. IF2; IF1 vs. YC and IF2 vs. YC). A P value<0.05 was considered to be statistically significant.
Results
Of the 41 patients included, two were excluded from the study due to inadequate blood and urine sampling. The descriptive data of the evaluated 39 patients, receiving a mean dose of 50 μg/kg, are listed in Table 1.
The proportion of the patients undergoing complex neuro-, cardio- or visceral surgical intervention was 95%. Postoperative requirement for mechanical ventilation was necessary in 46% of the patients, with the largest percentage found in group NB (75%).
The highest values of Cmax 15 min after piritramide application were found in group NB (Cmax, median=79 μg/l; range 5–723 μg/l). Median value of Cmax was reduced in IF1, but the range remained very large (Cmax=36 μg/l, range 6–855 μg/l). The lowest median was found in group IF2 (Cmax=12 μg/l, range 3–315 μg/l). In the YC group Cmax was similar to what would be expected in adults, and the range was small (Cmax=16 μg/l; range 9–35).
The intergroup differences in Cmax were of no statistical significance (see Fig. 1).
The median half-life of distribution \({\left( {t_{{1 \mathord{\left/ {\vphantom {1 {2{\text{ $ \alpha $ }}}}} \right. \kern-\nulldelimiterspace} {2{\text{ $ \alpha $ }}}}} } \right)}\) was rather long in the newborns (37 min, range 15–189 min) whereas in the other groups the half-life was shorter with rather smaller ranges: 8.4 min (range 3.6–18.1 min) in IF1, 13.2 min in IF2 (range 2.0–34.7 min) and 17.8 min in CY (range 7.5–38.0 min). The differences between the four groups were not statistically significant (see Fig. 2).
Comparison of the distribution half-life \({\left( {t_{{{\text{1}} \mathord{\left/ {\vphantom {{\text{1}} {{\text{2 $ \alpha $ }}}}} \right. \kern-\nulldelimiterspace} {{\text{2 $ \alpha $ }}}}} } \right)}\) of piritramide. Group 1 newborns (NB), 2 younger infants (IF1), 3 older infants (IF2) and 4 young children (YC)
The median elimination half-life \({\left( {t_{{1 \mathord{\left/ {\vphantom {1 {2{\text{ $ \beta $ }}}}} \right. \kern-\nulldelimiterspace} {2{\text{ $ \beta $ }}}}} } \right)}\) of piritramide displayed a distinct prolongation in the group of newborns based on two-compartmental model analysis with TOPFIT (see Fig. 3). The differences between group NB and the other three groups (IF1, IF2 and YC) were statistically significant (Mann-Whitney U, P<0.05).
Comparison of the terminal elimination half-life \({\left( {t_{{{\text{1}} \mathord{\left/ {\vphantom {{\text{1}} {{\text{2 $ \beta $ }}}}} \right. \kern-\nulldelimiterspace} {{\text{2 $ \beta $ }}}}} } \right)}\) of piritramide. Group 1 newborns (NB), 2 younger infants (IF1), 3 older infants (IF2) and4 young children (YC)
Only in the newborn group there was a difference between the elimination half-life calculated by a noncompartmental method and by a two-compartment method. The latter resulted in a much longer elimination half-life (702 versus 249 min). Considering the wide range of values and the small group sizes the differences between the newborn group and the other groups after non-compartmental analysis were not statistically significant.
The recovery of piritramide in the urine was small in all groups (1–4% of the actually given dose). Higher values may indicate that the children received piritramide before or during the operation (patients no. 9, 10, 26; Table 2).
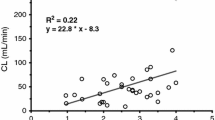
The distribution volume, Vdss, and the total clearance, Clt, were small in the newborn and young infant groups (values are median±SD) (2.0±4.93 l/kg and 15.9±16.7 ml/min in NB, 1.7±2.5 l/kg and 46.6±76.9 ml/min in IF1 respectively). In the two other groups distribution volume was about 7 l/kg and similar to the values of an adult patient (7.0±5.2 l/kg in IF2 and 6.7±2.2 l/kg in YC). Total clearances in the IF2 and YC group were 235.5±454.1 ml/min and 338.0±168.1 ml/min respectively. The differences relating to volume of distribution and total clearance between NB/IF1 vs. IF2/YC were statistically significant (see Figs. 4, 5).
In addition, NONMEM analysis was performed to assess maturational trends in the pharmacokinetics of piritramide. The analysis used 288 concentration observations from 39 subjects. Quality of fit of pharmacokinetic data and changes in clearance with age and weight are shown in Figs. 6, 7 and 8.
Discussion
In 1961 Paul A. J. Janssen first described piritramide as a potent analgesic with unusual chemical structure and typical morphine-like properties in a variety of pharmacological tests in animals [28]. Several human studies on the clinical effects of piritramide in the treatment of postoperative pain in comparison with the most common opioids and placebo were published through 1968 [9, 10, 22, 30, 34, 40, 42].
