Abstract
Cytomegalovirus (CMV) infection induces powerful and sustained T-cell responses against a few selected immunodominant antigenic epitopes. This immune response was named memory inflation, because it does not contract in the long term, and may even expand over months and years of virus latency. It is by now understood that memory inflation does not occur at the expense of the naïve T-cell pool, but rather as a competitive selection process within the effector pool, where viral antigens with higher avidity of TCR binding and with earlier expression patterns outcompete those that are expressed later and bind TCRs less efficiently. It is also understood that inflationary epitopes require processing by the constitutive proteasome in non-hematopoietic cells, and this likely implies that memory inflation is fuelled by direct low-level antigenic expression in latently infected cells. This review proposes that these conditions make inflationary epitopes the optimal candidates for adoptive immunotherapy of CMV disease in the immunocompromised host. At present, functional target CMV epitopes have been defined only for the most common HLA haplotypes. Mapping the uncharacterized inflationary epitopes in less frequent HLAs may, thus, be a strategy for the identification of optimal immunotherapeutic targets in patients with uncommon haplotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Memory inflation (MI) is among the most distinctive features of cytomegalovirus (CMV) infection. While the term is not formally defined, it is best described as the long-term maintenance or slight increase in the number of antigen-specific CD8 T cells exhibiting an effector, but not exhausted phenotype [1,2,3]. Inflationary cells were first described upon experimental mouse cytomegalovirus (MCMV) infection in the pulmonary infiltrates of latently infected mice [4], but dynamic monitoring of blood cells showed that tetramer-specific cells also accumulate over time in the circulating CD8 T-cell pool [5]. This phenomenon is not restricted to experimental mouse infection. Large virus-specific responses were also observed in human CMV (HCMV) seropositive people [6, 7], and HCMV responses against defined epitopes were shown to strengthen with advancing age in some [8, 9], although not in all studies [10, 11]. Similarly, robust CMV-specific T-cell responses were described in adult and aged rhesus monkeys [12]. Therefore, the persistence of large CMV-specific T-cell populations is a conserved phenomenon in numerous species that coevolved with this virus and, thus, a clinically relevant phenomenon that can be well represented in animal models.
Only some MCMV epitopes show inflationary traits [4, 13, 14]. Other ones, dominating the immune response at early times post-infection, contract over time, akin to the conventional immune response kinetics upon infection with non-persistent pathogens [2, 14]. This dichotomy of immune responses to the same pathogen, and occasionally to antigenic epitopes within the same viral gene [13], remain a subject of intense scientific interest. This review will focus on describing the specific properties of inflationary cells, the likely molecular mechanisms underlying this phenomenon, as well as the relevance of inflationary antigens as targets for antiviral immunotherapy.
Phenotype and functionality of T-cell subsets
Upon priming, CD8 T cells typically respond by vigorous antigen-driven proliferation of effector cells [15, 16], which gives rise over time to the two main subsets of primed cells, the effector or effector-memory (EM) and the central memory (CM) cells [16, 17]. CM cells are characterized by the expression of surface markers facilitating their homing to secondary lymphatic organs, such as CD62L or the chemokine receptor CCR7 [17], by the expression of costimulating receptors, such as CD27 or CD28 [18], but also by their ability to expand indefinitely in adoptive transfer settings [19] and mature into effector cells upon restimulation [17]. Effector cells, on the other hand, are less frequent in secondary lymphatic organs [20] and lose CD27, CD28, CCR7, and CD62L from their surface [17, 18], but respond more promptly to antigenic restimulation by secreting interferon gamma (IFNγ) [17] and have stronger cytolytic activity [21]. It was subsequently discovered that the CD62L− effector cells can be further subdivided into short-lived effector cells (SLEC) or the longer lived EM cells, based on their expression of the IL7 receptor beta chain (CD127) or the inhibitory receptor KLRG1. CD127 is lost and KLRG1 is expressed on the SLEC, whereas EM cells have the opposing phenotype [22]. It has been proposed that the KLRG1-CD127+ EM cells may act as memory precursor effector cells (MPEC) [22], although competing evidence has argued that CM cannot differentiate from effector cells, but rather that CM precede EM and SLECs during T-cell differentiation [23]. It remains unclear if one of these competing models is more accurate, or if they both reflect events that naturally occur in different T-cell priming conditions. In particular, it remains unclear whether KLRG1−CD127+ EM may convert to CM cells in conditions of intermittent exposure to antigen during virus latency, and no data are currently available to define this question. Therefore, this review will avoid the term MPECs, and refer to them as EM cell. Nevertheless, the consensus of the scientific community is that SLEC cells are the most differentiated subsets of T cells in both humans and mice, and that they have robust effector function but a restricted proliferative potential.
