Abstract
We studied the effects of a new regimen consisting of intravenous immune globulin (IVIG) combined with dexamethasone (DEX) on clinical outcome and serum levels of vascular endothelial growth factor (VEGF) in the initial treatment of Kawasaki disease (KD). A total of 46 KD patients received 0.3 mg/kg per day DEX plus heparin i.v. for 3 consecutive days, together with 2 g/kg IVIG over 4 to 5 days (DEX group). Low-dose acetylsalicylic acid was started after completion of DEX therapy. The control group consisted of 46 KD patients retrospectively treated earlier with 2 g/kg IVIG over 4 to 5 days plus higher dose acetylsalicylic acid (CONTROL group). No serious adverse effect was noted in either group. There were no differences in baseline and post-treatment laboratory data except for C-reactive protein between the groups. Post-treatment C-reactive protein in the DEX group (median 0.9 mg/dl, range 0.0 to 24.7 mg/dl) was lower than that (1.2 mg/dl, range 0.2 to 19.5 mg/dl) in the CONTROL group ( P =0.033 by Mann-Whitney U test). In addition, the mean duration of fever after the first IVIG infusion was 2.2 days (median 1 day, range 1 to 12 days) in the DEX group and 2.8 days (2 days, 1 to 16 days) in the CONTROL group ( P =0.015 by Mann-Whitney U test). The new regimen did not reduce VEGF levels. Two patients in each group developed small- or medium-sized coronary artery aneurysms. Conclusion:although this regimen did not affect coronary outcome, intravenous immune globulin therapy combined with dexamethasone for the initial treatment of Kawasaki disease was safe and may accelerate the resolution of systemic inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kawasaki disease (KD) is an acute febrile vasculitic syndrome of unknown aetiology [6]. Coronary artery aneurysms (CAA) develop in approximately 15% to 25% of untreated cases. KD is the leading cause of acquired coronary heart disease in children [15]. Intravenous immune globulin (IVIG) is clearly effective in the rapid resolution of KD inflammation; however, approximately 15% of patients had persistent or recurrent fevers after IVIG completion and are considered to have a higher risk of developing CAA, suggesting a need for a better treatment regimen [10, 13, 17,18]. We and others have demonstrated that vascular endothelial growth factor (VEGF) might play a role in the pathogenesis of KD. Severe oedema with plasma leakage has been documented in patients who were resistant to IVIG compared with those who responded [9, 12,18]. Dexamethasone (DEX) is reported to ameliorate brain oedema [4] and tracheal oedema [2], and to suppress the production of VEGF in vitro [7]. We investigated the effects of IVIG combined with DEX on clinical outcome and serum VEGF levels in the initial treatment of acute KD.
Subjects and methods
Subjects
From January 2001 to August 2001, we enrolled patients in the Chiba Municipal Kaihin, Teikyo University Ichihara, and Chiba University Hospitals after obtaining their informed consent. Diagnosis of KD was based on the criteria of the Japanese Kawasaki Disease Research Committee [5]. For the comparison of the efficacy and safety of the regimen, patients who had been treated with IVIG (2 g/kg over 4 to 5 days) and acetylsalicylic acid (30 mg/kg) in these hospitals before January 2001 were used as controls. To exclude selection bias, control patients were selected retrograde from January 2001 and the numbers of patients from each hospital were matched. In both groups, subjects were excluded if they did not receive IVIG nor had a baseline echocardiogram indicating coronary abnormalities.
Treatment protocols
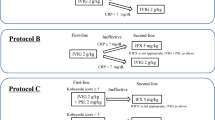
The protocols are indicated in Fig. 1. A total of 46 patients were treated intravenously with 0.15 mg/kg of DEX twice a day for 3 consecutive days together with 2 g/kg IVIG over 4 to 5 days (DEX group). DEX was administered twice a day by a single injection, 60 min before IVIG infusion and 60 min after IVIG completion. Patients of the DEX group received intravenous heparin infusion (10 U/kg per h) during DEX therapy as anti-coagulant therapy. Low-dose acetylsalicylic acid (10 mg/kg per day) was started after completion of DEX treatment. The 0.3 mg/kg per day dose of DEX used in this study has almost equivalent anti-inflammatory potency of 2 mg/kg per day prednisolone used in the previous investigation [1,14]. A group of 46 control KD patients received standard IVIG therapy, 2 g/kg IVIG for 4 to 5 consecutive days plus 30 mg/kg oral acetylsalicylic acid (CONTROL group). An axillary temperature was taken for each patient, with fever defined as >37.5°C. Patients who became afebrile within 5 days of starting IVIG were classified as IVIG-responsive. Patients who had persistent fever up to the 6th day of IVIG treatment were defined as IVIG-resistant. IVIG-resistant patients were treated with additional IVIG. None received ulinastatin during the course of their illness. Laboratory investigations were performed before the first IVIG dose and the following day after completion of initial IVIG treatment. CAA were defined with internal diameters of aneurysms >3.0 mm by two-dimensional echocardiogram 2 weeks after the onset of fever.
