Abstract
Background and Objectives
Intravenous immunoglobulin (IVIG) therapy for acute-stage Kawasaki disease (KD) is the first-line treatment for preventing the development of coronary artery aneurysms (CAA). Corticosteroids (prednisolone) and infliximab are often used in patients at a high risk of CAA or those with CAA at diagnosis; however, there are only a few reports of non-responders to corticosteroids as an adjuvant therapy or rescue alternative to IVIG. In this study, we compared the therapeutic effects of primary and secondary prednisolone with IVIG for KD.
Methods
We established the following three protocols: A was a secondary rescue prednisolone protocol; B was no prednisolone and second-line infliximab protocol, and C was the primary prednisolone protocol. The indication for prednisolone administration was based on the following: primary prednisolone administration, Kobayashi score; and secondary administration, Shizuoka score.
Results
Four hundred and sixty-nine patients were enrolled in the three protocols. A comparison between primary and secondary prednisolone and IVIG, as the first-line therapy revealed that the number of first non-responders in C group was 7 (8.3%), which was significantly lower than the 50 (20.9%) in A group. There was a significant difference in the first and second non-responders among the three groups, and the number of non-responders in A group was 6 (2.5%), which was significantly lower than the 13 (9.9%) in B group (p < 0.001, by Bonferroni test). The multivariate logistic regression analysis showed that IVIG non-responders among the protocol groups had an adjusted odds ratio of 6.47. Fifteen IVIG non-responders were administered infliximab as a second-line therapy, and of them, 9 (60%) showed therapy resistance. CAA occurred in 21 patients (4.6%). There was no significant difference among each protocol group.
Conclusions
The number of IVIG non-responders in the group with prednisolone administration was lower than that in the group without prednisolone administration. Secondary rescue infliximab therapy for IVIG non-responders resulted in a lower defervescence effect than the secondary rescue IVIG with prednisolone administration. Further prospective randomized studies are needed to identify factors useful for preventing IVIG non-responders and determine the optimal rescue therapy for preventing CAA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We aimed to compare the therapeutic effects of primary and secondary prednisolone with intravenous immunoglobulin for Kawasaki disease and established three treatment protocols (A, secondary rescue prednisolone protocol; B, no prednisolone and second-line infliximab protocol; and C, primary prednisolone protocol). |
Four hundred and sixty-nine patients were enrolled, with 245 in A group, 136 in B group, and 88 in C group. |
The number of non-responders in the group with prednisolone administration was lower than that in the group without prednisolone administration. Secondary rescue infliximab therapy for intravenous immunoglobulin non-responders resulted in a lower defervescence effect than secondary rescue intravenous immunoglobulin with prednisolone. |
1 Introduction
Kawasaki disease (KD) is the leading cause of acquired heart disease in children. It is an acute, self-limited, systemic vasculitis of unknown etiology that typically presents in early childhood. Coronary artery aneurysms (CAAs) occur in 15–25% of untreated patients [1]. One of the most widely administered therapies is intravenous immunoglobulin (IVIG), which substantially reduces the incidence of CAA [2, 3]. Randomized controlled studies and meta-analyses [4, 5] have confirmed that IVIG plus aspirin compared with aspirin alone reduces the risk of CAA, and thus, this treatment is now used as the standard therapy. However, some patients with KD do not respond to IVIG therapy and show persistent fever or the return of symptoms within 48 h. The proportion of patients with KD who did not respond to initial IVIG therapy was 11–23% [6,7,8]; the disease was complicated by CAA in 10–15% of these patients [9]. No prospective trial has been carried out to evaluate rescue therapy in initial IVIG non-responders for KD; thus, new more effective therapies are needed to prevent CAA.
A previous study reported that physicians are reluctant to administer corticosteroids for acute KD [10]; nonetheless, previously used drugs such as corticosteroids and drug combinations have been reassessed [11,12,13]. The Japanese Society of Pediatric Cardiology revised the Guidelines for Medical Treatment of Acute Kawasaki Disease based on the results of the Japanese RAISE study [14]; that is, corticosteroids can be used as primary therapy if a patient’s risk score for predicting an IVIG non-responder indicates severe KD. The study also endorsed corticosteroid as rescue therapy for IVIG non-responders [15]. A systematic review and meta-analysis of 16 controlled studies involving 2746 patients treated with IVIG plus corticosteroid versus IVIG alone concluded that the efficacy of corticosteroids for protecting against CAA was inversely related to the duration of illness before corticosteroid administration [16]. However, it remains unclear whether combination therapy is effective in a real-world clinical setting.