Phenotype and functionality of inflationary T cells
The vast majority of inflationary CMV specific CD8 T cells are not only CD62L deficient [4], but also exhibit a bona fide SLEC phenotype [2, 14, 24, 25]. The expansion of inflationary cells depends on the inoculum dose [26] and on the route of infection [27], and stronger responses tend to have a higher proportion of cells with SLEC phenotypes [26]. MI maintenance requires continuous production of SLECs in virus presence [24] and this cell proliferation is antigen-dependent [24], yet the inflationary cells require interleukin 15 (IL-15) for survival [28]. The cycling of inflationary cells involves and ongoing recruitment of naïve and CM [24], but also of EM cells [28, 29]. The cycling inflationary cells are predominantly Bcl-2 low [14, 30], additionally arguing for antigen-dependent T-cell proliferation maintaining the MI pool. While the cellular site of ongoing antigen production remains unknown in the human host, in murine experimental models, it was shown that these cells are in contact with the bloodstream [29], but that they are not of hematopoietic origin [30, 31]. It is presumed that endothelial cells (EC) may present a reservoir of viral antigen during latency [32], since latent virus could be demonstrated in microvasculature endothelial cells in the liver [33], but it remains unclear if EC are a relevant site of latent antigen expression, and if multiple cellular subsets are involved. While inflationary cells show obvious signs of in vivo proliferation in their native environment [14], they proliferate poorly upon in vitro antigenic restimulation [34, 35] or upon in vivo adoptive transfer [24], which has prompted speculations that they might be dysfunctional and senescent cells [36]. However, inflationary cells remain functional for life in terms of cytokine and granzyme responses [4, 14, 24, 37], even in demonstrably immunosenescent hosts [12]. Furthermore, introduction of heterologous antigenic epitopes into recombinant MCMV vectors has demonstrated that inflationary CD8 responses provide a highly efficient control of virus infections [38,39,40,41], but also of tumors [42, 43] in challenge settings. Thus, it is currently recognized that inflationary cells are highly functional [44, 45], sparking a wide interest in CMV-based vaccine vectors [46,47,48].
Antigen availability and memory inflation
It is generally assumed that MI is fuelled by CMV antigens that are intermittently expressed at low levels during virus latency [1, 32], although the evidence is largely circumstantial and a smoking gun proof remains elusive. Low-level MCMV transcription of immediate early (IE) genes persists in the lungs of latently infected mice [49, 50] and several natural inflationary epitopes are derived from IE genes [4, 13]. Furthermore, the insertion of exogenous epitopes into MCMV vectors induces stronger inflationary responses when the epitope is expressed from an IE gene [39, 40]. In BALB/c mice, IE1 encodes the immunodominant inflationary epitope YPHFMPTNL [4] and latent transcription is restricted to IE1 and IE2 genes [50], which are independently expressed during latency [51]. Genes expressed later in the virus cycle, such as the early gene M55 or the IE3 gene, a product of alternative splicing with the IE1 gene, are not detectable during latency [49, 52]. However, latent IE3 transcription occurs upon targeted mutagenesis of a single amino acid anchoring the YPHFMPTNL to the MHC-I molecule and loss of IE1-specific inflationary CD8 T-cell responses [53]. Similarly, in C57BL/6 mice, which do not express natural IE1- or IE2-derived epitopes, IE3 encodes an immunodominant inflationary epitope [13], which argues for its transcriptional activity in this mouse strain. Notably, viral IE transcription can be reversibly silenced by interferons [54, 55], including IFNγ [56], which is also the cytokine that is most abundantly secreted upon peptide restimulation of inflationary CD8 T cells [12, 45]. Therefore, it was proposed that inflationary CD8 T-cell and latent CMV transcription maintain a state of dynamic balance between the virus and the host [3, 32]. This balance assumes that the stochastic transcription of viral antigens in latency induces T-cell responses that represses further viral transcription and keeps a lid on virus reactivation from latency [32], thus providing a respite to T cells, which prevents their exhaustion and allows long-term virus control [3].