With the use of a new gas chromatographic method for sensitive determination of piritramide in human plasma [37] Kietzman et al. first reported the pharmacokinetics of piritramide after a single intravenous bolus in adults in 1996 [32] (Table 3). A few papers have provided pharmacokinetic and pharmacodynamic information about piritramide in adults after single bolus injections [4, 5, 31, 39] and prolonged administration [4, 5, 31, 39].
Up to the present there has been a lack of information about the pharmacokinetics in children and newborns, although piritramide is approved after the first year of age [13, 14].
In the present study, pharmacokinetic parameters of piritramide after single bolus application (dosage: 50 μg/kg) in newborns, infants and young children were calculated and compared among the groups by non-parametric Mann-Whitney U-test (NB vs. IF1; NB vs. IF2; NB vs. YC; IF1 vs. IF2; IF1 vs. YC and IF2 vs. YC).
The pharmacokinetic results of this study are listed along with the findings of previously published studies concerning the pharmacokinetic profile of piritramide in adults (Table 3).
The elimination half-life of piritramide in newborns (compartmental analysis) was significantly longer than that of any patient in any of the other groups (IF1, IF2, YC). The elimination half-lives in the other three groups (IF1, IF2 and YC) were distinctly shorter compared to those found in adults. Generally the half-life of opioid analgesics is correlated with the gestational age, reflecting maturity of physiologic systems [4, 5, 32].
The prolonged elimination half-life of piritramide in newborns may reflect the difference in body composition in newborns compared with infants, children and adults [41].
Greeley et al. compared the neonatal pharmacokinetic data in various age groups in the paediatric population. In this study \(t_{{1/2\beta }} \) of sufentanil was 737 min in neonates (0–1 month old) but 214 min in the infant group (1 month to 2 years). Increase in sufentanil clearance and decrease in elimination half-life may give evidence of the maturation of sufentanil kinetics during the first month [18, 19]. This phenomenon is consistent with the pharmacokinetic data found in the present study.
Developmental immaturity leads to decreases in protein binding, metabolism, and excretion, and increases in elimination [12]. Kart et al. [29] showed that the half-life and duration of action of morphine are prolonged in neonates mainly due to the decrease in metabolic capacity and total body clearance.
Generally the maturity of biotransformation in newborns is diminished mainly due to the decrease in metabolic capacity and enzymatic activity of the cytochrome P-450 system, sulphate conjugation and acetylation [16]. Although the metabolism of piritramide is not known, renal elimination is negligible, and it may be inferred that the elimination of piritramide depends almost exclusively on hepatic metabolism [32]. In this context surgery may cause changes in hepatic blood flow and cardiac output affecting volume of distribution, clearance [41] and terminal eliminaton half-life.
Gauntlett et al. demonstrated the impact of surgery on the pharmacokinetics of fentanyl in neonatal lambs. The authors suggested that the mechanism of decreased clearance of fentanyl is decreased hepatic function (decreased fentanyl extraction) rather than decreased hepatic flow [15, 33]. In this context another contributing factor might be post-natal patency of the ductus venosus that is partially draining the portal system as part of the fetal circulation and thereby shunting blood past the liver [17]. Hence, depending on the shunt fraction, significant amounts of the drug may be kept from hepatic metabolisation.
Moreover prolonged eliminaton of piritramide may lead to accumulation of the drug in newborns, suggesting that they require relatively smaller maintainance doses than adults. In contrast to the findings of Brack et al. [7] we suggest that the patient’s age is a determinant in postoperative opioid titration after surgery in these special groups.
It should be noted that there was a large variability in the maximum concentration of piritramide in the NB group (see Fig. 3) due to the clinical condition and severity of disease in this special group. We assume that the impact of severe disease on the constitution of body compartments may be more extreme in newborns than in older infants and children. The transition from fetal to extra-uterine circulation is not abrupt, and within the NB group, various types of shunts may have contributed to the wide range in measured Cmax. Although the differences in Cmax among the four groups showed no statistical significance, the highest concentrations were found in the NB group. Similar findings were reported by Bouwmeester et al. [6]. In this study significantly higher plasma concentrations of morphine were measured in neonates after intravenous morphine infusion and intravenous morphine bolus application. Moreover mechanical ventilation decreased morphine metabolism and clearance [6].
A confounding factor in this study was the fact that 75% of the newborns required mechanical ventilation postoperatively. Surgery and the clinical conditions as well as the immaturity of metabolic capacity may affect metabolic clearance [11].
Kietzman et al. [32] described a three-compartment model after intravenous bolus application in adults.