An important issue in the administration of corticosteroid regimens for KD therapy is whether it should be used as a primary therapy adjuvant to IVIG or as a rescue alternative to IVIG. We previously reported that serum C-reactive protein (CRP) level after initial IVIG therapy is a good predictor of IVIG non-responders in patients with KD [17]. Targeted use of prednisolone with IVIG as second-line therapy for refractory KD with a high serum CRP level (≥ 10 mg/dL after the first IVIG) was considerably more effective and appeared to lower the incidence of CAA than the second round of IVIG alone [18]. However, our previous research could not compare with the RAISE study [14] can be used as IVIG with primary corticosteroid therapy if a patient’s risk score for predicting an IVIG non-responder indicates severe KD. Various corticosteroid regimens are used in patients who fail to respond to IVIG therapy. Reports on the advantages and disadvantages of corticosteroid regimens, including primary or rescue corticosteroid regimens, for KD are limited. We conducted this study to investigate appropriate timing to add prednisolone with KD therapy. Additionally, options for rescue therapy are based on agents with established effectiveness in other vasculitides, including inhibitors of tumor necrosis factor (TNF-α) [19], immunosuppressive agents, and plasmapheresis [20]. This study was conducted to compare the therapeutic effects of prednisolone with IVIG for KD and therapeutic options for IVIG non-responders.
2 Patients and Methods
2.1 Study Population
The subjects received a diagnosis of KD based on the Diagnostic Guideline for KD by the attending physicians of institutions involved in the Shizuoka Kawasaki Disease Study Group (SKDSG). Patients who met at least five criteria for typical KD or four criteria for atypical KD with CAA were diagnosed as definitely having KD; those who met less than four criteria and had no other diagnoses were diagnosed as having incomplete KD [21]. Patients with typical and atypical KD were included in this study. This trial was conducted in accordance with the ethical principles of the Declaration of Helsinki and in compliance with Good Clinical Practice and related regulations. The study was reviewed and approved by the ethics committee of Shizuoka Children’s Hospital and all participating institutions. Patients and their guardians provided written informed consent before enrollment or informed consent was obtained as an opt-out and inclusion agreement based on the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. Some institutions posted an explanation of this study on their web homepage. When a subject was not willing to participate, his/her data were excluded from the analysis.
2.2 Methods
This was a prospective cohort study that aimed to compare the therapeutic effects of prednisolone with those of IVIG for KD and identify therapeutic options for IVIG non-responders. This study was registered as a clinical trial at UMIN (UMIN000025707). This multicenter study was carried out from October 1, 2016 to March 31, 2019 by the SKDSG, which consists of the following 12 institutions: Shizuoka Children’s Hospital, Shizuoka General Hospital, Shizuoka Saiseikai General Hospital, Shizuoka City Shimizu Hospital, Shizuoka City Hospital, Fuji City General Hospital, Seirei Numazu Hospital, Shizuoka Kosei Hospital, Shizuoka Red Cross Hospital, Yaizu City Hospital, Fujieda Municipal General Hospital, and Chutoen General Medical Center. Each hospital independently decided to select the one protocol from the three protocols (A, B, and C) for KD therapy before participating in the study.
2.3 Protocol
In Japan, clinical risk scores are used in clinical practice to identify patients at a high risk of not responding to IVIG and developing CAA [22,23,24]. Recently, patients with KD have been treated following the RAISE protocol at numerous institutions using the scoring system [14, 25]. In the SKDSG, before establishing the protocol for KD therapy in the present study, we discussed the following: (1) issues associated with corticosteroid regimens for KD therapy; (2) appropriate timing to add prednisolone with KD therapy; (3) RAISE protocol that recommends primary prednisolone protocol; and (4) previously reported secondary prednisolone protocol [17, 18]. Furthermore, we discussed whether second-line infliximab should be used as a therapeutic option for IVIG non-responders based on agents with established effectiveness in other vasculitides, including inhibitors of TNF-α [19]. Based on these aspects, we established the following three protocols:
Protocol A: This protocol involved the use of secondary prednisolone with IVIG. Patients resistant to first-line IVIG therapy (2 g/kg) were divided into two groups based on the Shizuoka score—high serum CRP (≥ 7.0 mg/dL) and low serum CRP (< 7.0 mg/dL) groups—depending on the serum CRP level examined at approximately 48 h after the initiation of IVIG therapy. The high serum CRP group was treated by intensified, co-administration of prednisolone and a second IVIG course (2 g/kg). Targeted use of prednisolone with IVIG for refractory KD with high serum CRP levels (≥ 10 mg/dL after first IVIG) as a second-line therapy was considerably more effective and appeared to lower the incidence of CAA than the second round of IVIG alone [18]. This indicated that secondary prednisolone is administered to patients with a CRP level of 7.0 mg/dL instead of 10.0 mg/dL after the first-line therapy because the sensitivity of IVIG non-responders to first-line therapy decreased with CRP level (based on previous data). The sensitivity and specificity of the predicted second IVIG non-responders with CRP level ≥ 8 mg/dL were 76.0% and 63.6% [17], with CRP level 8.89 mg/dL were 58.3% and 73.0%, and with CRP level 10 mg/dL were 51.1% and 79.6%, respectively [18].
Protocol B: This protocol involved the use of secondary infliximab instead of secondary prednisolone for first non-responders. The secondary infliximab was modified from our previous protocol based on a high level of CRP (7.0 mg/dL).