The full latent transcriptome of MCMV remains unknown, but two recent studies on HCMV transcriptome have provided insights into the program of latent HCMV gene expression [57, 58]. These results were somewhat discordant, probably due to differences in tissues being examined and methods applied. On one hand, the transcriptome of bulk CD34+ populations of naturally or experimentally infected latent cells indicated a focused latent transcriptional program that is distinguishable from lytic viral gene expression [58]. On the other, single-cell transcriptome of cells obtained from a variety of tissues argued that, in cells expressing viral genes, the transcriptome resembles either the late stage of the virus cycle in some cells or the immediate early one in other ones [57]. It is notable that CD8 epitopes to the human CMV may be encoded by numerous viral genes [6], but these do not correspond to the focused latent transcriptome in bulk CD34+ cells. Thus, the aforementioned restriction of inflationary antigen expression to non-hematopoietic cells in the MCMV system [30, 31] is consistent with the observations in HCMV latency and immune responses, where the recognized antigens seem not to be derived from the viral genes expressed in hematopoietic cells. The discrepancy between the prevalent HCMV epitopes and the latent transcriptome in hematopoietic cells may point either to additional sites of HCMV latency in non-hematopoietic cells, or to a model where inflationary cells are induced only when the virus moves out of latency with a regulated gene-expression program and initiates a bonafide lytic cycle. Very little evidence is available on HCMV latency outside of the hematopoietic system [57, 59], likely due to ethical and practical difficulties in the isolation of viable cells from solid human organs. Therefore, the current evidence on HCMV latency may have been slanted towards cells that are easily available during routine diagnostic procedures, such as blood cells. More studies are required to define if additional sites of latency play a role in HCMV maintenance and memory inflation.
The conditions of primary infection determine the size of the latent MCMV reservoir and its ability to reactivate from latency [60]. Conditions that result in a more vigorous primary virus replication in an organ will establish a larger latent reservoir in the same organ [61, 62]. Therefore, a larger dose of MCMV results in more latent genomes [26]. Similarly, the inoculum size defines the size and the phenotype of the responding inflationary cells [26], where an increase in latent genomes translates into more memory inflation and a phenotype that is slanted toward terminal T-cell differentiation [26]. While this evidence is in its essence a correlative one, the simplest explanation for these correlations is that the larger number of viral genomes in latency yields an increase in latent transcripts and thus in more antigen expression and stronger CD8 activation.
MHC-restricted antigenic epitopes do not only need to be expressed, but also processed and presented on the cell surface. While CMVs are notorious for their ability to interfere with antigenic presentation [63, 64], the viral repressors of antigenic presentation (VRAP) are early genes which are expressed during primary lytic infection, but not during latency. Hence, their expression enhances the primary virus growth and spread and thus, paradoxically, increases the overall latent reservoir [61] and thus decreases the overall inflationary response [65]. However, in line with the idea that memory inflation depends on antigen expression during latency, the hierarchy of responding genes in memory inflation appeared not to be significantly affected by VRAP presence or absence [66]. A much more striking inflationary phenotype was observed in mice lacking the LMP7 subunit of the immunoproteasome. While the CD8 responses to non-inflationary epitopes expressed within MCMV genes M45 and M57 were severely impaired in LMP7-deficient mice, inflationary responses were essentially maintained [67]. Shifting the non-inflationary epitope HGIRNASFI from its native position in the M45 gene to the C-terminus of the same gene rendered it accessible to the constitutive proteasome for processing and resulted in inflationary responses [43]. Therefore, inflationary responses do not only depend on peptide expression patterns, but also on their accessibility to the constitutive proteasome, which improved the efficacy of antigen processing. This observation may have implications for the ability of T cells to recognize the virus-infected cells, which will be explored in more detail in the chapter on immune protection.
Memory inflation as competitive selection process
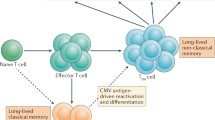
It is intriguing that memory inflation, a widely accepted and intuitive shorthand to describe the persistent expansion of CMV cells, is strictly speaking a misnomer. The cells involved in memory inflation, are not memory cells, but rather, the short-lived effectors. Furthermore, even the SLEC compartment does not progressively inflate in latent MCMV infection. Rather, lifelong monitoring of infected mice has shown that the effector and EM pool expand rapidly upon infection and remain high for life [68]. Inflationary cells, predominantly SLECs and EMs, continued to accumulate slowly in the same mice over long periods, although the pool remained flat (Fig. 1, adapted from [68]).