In comparison to the pharmacokinetic model previously published in adults, in this study the collection of blood samples was limited in amount and frequency due to ethical concerns and clinical rational. The time–concentration profile was best fitted to a two-compartment model (TOPFIT and NONMEM analysis), although the first period of distribution (15 min) after bolus application without any intermediate measurement led to a lack of information.
Conclusion
In the present study we gathered pharmacokinetic information about the disposition of piritramide in a paediatric patient collective since there is a lack of pharmacokinietic information piritramide in newborns, infants and children.
Distribution and elimination of piritramide were significantly prolonged in the NB group and decreased in the other three groups (IF1, IF2 and YC).
Prolonged elimination and decreased total clearance of piritramide may lead to accumulation in newborns, and the dosage needed to treat postoperative pain should be reduced. In infants and young children the elimination of piritramide is increased compared to adults, so that a shorter clinical effect may occur.
Further studies for dose adjustment of piritramide in paediatric patients will have to be accomplished.
Abbreviations
- ICU:
-
Intensive care unit
- Cmax :
-
Highest observed serum concentration (μg/l)
- i.v.:
-
Intravenous
- HPLC:
-
High performance liquid chromatography
- \(t_{{1 \mathord{\left/ {\vphantom {1 {2{\text{ $ \alpha $ }}}}} \right. \kern-\nulldelimiterspace} {2{\text{ $ \alpha $ }}}}} \) :
-
Half-life of drug during distribution (min)
- \(t_{{1 \mathord{\left/ {\vphantom {1 {2{\text{ $ \beta $ }}}}} \right. \kern-\nulldelimiterspace} {2{\text{ $ \beta $ }}}}} \) :
-
Half-life of drug during terminal phase (min)
- Vdss :
-
Steady-state volume of distribution after intravenous administration (l)
- PIR:
-
Piritramide
- PK:
-
Pharmacokinetics
- SD:
-
Standard deviation
References
Anon (1991) Piritramide (monograph 778). Drugs available abroad. A guide to therapeutic drugs available and approved outside the U.S. Derwent Publications, London, UK
Beal SL, Sheiner LB, Boekmann A (1998) NONMEM user’s guide. Division of Pharmacology, University of California, San Francisco
Blumer JL (ed) (1999) The therapeutic orphan-30 years later. Proceedings of a joint conference of the Pediatric Pharmacology Research Unit Network, the European Society of Developmental Pharmacology, and the National Institute of Child Health and Human Development. Pediatrics 104(3 Pt 2):581–645
Bouillon T, Groeger P, Kietzmann D (2004) The pharmacokinetics of piritramide after prolonged administration to intensive care patients. Eur J Anaesth 21:673–678
Boullion T, Kietzmann D, Port R, Meineke I, Hoeft A (1999) Population pharmacokinetics of piritramide in surgical patients. Anesthesiology 90:7–15
Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NHG (2004) Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth 92(2):208–217
Brack A, Böttiger BW, Schäfer M (2004) New insights of postoperative pain therapy. Anästhesiol Intensivmed Notfallmed Schmerzther 39:157–164
Büttner W (1994) Die Erfassung des postoperativen Schmerzes beim Kleinkind. Habilitations-Schrift, Ruhr-Universität Bochum. Arcis-Verlag, Munich
Büttner W, Finke W (2000) Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants and young children: a comprehensive report on seven consecutive studies. Paediatr Anaesth 10:303–318
Büttner W, Finke W, Schwanitz M (1990) Nalbuphine and piritramide in the postoperative period in small children. Part 2. Breathing patterns. Anaesthesist 39:258–263
Dagan O, Klein J, Bohn D, Barker G, Koren G (1993) Morphine pharmacokinetics in children following cardiac surgery: effects of disease and inotropic support. J Cardiothorac Vasc Anesth 7(4):396–398
Davis PJ, Killian A, Stiller RL, Cook DR, Guthrie RD, Scierka AM (1989) Pharmacokinetics of alfentanil in newborn premature infants and older children. Dev Pharmacol Ther 13(1):21–27
Hutchinson TA. Shahan DR (2004) Drugdex system. Healthcare series vol. 121. Micromedex, Greenwood Village, CO
Fachinformation (2002) Dipidolor. Bundesverband der Pharmazeutischen Industrie
Gauntlett IS, Fisher DM, Hertzka RE, Kuhls E, Spellman MJ, Rudolph C (1988) Pharmacokinetics of fentanyl in neonatal humans and lambs: effects of age. Anesthesiology 69:683–687
Gladtke E (1979) The importance of pharmacokinetics for paediatrics. Eur J Pediatr 131(2):85–91