Protocol C: This was a primary corticosteroid protocol for IVIG non-responders predicted using the Kobayashi score [14] (RAISE protocol). Patients were stratified by the Kobayashi score into predicted IVIG non-responders (Kobayashi score ≥ 5) and predicted IVIG responders (Kobayashi score < 5). The scoring and cut-off values for the Kobayashi score were as follows: two points each for serum sodium concentration of ≤ 133 mmol/L, ≤ 4 days of illness at diagnosis, aspartate aminotransferase concentration of ≥ 100 units per L, and neutrophil count of ≥ 80%; and one point each for a platelet count of ≤ 30 × 104 cells per μL, CRP level of ≥ 100 mg/L, and age ≤ 12 months. Patients resistant to the first-line therapy of protocol C were administered second-line infliximab.
The treatment protocols are shown in Fig. 1. If patients in B and C groups did not meet the criteria for infliximab administration (such as patients aged less than 1 year, patients who received Bacillus Calmette–Guérin vaccination within 6 months or live vaccination within 3 months before enrollment, patients with suspected coexistence of an active infection, patients with a history of infliximab-complicated abnormal laboratory results, patients with immunodeficiency or serious complications requiring hospitalization, and if the attending physician did not allow infliximab administration), they were treated similar to those in A group and with IVIG plus prednisolone, respectively. If the patients did not respond to secondary therapy, the physician selected a rescue therapy, such as additional IVIG therapy (2 g/kg), steroid therapy, infliximab, plasma exchange [15], and cyclosporin [26]. The selection of rescue therapy after secondary therapy for IVIG non-responders was dependent on the institutes involved in the working group. The regimen for prednisolone comprised 2 mg/kg/day intravenous (IV) therapy in three divided doses until fever resolved and serum CRP level decreased to < 1.0 mg/dL. Subsequently, prednisolone was tapered gradually to 1.5, 1.0, and 0.5 mg/kg/day every 3–5 days to maintain a continuous decrease in serum CRP. Prednisolone was discontinued if the serum CRP level remained within the normal range (< 0.5 mg/dL) at the 0.5 mg/kg/day prednisolone dose. Prednisolone was orally administered when the patient’s conditions improved. All patients were treated with 30 mg/kg aspirin during the acute phase and 5 mg/kg aspirin after the control of inflammation.
Study flowchart and therapy protocol. We established three protocols: primary (Protocol C) and secondary rescue prednisolone (Protocol A) protocols and no prednisolone protocol and second-line infliximab protocol (Protocol B). CRP C-reactive protein, IVIG intravenous immunoglobulin, PSL prednisolone, IFX infliximab
2.4 Measurements and Definitions
2.4.1 Fever Investigation
Fever was defined as an axillary temperature of ≥ 37.5 °C, and defervescence was defined as a decrease in body temperature to < 37.5 °C. Fever was monitored 2–3 times per day until discharge in each institution.
2.4.2 Laboratory Tests
Laboratory tests were performed before and after initial IVIG including measurement of blood characteristics. If laboratory tests were performed more than once before the first therapy, the worst value was used for analysis.
2.4.3 CAA Definitions
CAA was defined in accordance with the report of the Japanese Ministry of Health and Welfare. It describes the Guide for KD criteria as an actual internal diameter of 3 mm or more in a child younger than 5 years or 4 mm or more in a child 5 years or older, with the internal diameter of the segment at least 1.5-fold greater than that of an adjacent segment or a clearly irregular luminal contour [27], which was examined approximately 1 month after onset by two-dimensional echocardiograms. The diameter of the proximal right coronary artery (CA), proximal left main CA, proximal left anterior descending CA, and proximal left circumflex CA was determined by two-dimensional echocardiograms. The results were converted to z score using a model derived with the Lambda-mu-sigma method [28]. From the CA data, the maximum diameter was defined as the maximum CA diameter of each branch.
2.4.4 Outcome Analysis
Outcomes were defined as follows. The primary outcome was the number of non-responders after therapy within 48 h of administration of first- and second-line therapies among the three protocol groups. Next, we compared the number of non-responders between the groups with and without prednisolone administration.
Non-responders were defined as those who remained febrile 48 h after the administration of first- or second-line therapy or had recrudescent fever after first- or second-line therapy. These patients were divided into two types. The first non-responders were defined as those who remained febrile 48 h after the administration of first-line therapy or had recrudescent fever after first-line therapy. The second non-responders were defined as those who remained febrile 48 h after the administration of second-line therapy or had recrudescent fever after second-line therapy.
As a secondary outcome, we compared the number of second non-responders to each second-line therapy and incidence of CAA determined by two-dimensional echocardiography at 1 month among the three protocol groups and between the groups with and without prednisolone administration.
2.5 Statistical Analysis
The results are expressed as median and interquartile range. To determine differences in numerical data, two-side comparisons were performed using Mann–Whitney U test. Comparisons of three factors were performed using Kruskal–Wallis test. Fisher’s exact test was used to evaluate differences in incidence. Post hoc analyses were performed using the Bonferroni test. For all statistical analyses, the results with p < 0.05 were considered significant. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Tochigi, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing) [29].