Memory inflation is not an inflation of the effector-memory compartment. DBA/2 mice were infected with MCMV and blood CD8 T cells were analysed by flow cytometry at indicated time points. EM are defined as CD11a+ CD62L−; IE1+ cells were defined by Tetramer staining (
Consequently, we proposed that memory inflation is not an accumulation of CMV-specific cells, but rather the process of focusing towards the epitopes that are most efficient at stimulating CD8 T cells and that outcompete the less-efficient antigenic targets [68]. This competition was empirically demonstrated by introducing additional epitopes to CMV antigens into IE genes and measuring responses to endogenous epitopes [39, 69]. Responses to the IE3 epitope, or other immunodominant inflationary epitopes in C57BL/6 mice, are robustly reduced if epitopes SIINFEKL or SIEEFARL, known to induce high-avidity T-cell responses, are introduced into the ie2 gene sequence of recombinant viruses [39, 69], but not when the low-avidity KCSRNRQYL peptide is inserted at the same site [40]. Notably, this reduction in inflationary responses to endogenous epitopes does not occur if wild-type MCMV is co-inoculated with the recombinant virus [69], which fits a model where the responses to the immunodominant epitope may only restrict the transcription of subdominant ones if the dominant epitope precedes the expression of the subdominant ones within the same latent cell. Accordingly, the additional immunodominant epitope did not affect the inflationary responses to the endogenous epitopes when introduced into an early viral gene [39]. Furthermore, peptide competition affects exclusively the EM, but not the CM subset of antigen-specific inflationary cells [40]. This implied that the homeostatic proliferation of CM cells is unaffected by competition, providing additional evidence that competitive expansions of peptide-specific CD8 populations depend on antigen-driven proliferation and, Thus, on antigen expression during latency. The ideal epitope that outcompetes other ones is defined both by the avidity of binding of the responding TCRs to the peptide MHC complex and by the context of its gene expression, where earlier expression of high avidity epitopes outcompetes the responses to epitopes expressed later in the virus cycle [40]. In conclusion, inflationary responses are limited by CD8 competition for inflationary epitopes. As long as the inoculum size remains constant, the expression of additional epitopes will not alter the overall inflationary response to MCMV.
Memory inflation as immune protection principle
CD8 T cells recognizing CMV epitopes are increasingly used in immunotherapeutic settings of HCMV disease in the immunocompromised host [70,71,72], in line with early experiments in the mouse model [73]. Since CD8 populations have to be harvested from individuals that are matched on major HLA haplotypes, but minor histocompatibility differences cannot be excluded (except in rare cases of donors who are identical twins), adoptive T-cell transfers of polyclonal populations may result in graft versus host disease and are, thus, avoided. Transfers of populations of T cells recognizing defined CMV antigens have been pursued with variable doses of success [71], but the choice of optimal antigenic targets has remained unclear. Some evidence suggested that natural CD8 responses to IE-derived epitopes may provide better immune control of HCMV replication and disease in kidney transplant settings than responses to the HLA-A02 restricted, pp65-derived epitope [74]. Other studies showed robust immune control of HCMV infection in stem cell recipients by adoptive transfer of CD8 T cells recognizing the same pp65-derived epitope [72]. Therefore, immune protection may depend on the viral epitope, but also on the overall context of disease, immunosuppressive regimen, or site of virus replication. Nevertheless, it remained unclear which epitopes might be ideal targets for adoptive immunotherapy in less common and hitherto uncharacterized HLA haplotypes. Therefore, identifying patterns that predict the protective potential of newly discovered epitopes would fill an important clinical need.