Gow PJ, Ghabrial H, Smallwood RA, Morgan DJ, Ching MS (2001) Neonatal hepatic drug elimination. Pharmacol Toxicol 88(1):3–15
Greeley WJ, de Bruin NP, Davis DP (1987) Sufentanil pharmacokinetics in pediatric cardiovascular patients. Anesth Analg 66:1067–1072
Guay J, Gaudreault P, Tang A, Goulet B, Varin F (1992) Pharmacokinetics of sufentanil in normal children. Can J Anaesth 39:14–20
Hartwig S, Roth B, Theisohn M (1991) Clinical experience with continuous intravenous sedation using midazolam and fentanyl in the paediatric intensive care unit. Eur J Pediatr 150(11):784–788
Heinzel G, Wolosczak R, Thomann P (1993) TopFit version 2.0: pharmacokinetic and pharmacodynamic data analysis system. Gustav Fischer Verlag, Stuttgart
Henschel WF, Buhr G, Fernanadez R (1968) Clinical tests with a new long acting analgesic. In: Progress in anaesthesiology (Proceedings of the fourth World Congress of Anaesthesiologists), Amsterdam, Excerpta Medica International Congress Series No 200 74:887
Höhne C, Donaubauer B, Kaisers U (2004) Opioids during anesthesia in liver and renal failure. Anaesthesist 53:291–303
Hughes M, Glass P, Jacobs J (1992) Context-sensitive half-time in multicompartment pharmacokinetic models for intravenous anesthetic drugs. Anesthesiology 76:334–341
Hunt A, Joel S, Dick G, Goldman A (1999) Population pharmacokinetics of oral morphine and its glucuronids in children receiving morphine as immediate-release liquid or sustained-release tablets for cancer pain. J Pediatr 135:47-55
Jacqz-Aigrain E, Burtin P (1996) Clinical pharmacokinetics of sedatives in neonates. Clin Pharmacokinet 31:423–443
Janssen PAJ (1982) Potent, new analgesics, tailor-made for different purposes. Acta Anaesth Scand 26:262–268
Janssen PAJ (1961) Piritramide (R 3365) a potent analgesic with unusual chemical structure. J Pharm Pharmacol 13:513–530
Kart T, Christrup LL, Rasmussen M (1997) Recommended use of morphine in neonates, infants and children based on a literature review. Part 1—pharmacokinetics. Paediatr Anaesth 7(1):5–11
Kay B (1971) A clinical investigation of piritramide in the treatment of postoperative pain. Br J Anaesth 43:1167–1171
Kietzmann D, Bouillon T, Hamm C, Schwabe K, Schenk H, Gundert-Remy U, Kettler D (1997) Pharmacodynamic modelling of the analgesic effects of piritramide in postoperative patients. Acta Anaesthesiol Scand 41:888–894
Kietzmann D, Briede I, Bouillon T, Gundert-Remy U, Kettler D (1996) Pharmacokinetics of piritramide after an intravenous bolus in surgical patients. Acta Anaesthesiol Scand 40:898–903
Kuhls E, Gauntlett IS, Lau M, Brown R, Rudolph CD, Teitel DF, Fisher DM (1995) Effect of increased intra-abdominal pressure on hepatic extraction and clearance of fentanyl in neonatal lambs. J Pharmacol Exp Ther 274(1):115–119
Kumar N, Rowbotham DJ (1999) Editorial II. Piritramide. Br J Anaesth 82(1):3–5
Latasch L, Freye E (2002) Pain and opioids in the preterm and the neonate. Anaesthesist 51:272–284
Martens-Lobenhoffer L, Römhild W (2003) Quantitative determination of piritramide in human serum applying liquid chromatography-two-stage mass spectrometry. J Chromatogr B 783:53–59
Michaelis HC, Kietzmann D, Neurath H, Jongepieper U, Schilling B (1991) Sensitive determination of piritramide in human plasma by gas chromatography. J Chromatogr 571:257–262
Misztal G (1991) Determination of piritramide in plasma using high pressure liquid chromatography methods. Acta Pol Pharm 48(5–6):1–2
Saarenmaa E, Neuvonen PJ, Fellman V (2000) Gestational age and birth effects on plasma clearance of fentanyl in newborn infants. J Pediatr 136(6):767–770
Saarne A (1969) Clinical evaluation of the new analgesic piritramide. Acta Anaesth Scand 13:11–19
Taddio A (2002) Opioid analgesia for infants in the neonatal intensive care unit. Clin Perinatol 29:493–509
Weyne F, Schluter J, Lust P (1968) Piritramide, a potent postoperative analgesic with unusually low respiratory depressant, cardiovascular and emetic effects. Acta Anaesth Belg 19:33–45
Acknowledgement
The authors appreciated strongly the help of the nurses and physicians of the Children’s hospitals to maintain the study, filling out the case report formulas and giving support and general assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, C., Kremer, W., Harlfinger, S. et al. Pharmacokinetics of piritramide in newborns, infants and young children in intensive care units. Eur J Pediatr 165, 229–239 (2006). https://doi.org/10.1007/s00431-005-0021-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-005-0021-z