3 Results
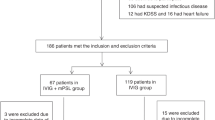
The study flowchart is shown in Fig. 2. A total of 503 patients entered this study. Thirty-four patients were excluded because they were not administered IVIG. Thus, 469 patients were enrolled, with 245 in A group, 136 in B group, and 88 in C group. Eleven patients in A and B groups and 4 patients in C group were excluded because other therapies were performed as defined by each protocol.
Study flowchart. Five hundred and three patients were enrolled in this study. Thirty-four patients were excluded because they did not receive intravenous immunoglobulin (IVIG) treatment. Thus, 469 patients were enrolled in each protocol, with 245 in A group, 136 in B group, and 88 in C group. Eleven patients in A and B groups, and 4 patients in C group were excluded because other therapies were not administered as defined by each protocol
3.1 Primary Outcome
The baseline characteristics of each protocol group are shown in Table 1. Although there were significant differences in the number of diagnostic criteria in C group compared with A and B groups, most cases had more than four diagnostic criteria as specified by each protocol. Based on blood examination before the initial therapy, more severe cases were treated by protocol C than by protocols A and B according to platelet count, aspartate aminotransferase level, total bilirubin, sodium content, and Kobayashi score. These results were similar after the initial therapy. The high-risk (HR) rate with Kobayashi scoring did not differ significantly among each protocol group.
A comparison of primary and secondary prednisolone with IVIG, as the first-line therapy, and that between groups with and without secondary prednisolone administration, in the second-line therapy, are shown in Table 2. A comparison of non-responders between the groups with and without prednisolone administration is shown in Table 3. There was a significant difference in the number of diagnostic criteria between the groups with and without prednisolone administration. From each blood examination before the initial therapy, the protocol with prednisolone administration appeared to be used to treat more severe cases according to CRP, aspartate aminotransferase, alanine aminotransferase, and sodium levels. The HR rate of the Kobayashi score was not significantly different between the groups. Table 4 presents the crude and adjusted non-responder estimates for the groups with and without prednisolone administration. In univariate analysis, the first and second non-responders in the group without prednisolone administration was significantly higher than that in the group with prednisolone administration, with a crude odds ratio of 3.83 (95% CI 1.47–10.44; p = 0.003). Multivariate logistic regression analysis was performed to compare the first and second non-responders between groups with and without prednisolone administration after adjusting for possible confounders, Kobayashi score HR, Shizuoka score, and HR. The combined first and second non-responders in the group without prednisolone administration was significantly higher than that in the group with prednisolone administration, with an adjusted odds ratio of 6.47 (95% CI 2.03–20.60; p = 0.002).
3.2 Secondary Outcomes
Figure 3 shows the first non-response of patients to second-line rescue therapy. In the second-line therapy, all patients with a high CRP level were successfully treated with IVIG plus prednisolone following protocol A. However, in B group, there were 10 second non-responders with a CRP level > 7 mg/dL after initial IVIG. Seven of nine patients treated with infliximab as second-line therapy showed resistance. In C group, two of six patients treated with infliximab were resistant to the therapy. Fifteen of the first non-responders were treated with infliximab as second-line therapy, among whom nine patients showed resistance. Among the second non-responders, 14 (12 in A group, 1 in B group, 1 in C group) were treated using IVIG with prednisolone as second-line therapy, with 1 patient showing resistance. There was a significant difference in second responders between second-line infliximab and second-line IVIG with prednisolone (p < 0.005).
Second step outcome. Efficacy rate of second-line rescue therapy. In A group, there were 6/50 non-responders (12.0%), in B group, there were 13/22 non-responders (59.1%), and in C group, there were 2/7 non-responders (28.6%). CRP C-reactive protein, IVIG intravenous immunoglobulin, PSL prednisolone, IFX infliximab
Twenty-one patients were administered an additional rescue therapy after second-line therapy; 8 patients were treated with IVIG (all protocol B), 2 with IVIG plus steroids (all protocol B), 8 with steroids (6 protocol A and 2 protocol B), 2 with plasma exchange (all protocol C), and 1 with infliximab (protocol B). Six patients were administered additional therapy after third-line therapy: 1 with cyclosporin A (protocol B), 3 with IVIG (2 protocol A and 1 protocol B), and 2 with steroids (all protocol B).
3.3 Patients Complicated with CAA
CAA was observed in 21 patients (4.6%) according to the Japanese criteria; there was no significant difference among the protocol groups. Similarly, 53 patients (11.7%) and 8 patients (1.8%) had z scores of over 2.5 and 5.0 in the CAs, respectively, with no significant difference among the protocol groups. There were also no significant differences among A, B, and C groups or between groups with and without prednisolone administration. Additionally, there was no significant difference between protocols with and without prednisolone administration in patients who were less than 1 year old at the onset, based on the Kobayashi score.
3.4 Total Prednisolone Administration in Each Protocol
Prednisolone administration was based on the Kobayashi and Shizuoka scores in C and A groups. We compared total prednisolone administration between each group. The total administration rate of prednisolone in first- and second-line therapies in C group was 38.1%, which was significantly higher than that in A and B groups (Table 2).