Protocols of regimens used in this study. The DEX group patients received 2 g/kg IVIG over 4 to 5 days, together with 0.3 mg/kg per day DEX for 3 days. The study group also received intravenous heparin infusion (10 U/kg per h), but not acetylsalicylic acid, during the first 3 days. Low-dose acetylsalicylic acid (10 mg/kg per day) was started after completion of DEX treatment. Control KD patients received IVIG, 2 g/kg over 4 to 5 days, with 30 mg/kg per day acetylsalicylic acid
Measurements of serum vascular endothelial growth factor levels
Serum levels of free VEGF165 were assayed using an ELISA kit (R and D Systems) as previously reported [18,20]. The IVIG solution did not contain VEGF protein, as determined with this assay. Serum samples before the first IVIG dose and the following day after completion of the initial IVIG dose (2 g/kg) were collected and stored frozen at −80°C until use. In this study, paired serum samples taken before and after initial IVIG were available in 16 patients of the DEX group and 14 patients of the CONTROL group, respectively. Of these, one DEX and two CONTROL patients were IVIG-resistant.
Statistical analysis
The results are presented as the mean ± SD. For comparisons of the two groups, numeric values were analysed using the Student or Welch t test for unpaired values. Comparisons between paired VEGF values were performed with a paired t test. Data that did not show a normal distribution were analysed by the Mann-Whitney U test. Categorical data were assessed by the chi-squared test. A value of P < 0.05 was considered significant. The statistical analyses were conducted with the Prism Software, version 4 (Graphpad Software, San Diego).
Results
Demographic data for the DEX and CONTROL groups
The demographic data for both groups are summarised in Table 1. There were no differences with respect to age (2.4±2.0 versus 2.2±1.5 years old; P =0.60 by Welch’s t test), sex distribution (male/female, 23/23 versus 22/24; P =0.83 by chi-squared test), body weight (median 11.3 kg, range 5.9 to 22.8 kg versus median 9.7 kg, range 5.6 to 20.5 kg; P =0.56 by Mann-Whitney U test) or duration of illness on admission (5.3±1.6 versus 5.2±1.5 days P =0.73 by unpaired Student’s t test) between the DEX and CONTROL groups.
Clinical outcome
No serious adverse effects were noted in patients of either group. No hypertension, glycosuria or thromboembolic event was noted in any individual. The mean duration of fever after the start of initial treatment was 2.2 days (median 1 day, range 1 to 12 days) in the DEX group and 2.8 days in the CONTROL group (median 2 days, range 1 to 16 days; P =0.015 by Mann-Whitney U test) (Table 2). In this series, 6 of 46 patients in each group were IVIG-resistant and were treated with additional IVIG. The additional doses were 1728±413 mg in the DEX group and 1740±958 mg in the CONTROL group ( P =0.98 by unpaired Welch’s t test). The duration of re-treated IVIG was 3.3±1.5 days and 2.7±1.4 days, respectively ( P =0.44 by unpaired Student’s t test). In 12 IVIG-resistant patients, the mean duration of fever after the start of initial treatment was 8.3±2.7 days in the DEX group and 9.2±3.8 days in the CONTROL group ( P =0.67 by unpaired Student’s t test). All IVIG-resistant patients responded to additional IVIG, but 2 of the 46 in each group (a total of four patients) developed CAA. Follow-up echocardiographic and angiographic examinations confirmed the regression of CAA within 8 months after illness in all these patients.
Laboratory data for the DEX and CONTROL groups
There were no differences in baseline and post-treatment haematcrit, platelets, total protein and albumin between the groups (Table 3). Circulating white blood cell counts in the DEX group were higher than those in the CONTROL group, probably due to corticosteroid effects. After initial IVIG completion, the mean level of C-reactive protein was 2.6 mg/dl (median 0.9 mg/dl, range 0.0 to 24.7 mg/dl) in the DEX group and 2.7 mg/dl (median 1.2 mg/dl, range 0.2 to 19.5 mg/dl) in the CONTROL group ( P =0.033 by Mann-Whitney U test).