I propose here that inflationary epitopes are likely to offer ideal targets for immune control of CMV infections. This idea is based on several lines of experimental evidence and logical deductions. In the mouse model, adoptive transfer of CD8 T cells recognizing the inflationary IE1 derived epitope were protective [75], but CD8 cells against the non-inflationary Db restricted M45 epitope were not [76]. This correlative evidence was explained by the fact that the M45-derived epitope is poorly recognized by CD8 T cells on the surface of virus-infected cells [77], yet this may be improved by IFN pretreatment [78], or in the absence of VRAP expression [76]. The transfer of this M45 epitope from its natural location to the C-terminus of the M45 gene resulted in a strong increase in peptide presentation on MHC molecules and CD8 T-cell recognition [43]. The processing of the peptide on the M45 C-terminus was proteasome-dependent, but resulted in an immunoproteasome-independent memory inflation [43]. Conversely, in vivo response to the same peptide encoded at its native site in the M45 gene is immunoproteasome-dependent [43, 67]. IFN pretreatment, which is known to activate the immunoproteasome expression, allowed the presentation of the native M45 peptide on cell surface and direct recognition of infected cells by cocultured CD8 T cells [78]. Most importantly, co-culture of virus infected endothelial cells with a CTL line recognizing the M45-derived epitope resulted in efficient control of MCMV replication only in the case of the recombinant virus expressing the epitope independently of the immunoproteasome (Fig. 2). Therefore, the availability of a peptide to the constitutive proteasome processing enables its presentation on the surface of infected cells, recognition by peptide-specific CD8 T cells, CD8-mediated virus control in vitro, and inflationary memory responses in vivo. While it was shown that CD8 T cells recognizing subdominant epitopes are also sufficient for immune protection against MCMV [79], the present model predicts that these will be necessarily epitopes with inflationary potential and processed by the constitutive proteasome, but outcompeted by the dominant inflationary epitopes.
Peptide processing determined the protective potential of cognate CD8 T cells. C57BL/6 LSEC were infected at MOI of 0.1 with indicated viruses (see Dekhtiarenko et al. [43] for details) and Db-HGIRNASFI-specific CTL cells were added 1h later. Virus titers in supernatants were established on indicated days by plaque assay on MEFs and means ± SD of biological triplicates are shown. DL detection limit
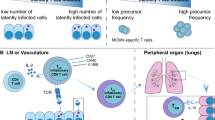
At this point, one should remember that inflationary responses depend on antigen presentation on non-hematopoietic cells [30, 31]. Like all cells, they express the constitutive proteasome, and in the absence of inflammatory stimuli, they will not express the immunoproteasome components.
From these considerations, emerges a model (Fig. 3), where latent CMV in non-hematopoietic cells occasionally expresses IE genes during latency. The epitopes that are the first to be expressed and that possess intrinsic properties that foster CD8 T-cell recognition (i.e., higher avidity of binding to the TCR) may outcompete subdominant ones and induce inflationary responses, but only if they are available to the constitutive proteasome for processing. In that case, the direct presentation of antigens that drives memory inflation will also select for higher avidity T-cell responses to control the virus replication cycle as early as possible, prior to the expression of VRAPs. This allows for a détente between the virus and the host that would induce minimal inflammatory damage, since epitopes can be recognized in the absence of IFN mediated upregulation of the immunoproteasome. If the immunoproteasome had to be upregulated to present the epitopes to CD8 T cells, then a round of IFN response would have to precede the CD8 T-cell-mediated recognition of infected cells, and this initial response would necessarily be non-specific and, thus, affecting a lot of uninfected bystander tissue. Direct presentation of epitopes on latent cells, in the absence of a boost by the innate immune system, may also explain why CMV accurately senses deficiencies of the adaptive immune system. In that case, the absence of inflationary cells would prompt CMV to reactivate from latency, which is consistent with clinical and experimental observations, where T-cell loss results in CMV reactivation [80]. Finally, this scenario would predict that inflated HCMV epitopes would also be immunoproteasome-independent and, thus, better targets for protection in immunotherapeutic settings.
In conclusion, if inflationary CD8 T cells are, indeed, sustained by ongoing direct presentation of viral epitopes by the constitutive proteasome, inflationary epitopes, rather than the conventional or subdominant ones may also be the logical optimal target for immunotherapeutic strategies. This idea should be addressed in future experimental models and clinical studies.