4 Discussion
4.1 Targeted Administration of Prednisolone for IVIG Non-responders
In the present prospective study, we estimated the effectiveness of protocols A, B, and C (RAISE protocol) to determine the optimal use of prednisolone to achieve the maximum effects and safety. As reported previously [14, 25], the C group had a lower number of first non-responders than the IVIG alone group (protocols A and B). However, intensifying second-line therapy using IVIG with prednisolone in patients with a persistently high CRP level in A group showed a similar response as C group until second-line therapy. Moreover, we compared the effectiveness of protocols with and without prednisolone administration. Our data showed that the first and second non-responders in the group with prednisolone administration (protocols A and C) were significantly lower than those in the group without prednisolone administration (protocol B). The multivariate logistic regression analysis showed that the first and second non-responders in groups with prednisolone administration as first- or second-line therapy were significantly higher than those in the group without prednisolone administration, with an adjusted odds ratio of 6.47. Previously, there were insufficient data to determine whether first-versus second-line administration of prednisolone had favorable effects in IVIG non-responders [30]. Our data showed that both first- and second-line administration of prednisolone had similar favorable effects in IVIG non-responders.
The rate of prednisolone administration in A group was 5.0%, which was considerably lower than that (38.1%) in C group. A previous study reported that adrenal suppression occurred in a large proportion of children with KD treated with IVIG plus prednisolone, despite the short duration and relatively small amounts of prednisolone administered [31]. Even inhaled corticosteroids, which were previously considered to have no systemic adverse effects, have recently been reported to affect linear growth in children, particularly in the first year of treatment [32]. A meta-analysis reported that steroids plus non-steroidal anti-inflammatory drugs synergistically increased gastrointestinal complications [33]. Based on these studies, it can be suggested that the systemic administration of prednisolone should be limited as much as possible. Our protocol A with reduced levels of prednisolone achieved the maximum effect in IVIG non-responders; however, we could not evaluate the hospitalization duration required to reduce prednisolone administration and/or medical costs. It is important to balance the adverse effects and benefits of therapy strategies for KD involving prednisolone in the acute phase.
4.2 Second-line Infliximab for IVIG Non-responders
We administered infliximab in protocols B and C. In B group, seven patients treated with second-line infliximab showed resistance. In C group, two patients showed resistance to this therapy. Fifteen IVIG non-responders were treated with second-line infliximab and nine patients showed resistance. The efficacy rate of this treatment was 40%, which was lower than that reported previously [19, 34, 35]. Infliximab, a monoclonal antibody, is a selective anti-inflammatory molecule that functions by blocking TNF. The pro-inflammatory cytokine TNF-α has been shown to be elevated in patients with KD, with the highest levels observed in patients with CAA [36]. Infliximab therapy for KD has been reported to decrease serum soluble TNF receptor 1 and IL-6 levels [37] and regulate signaling pathways related to KD inflammation and IVIG resistance factors [38]. In a previous study, primary adjunctive treatment with infliximab decreased the duration of fever but was not associated with a decreased risk of non-response to IVIG [39]. Our data showed that the efficacy rate of second-line infliximab therapy for IVIG non-responders was limited and lower than that of second-line IVIG with prednisolone therapy. Dionne et al reported that the rate of initial IVIG non-responders was lower in the group administered additional corticosteroids than in the group administered additional infliximab or IVIG alone [40]. Thus, second-line infliximab therapy for IVIG non-responders may have lower defervescence effects than prednisolone.
4.3 Complicated with CAAs
Our data showed no significant difference in the percentage of CAA among A, B, and C groups, and CAA at 1 month and z score of more than 5 indicated that the risk of persistent CAA was 3 in A group, 2 in B group, and 3 in C group. We could not compare the CAA rate among the different protocol groups, because the observational period was too short to estimate cases complicated by CAA. Nevertheless, recently, the administration of corticosteroids or infliximab for KD showed a favorable long-term effect on complicated CAA. Dionne et al reported that adjunctive primary therapy with prednisolone or infliximab may improve coronary outcomes in patients with a coronary z score of more than 2.5 [50]. Another study reported that CAA regression rates among IVIG non-responders were higher in patients administered infliximab than in those not administered infliximab therapy. Infliximab therapy was associated with significant early regression of CAA in patients presenting with refractory KD. Infliximab not only suppresses inflammatory responses that lead to the development of CAAs, but additionally assists with/achieves vascular remodeling following early CAA regression [41]. Our protocol C involved the administration of both prednisolone and infliximab. Further long-term observational studies are needed to confirm our results.
4.4 Limitations
This study was a multicenter prospective investigation with a relatively small number of patients. Echocardiographic examination was performed by different observers and with different equipment in each hospital. Thus, there may have been information bias in the echocardiography results because the interpretation by pediatric cardiologists at each institution could not be completely blinded. However, the echocardiogram techniques and assessment might have varied among the participating hospitals, although all examiners followed the standardized method [42].