Serum levels of vascular endothelial growth factor
There were no differences in the age (3.0±2.4 versus 2.5±2.0 years old; P =0.57 by unpaired Student’s t test), sex distribution (male/female, 9/7 versus 6/8; P =0.46 by chi-squared test), body weight (13.8±1.3 versus 11.5±4.8 kg; P =0.22 by unpaired Student’s t test) or duration of illness on admission (4.6±1.0 versus 5.1±0.7 days; P =0.12 by unpaired Welch’s t test) between the DEX and CONTROL groups. Baseline levels of VEGF (862±501 versus 927±524 pg/ml; P =0.73 by unpaired Student’s t test) and post-treatment levels (1263±588 versus 1101±680 pg/ml; P =0.49 by unpaired Student’s t test) were similar between the DEX and CONTROL groups (Table 3). The VEGF levels before and after treatment were significant in the DEX group ( P =0.0015 by paired t test), but not in the CONTROL group ( P =0.24 by paired t test). Of these 30 patients studied VEGF levels, three patients were IVIG-resistant. Serum VEGF level after treatment increased from 291 to 621 pg/ml in the patient resistant to DEX regimen and also increased from 1511 to 2899 pg/ml or from 821 to 1167 pg/ml in the two patients resistant to CONTROL regimen, respectively.
Discussion
Pathological findings of KD vasculitis are characterised by systemic oedema and mononuclear cell infiltrates. Vascular leakage due to increased vascular permeability is evident in tissues from patients with fatal KD [20], and VEGF may play an important role in this leakage [18,20]. Oedematous changes with cellular infiltration are often found in the systemic arteries of these patients, including the pulmonary, iliac, hepatic, splenic, renal and coronary arteries [20]. Additionally, the venous system is also characterised by oedematous change with cellular infiltration. Since systemic oedema seems to be a characteristic feature of KD, it is important to know whether steroid therapy would be effective in the resolution of inflammation in KD. However, the effects of steroid therapy remain to be elucidated.
To our knowledge, this is the first trial using DEX for the initial treatment of acute KD, although prednisolone or pulse methylprednisolone have been used for KD therapy [3, 11, 14, 16,19]. DEX is known to ameliorate tracheal oedema in preterm infants with respiratory failure [2]. Other reports have documented that DEX ameliorates brain oedema in experimental brain tumours and in peritoneal brain oedema [4], and reduces VEGF expression in growth plate chondrocytes in vitro [7]. In the present study, the duration of fever in the DEX group was shorter than that in the CONTROL group. In addition, post-treatment C-reactive protein in the DEX group was lower than that in the CONTROL group. These were the differences found in the clinical effects of the studied regimens. Similar effects were reported by Shinohara et al. [14] in studies using prednisolone in the treatment of acute KD. The serum levels of VEGF did not differ between patients treated with and without DEX. Similar results were reported in a study of preterm infants with respiratory distress syndrome [8]. In that study, early post-natal DEX treatment decreased the amounts of hepatocyte growth factor but not VEGF in tracheal aspirate fluids. In our study, the DEX regimen did not reduce serum VEGF levels.
We have preliminarily demonstrated that IVIG therapy, combined with DEX and heparin (but not acetylsalicylic acid), is safe in the initial treatment of acute KD and may shorten the duration of fever. The results do not support an effect of DEX on the reduction of serum VEGF levels. Although a single IVIG dose regimen is recognised as more effective compared with multiple infusions [10,13], the health insurance did not cover 2 g/kg single dose of IVIG for the initial treatment of acute KD in Japan during the study period. Further analyses using larger numbers of patients treated with single IVIG dose plus steroids may be necessary to confirm the efficacy of IVIG plus steroids.