In the present study, the indication for prednisolone administration was based on the Kobayashi and Shizuoka scores in A and C groups. Predicting the non-response to IVIG before first-line IVIG and additional corticosteroid therapy for KD therapy is used in Japan but has not been globally accepted [43,44,45,46,47,48,49,50,51]. Retrospectively, in the present study, based on the HR Kobayashi scores, 29 (40.8%) IVIG non-responders were observed in A group, 16 (44.4%) in B group, and 2 (6.3%) in C group. IVIG non-responders in C group were lower than those in A and B groups (p < 0.001). There were no significant differences in IVIG non-responders among the three groups based on the low-risk Kobayashi scores (p = 0.296). For A and B groups, the sensitivity and specificity of the Kobayashi score were 62.5% and 79.2%, respectively. Our data showed that the Kobayashi score is still useful for preventing an initial non-response to IVIG. However, a validation study is needed to establish the predicting IVIG non-responders for KD therapy.
5 Conclusion
In conclusion, the number of non-responders in the group with prednisolone administration was lower than that in the group without prednisolone administration. Secondary rescue infliximab therapy for IVIG non-responders resulted in a lower defervescence effect than secondary rescue IVIG with prednisolone. Further prospective randomized studies are needed to identify the factors useful for preventing IVIG non-responders at an early stage and determine the optimal rescue therapy for preventing CAA.
References
McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, Kobayashi T, Wu MH, Saji TT, Pahl E. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–99.
Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1982;2:1359.
Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, Glode MP, Mason WH, Reddy V, Sanders SP. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–7.
Durongpisitkul K, Gururaj VJ, Park JM, Martin CF. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics. 1995;96:1057–61.
Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr. 1997;131:888.
Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000;105:E78.
Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr Infect Dis J. 1998;17:1144–8.
Han RK, Silverman ED, Newman A, McCrindle BW. Management and outcome of persistent or recurrent fever after initial intravenous gamma globulin therapy in acute Kawasaki disease. Arch Pediatr Adolesc Med. 2000;154:694–9.
Durongpisitkul K, Soongswang J, Laohaprasitiporn D, Nana A, Prachuabmoh C, Kangkagate C. Immunoglobulin failure and retreatment in Kawasaki disease. Pediatr Cardiol. 2003;24:145–8.
Kato H, Koike S, Yokoyama T. Kawasaki disease: effect of treatment on coronary artery involvement. Pediatrics. 1979;63:175e9.
Shinohara M, Sone K, Tomomasa T, Morikawa A. Corticosteroids in the treatment of the acute phase of Kawasaki disease. J Pediatr. 1999;135:411–3.
Jibiki T, Terai M, Kurosaki T, Nakajima H, Suzuki K, Inomata H, Terashima I, Honda T, Yasukawa K, Hamada H, Kohno Y. Efficacy of intravenous immune globulin therapy combined with dexamethasone for the initial treatment of acute Kawasaki disease. Eur J Pediatr. 2004;163:229–33.
Sundel RP, Baker AL, Fulton DR, Newburger JW. Corticosteroids in the initial treatment of Kawasaki disease: report of a randomized trial. J Pediatr. 2003;142:611–6.
Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, RAISE study group investigators, et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. 2012;379:1613–20.
Guidelines for Medical Treatment of Acute Kawasaki disease. Report of the Research Committee of the Japanese Society of Pediatric Cardiology and Cardiac Surgery (2012 revised version). Pediatr Int. 2014;56:135–58.
Chen S, Dong Y, Kiuchi MG, Wang J, Li R, Ling Z, Zhou T, Wang Z, Martinek M, Pürerfellner H, Liu S, Krucoff MW. Coronary artery complication in Kawasaki Disease and the importance of early intervention: a systematic review and meta-analysis. JAMA Pediatr. 2016;170:1156–63.
Iwashima S, Kimura M, Ishikawa T, Ohzeki T. Importance of C-reactive protein level in predicting non-response to additional intravenous immunoglobulin treatment in children with Kawasaki disease: a retrospective study. Clin Drug Investig. 2011;31:191–9.
Kimura M, Harazaki M, Fukuoka T, Asakura I, Sakai H, Kamimaki T, Ohkawara I, Akiyama N, Tsurui S, Iwashima S, Shimomura M, Morishita H, Meguro T, Seto S. Targeted use of prednisolone with the second IVIG dose for refractory Kawasaki disease. Pediatr Int. 2017;59:397–403.
Burns JC, Mason WH, Hauger SB, Janai H, Bastian JF, Wohrley JD, Balfour I, Shen CA, Michel ED, Shulman ST, Melish ME. Infliximab treatment for refractory Kawasaki syndrome. J Pediatr. 2005;146:662–7.
Son MB, Gauvreau K, Ma L, Baker AL, Sundel RP, Fulton DR, Newburger JW. Treatment of Kawasaki disease: analysis of 27 US pediatric hospitals from 2001 to 2006. Pediatrics. 2009;124:1–8.
Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, Ishii M, Harada K. Revision of diagnostic guidelines for Kawasaki disease. Pediatr Int. 2005;47:232–4.
Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, Matsuishi T. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006;149:237–40.
Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, Kobayashi T, Morikawa A. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–12.
Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, Kogaki S, Hara J. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. 2007;166:131–7.
Miyata K, Kaneko T, Morikawa Y, Sakakibara H, Matsushima T, Misawa M, Takahashi T, Nakazawa M, Tamame T, Tsuchihashi T, Yamashita Y, Obonai T, Chiga M, Hori N, Komiyama O, Yamagishi H, Miura M, Post RAISE Group. Efficacy and safety of intravenous immunoglobulin plus prednisolone therapy in patients with Kawasaki disease (Post RAISE): a multicentre, prospective cohort study. Lancet Child Adolesc Health. 2018;2:855–62.
Suzuki H, Terai M, Hamada H, Honda T, Suenaga T, Takeuchi T, Yoshikawa N, Shibuta S, Miyawaki M, Oishi K, Yamaga H, Aoyagi N, Iwahashi S, Miyashita R, Onouchi Y, Sasago K, Suzuki Y, Hata A. Cyclosporin A treatment for Kawasaki disease refractory to initial and additional intravenous immunoglobulin. Pediatr Infect Dis J. 2011;30:871–6.
Research Committee on Kawasaki Disease. Report of subcommittee on standardization of diagnostic criteria and reporting of coronary artery lesions in Kawasaki disease. Tokyo, Japan: Ministry of Health and Welfare; 1984. ((in Japanese)).
Kobayashi T, Fuse S, Sakamoto N, Mikami M, Ogawa S, Hamaoka K, Arakaki Y, Nakamura T, Nagasawa H, Kato T, Jibiki T, Iwashima S, Yamakawa M, Ohkubo T, Shimoyama S, Aso K, Sato S, Saji T, Z Score Project Investigators. A new Z-Score curve of the coronary arterial internal diameter using the lambda-mu-sigma method in a pediatric population. J Am Soc Echocardiogr. 2016;29:794–801.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Wardle AJ, Connolly GM, Seager MJ, Tulloh RM. Corticosteroids for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev. 2017;1:CD011188.
Goto M, Miyagawa N, Kikunaga K, Miura M, Hasegawa Y. High incidence of adrenal suppression in children with Kawasaki disease treated with intravenous immunoglobulin plus prednisolone. Endocr J. 2015;62:145–51.
Zhang L, Priestch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: effects on growth. Cochrane Database Syst Rev. 2014;7:CD009471.
Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med. 1991;115:787–96.
Son MB, Gauvreau K, Burns JC, Corinaldesi E, Tremoulet AH, Watson VE, Baker A, Fulton DR, Sundel RP, Newburger JW. Infliximab for intravenous immunoglobulin resistance in Kawasaki disease: a retrospective study. J Pediatr. 2011;158:644–9.
Mori M, Hara T, Kikuchi M, Shimizu H, Miyamoto T, Iwashima S, Oonishi T, Hashimoto K, Kobayashi N, Waki K, Suzuki Y, Otsubo Y, Yamada H, Ishikawa C, Kato T, Fuse S. Infliximab versus intravenous immunoglobulin for refractory Kawasaki disease: a phase 3, randomized, open-label, active-controlled, parallel-group, multicenter trial. Sci Rep. 2018;8:1994.
Matsubara T, Furukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol. 1990;56:29–36.
Hirono K, Kemmotsu Y, Wittkowski H, Foell D, Saito K, Ibuki K, Watanabe K, Watanabe S, Uese K, Kanegane H, Origasa H, Ichida F, Roth J, Miyawaki T, Saji T. Infliximab reduces the cytokine-mediated inflammation but does not suppress cellular infiltration of the vessel wall in refractory Kawasaki disease. Pediatr Res. 2009;65:696–701.
Ogihara Y, Ogata S, Nomoto K, Ebato T, Sato K, Kokubo K, Kobayashi H, Ishii M. Transcriptional regulation by infliximab therapy in Kawasaki disease patients with immunoglobulin resistance. Pediatr Res. 2014;76:287–93.
Tremoulet AH, Jain S, Jaggi P, Jimenez-Fernandez S, Pancheri JM, Sun X, Kanegaye JT, Kovalchin JP, Printz BF, Ramilo O, Burns JC. Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet. 2014;383:1731–8.
Dionne A, Burns JC, Dahdah N, Tremoulet AH, Gauvreau K, de Ferranti SD, Baker AL, Son MB, Gould P, Fournier A, Newburger JW, Friedman KG. Treatment intensification in patients with Kawasaki disease and coronary aneurysm at diagnosis. Pediatrics. 2019;143:e20183341.
Nagatomo Y, Muneuchi J, Nakashima Y, Nanishi E, Shirozu H, Watanabe M, Uike K, Nagata H, Hirata Y, Yamamura K, Takahashi Y, Okada S, Suzuki Y, Hasegawa S, Ohga S. Effective infliximab therapy for the early regression of coronary artery aneurysm in Kawasaki disease. Int J Cardiol. 2018;271:317–21.