Abbreviations
- CAA :
-
coronary artery aneurysms
- DEX :
-
dexamethasone
- IVIG :
-
intravenous immune globulin
- KD :
-
Kawasaki disease
- VEGF :
-
vascular endothelial growth factor
References
Chrousos GP, Margioris AN (2000) Adrenocorticosteroids and adrenocortical antagonists. In: Katzung BG (ed) Basic and clinical pharmacology. Lange Medical Books/McGraw-Hill, New York, pp 660–678
Couser RJ, Ferrara TB, Falde B, Johnson K, Schilling CG, Hoekstra RE (1992) Effectiveness of dexamethasone in preventing extubation failure in preterm infant at increased risk for airway edema. J Pediatr 121: 591–596
Hashino K, Ishii M, Iemura M, Akagi T, Kato H (2001) Re-treatment for immune globulin-resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatr Int 43: 211–217
Ikeda Y, Carson BS, Lauer JA, Long DM (1993) Therapeutic effects of local delivery of dexamethasone on experimental brain tumors and peritoneal brain edema. J Neurosurg 79: 716–721
Japan Kawasaki Disease Research Committee (1984) 1984 Diagnostic guidelines of Kawasaki disease, 4th rev. edn. Japan Kawasaki Disease Research Committee, Tokyo
Kawasaki T (1967) Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children: clinical observations of 50 cases (in Japanese). Arerugi 16: 178–222
Koedam JA, Smink JJ, van Buul-Offers SC (2002) Glucocorticoids inhibit vascular endothelial growth factor expression in growth plate chondrocytes. Mol Cell Endocrinol 197: 35–44
Lassus P, Nupponen I, Kari A, Pohjavuori M, Andersson S (2002) Early postnatal dexamethasone decreases hepatocyte growth factor in tracheal aspirate fluid from premature infants. Pediatrics 110: 768–771
Maeno N, Takei S, Masuda K, Akaike H, Matsuo K, Kitajima I, Maruyama I, Miyata K (1998) Increased serum levels of vascular endothelial growth factor in Kawasaki disease. Pediatr Res 44: 596–599
Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, Colan SD, Duffy CE , Fulton DR, Glode MP, Mason WH, Meissner HC, Rowley AH, Shulman ST, Reddy V, Sundel RP, Wiggins JW, Colton T, Melish ME, Rosen FS (1991) A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med 324: 1633-1639
Nonaka Z, Maekawa K, Okabe T, Eto Y, Kubo M (1995) Randomized controlled study of intravenous prednisolone and gammaglobulin treatment in 100 cases with Kawasaki disease. In: Kato H (ed) Kawasaki disease. Proceedings of the 5th International Kawasaki Disease Symposium. Elsvier Science BV, Amsterdam, pp 328–331
Ohno T, Yuge T, Kariyazono H, Igarashi H, Joh-o K, Kinugawa N, Kusuhara K, Hara T (2002) Serum hepatocyte growth factor combined with vascular endothelial growth factor as a predictive indicator for the occurrence of coronary artery lesions in Kawasaki disease. Eur J Pediatr 161: 105–111
Sato N, Sugimura T, Akagi T, Yamakawa R, Hashino K, Eto G, Iemura M, Ishii M, Kato H (1999) Selective high dose gamma-globulin treatment in Kawasaki disease: assessment of clinical aspects and cost effectiveness. Pediatr Int 41: 1–7
Shinohara M, Sone K, Tomomasa T, Morikawa A (1999) Corticosteroids in the treatment of the acute phase of Kawasaki disease. J Pediatr 135:465–469
Shulman ST, De Inocencio J, Hirsch R (1995) Kawasaki disease. Pediatr Clin North Am 42: 1205–1222
Sundel RP, Baker AL, Fulton DR, Newberger JW (2003) Corticosteroids in the initial treatment of Kawasaki disease: report of a randomized trial. J Pediatr 142: 611–616
Terai M, Shulman ST (1997) Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent on salicylate dose. J Pediatr 131: 888–893
Terai M, Honda T, Yasukawa K, Higashi K, Hamada H, Kohno Y (2003) Prognostic impact of vascular leakage in acute Kawasaki disease. Circulation 108: 325–330
Wright DA, Newburger JW, Baker A, Sundel RP (1996) Treatment of immune globulin-resistant Kawasaki disease with pulsed doses of corticosteroids. J Pediatr 128: 146–149
Yasukawa K, Terai M, Shulman ST, Toyozaki T, Yajima S, Kohno Y, Rowley AH (2002) Systemic production of vascular endothelial growth factor and fms-like tyrosine kinase-1 receptor in acute Kawasaki disease. Circulation 105: 766–769
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jibiki, T., Terai, M., Kurosaki, T. et al. Efficacy of intravenous immune globulin therapy combined with dexamethasone for the initial treatment of acute Kawasaki disease. Eur J Pediatr 163, 229–233 (2004). https://doi.org/10.1007/s00431-003-1386-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-003-1386-5