Fuse S, Kobayashi T, Arakaki Y, Ogawa S, Katoh H, Sakamoto N, Hamaoka K, Saji T. Standard method for ultrasound imaging of coronary artery in children. Pediatr Int. 2010;52:876–82.
Newburger JW, Sleeper LA, McCrindle BW, Minich LL, Gersony W, Vetter VL, Atz AM, Li JS, Takahashi M, Baker AL, Colan SD, Mitchell PD, Klein GL, Sundel RP. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med. 2007;356:663–75.
Sleeper LA, Minich LL, McCrindle BM, Li JS, Mason W, Colan SD, Atz AM, Printz BF, Baker A, Vetter VL, Newburger JW, Pediatric Heart Network Investigators. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr. 2011;158:831–5.
Jakob A, von Kries R, Horstmann J, Hufnagel M, Stiller B, Berner R, Schachinger E, Meyer K, Obermeier V. Failure to predict high-risk Kawasaki disease patients in a population-based study cohort in Germany. Pediatr Infect Dis J. 2018;37:850–5.
Fabi M, Andreozzi L, Corinaldesi E, Bodnar T, Lami F, Cicero C, Tchana B, Landini C, Sprocati M, Bigucci B, Balsamo C, Sogno Valin P, Di Fazzio G, Iughetti L, Valletta E, Marchetti F, Donti A, Lanari M. Inability of Asian risk scoring systems to predict intravenous immunoglobulin resistance and coronary lesions in Kawasaki disease in an Italian cohort. Eur J Pediatr. 2019;178:315–22.
Arane K, Mendelsohn K, Mimouni M, Mimouni F, Koren Y, Brik Simon D, Bahat H, Hanna Helou M, Mendelson A, Hezkelo N, Glatstein M, Berkun Y, Eisenstein E, Butbul YA, Brik R, Hashkes PJ, Uziel Y, Harel L, Amarilyo G. Japanese scoring systems to predict resistance to intravenous immunoglobulin in Kawasaki disease were unreliable for Caucasian Israeli children. Acta Paediatr. 2018;107:2179–84.
Kim BY, Kim D, Kim YH, Ryoo E, Sun YH, Jeon IS, Jung MJ, Cho HK, Tchah H, Choi DY, Kim NY. Non-responders to intravenous immunoglobulin and coronary artery dilatation in Kawasaki disease: predictive parameters in Korean children. Korean Circ J. 2016;46:542–9.
Son MBF, Gauvreau K, Kim S, Tang A, Dedeoglu F, Fulton DR, Lo MS, Baker AL, Sundel RP, Newburger JW. Predicting coronary artery aneurysms in Kawasaki disease at a North American Center: an assessment of baseline z scores. J Am Heart Assoc. 2017;6:e005378.
Davies S, Sutton N, Blackstock S, Gormley S, Hoggart CJ, Levin M, Herberg JA. Predicting IVIG resistance in UK Kawasaki disease. Arch Dis Child. 2015;100:366–8.
Berdej-Szczot E, Małecka-Tendera E, Gawlik T, Firek-Pędras M, Szydłowski L, Gawlik A. Risk factors of immunoglobulin resistance and coronary complications in children with Kawasaki disease. Kardiol Pol. 2017;75:261–6.
Acknowledgements
We thank the following current and ex-members of the Shizuoka Kawasaki Disease Study Group for their cooperation in carrying out the study. We also thank Takaaki Megro, MD, Shizuoka Children`s Hospital; Tutomu Kamimaki, MD, Shizuoka City Shimizu Hospital; Tomoko Kawasaki, MD, Seirei Numazu Hospital; Toshihiro Tanaka, MD, Shizuoka Kosei Hospital; Ichiro Ohkawara, MD, Shizuoka Red Cross Hospital; Nobutaka Shimizu, MD, Yaizu City Hospital; Kou Asakura, MD, Fujieda Muncipal General Hospital, and Mituaki Kimura, PhD, former chairman of the Shizuoka Kawasaki Disease Study Group for their assistance in the treatment of the patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions
All authors made substantial contributions to the conception of the study. All authors read and approved the final manuscript. H.S., S.I., S.S., and M.H. interpreted the data and wrote the paper.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for profit sectors.
Ethics approval
The study was reviewed and approved by the ethics committee of Shizuoka Children’s Hospital (ID: R000014887) and all participating institutions.
Consent to participate
Patients and their guardians provided written informed consent before enrolment or informed consent was obtained as an opt-out and inclusion agreement based on the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. Some institutions posted an explanation of this study on their web homepage. When a subject was not willing to participate, his/her data were excluded from the analysis.
Consent for publication
Not applicable.
Availability of data and material
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Code availability
This study was registered as a clinical trial at UMIN (UMIN000025707).
Rights and permissions
About this article
Cite this article
Sakai, H., Iwashima, S., Sano, S. et al. Targeted Use of Prednisolone with Intravenous Immunoglobulin for Kawasaki Disease. Clin Drug Investig 41, 77–88 (2021). https://doi.org/10.1007/s40261-020-00984-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00984-6