References
Klenerman P, Oxenius A (2016) T cell responses to cytomegalovirus. Nat Rev Immunol 16(6):367–377. https://doi.org/10.1038/nri.2016.38
O'Hara GA, Welten SP, Klenerman P, Arens R (2012) Memory T cell inflation: understanding cause and effect. Trends Immunol 33(2):84–90. https://doi.org/10.1016/j.it.2011.11.005
Cicin-Sain L, Arens R (2018) Exhaustion and inflation at antipodes of T cell responses to chronic virus infection. Trends Microbiol 26(6):498–509. https://doi.org/10.1016/j.tim.2017.11.012
Holtappels R, Pahl-Seibert MF, Thomas D, Reddehase MJ (2000) Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L(lo) memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J Virol 74(24):11495–11503
Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P (2003) Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol 170(4):2022–2029
Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ (2005) Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 202(5):673–685. https://doi.org/10.1084/jem.20050882
Weekes MP, Wills MR, Mynard K, Carmichael AJ, Sissons JG (1999) The memory cytotoxic T-lymphocyte (CTL) response to human cytomegalovirus infection contains individual peptide-specific CTL clones that have undergone extensive expansion in vivo. J Virol 73(3):2099–2108
Komatsu H, Sierro S, Klenerman P (2003) Population analysis of antiviral T cell responses using MHC class I-peptide tetramers. Clin Exp Immunol 134(1):9–12
Hosie L, Pachnio A, Zuo J, Pearce H, Riddell S, Moss P (2017) Cytomegalovirus-specific T cells restricted by HLA-Cw*0702 Increase markedly with age and dominate the CD8(+) T-cell repertoire in older people. Front Immunol 8:1776. https://doi.org/10.3389/fimmu.2017.01776
Jackson SE, Sedikides GX, Okecha G, Poole EL, Sinclair JH, Wills MR (2017) Latent cytomegalovirus (CMV) infection does not detrimentally alter T cell responses in the healthy old, but increased latent CMV carriage is related to expanded CMV-specific T cells. Front Immunol 8:733. https://doi.org/10.3389/fimmu.2017.00733
Jackson SE, Mason GM, Okecha G, Sissons JG, Wills MR (2014) Diverse specificities, phenotypes, and antiviral activities of cytomegalovirus-specific CD8+ T cells. J Virol 88(18):10894–10908. https://doi.org/10.1128/jvi.01477-14
Cicin-Sain L, Sylwester AW, Hagen SI, Siess DC, Currier N, Legasse AW, Fischer MB, Koudelka CW, Axthelm MK, Nikolich-Zugich J, Picker LJ (2011) Cytomegalovirus-specific T cell immunity is maintained in immunosenescent rhesus macaques. J Immunol 187(4):1722–1732. https://doi.org/10.4049/jimmunol.1100560
Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB (2006) Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. Journal of immunology 177(1):450–458
Sierro S, Rothkopf R, Klenerman P (2005) Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. European journal of immunology 35(4):1113–1123. https://doi.org/10.1002/eji.200425534
Youngblood B, Hale JS, Ahmed R (2015) Memory CD8 T cell transcriptional plasticity. F1000prime Rep 7:38. https://doi.org/10.12703/p7-38
Opferman JT, Ober BT, Ashton-Rickardt PG (1999) Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science (New York, NY) 283(5408):1745–1748
Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401(6754):708–712. https://doi.org/10.1038/44385
Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA (1997) Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med 186(9):1407–1418
Graef P, Buchholz VR, Stemberger C, Flossdorf M, Henkel L, Schiemann M, Drexler I, Hofer T, Riddell SR, Busch DH (2014) Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8(+) central memory T cells. Immunity 41(1):116–126. https://doi.org/10.1016/j.immuni.2014.05.018
Weninger W, Crowley MA, Manjunath N, von Andrian UH (2001) Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med 194(7):953–966
Wolint P, Betts MR, Koup RA, Oxenius A (2004) Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J Exp Med 199(7):925–936. https://doi.org/10.1084/jem.20031799
Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM (2007) Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27(2):281–295. https://doi.org/10.1016/j.immuni.2007.07.010
Buchholz VR, Flossdorf M, Hensel I, Kretschmer L, Weissbrich B, Graf P, Verschoor A, Schiemann M, Hofer T, Busch DH (2013) Disparate individual fates compose robust CD8+ T cell immunity. Science (New York, NY) 340(6132):630–635. https://doi.org/10.1126/science.1235454
Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB (2008) Memory inflation during chronic viral infection is maintained by continuous production of short-lived functional t cells. Immunity 29(4):650–659. https://doi.org/10.1016/j.immuni.2008.07.017
Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL (2002) Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 8(4):379–385. https://doi.org/10.1038/nm0402-379
Redeker A, Welten SP, Arens R (2014) Viral inoculum dose impacts memory T-cell inflation. Eur J Immunol 44(4):1046–1057. https://doi.org/10.1002/eji.201343946
Oduro JD, Redeker A, Lemmermann NA, Ebermann L, Marandu TF, Dekhtiarenko I, Holzki JK, Busch DH, Arens R, Cicin-Sain L (2016) Murine cytomegalovirus (CMV) infection via the intranasal route offers a robust model of immunity upon mucosal CMV infection. J Gen Virol 97(1):185–195. https://doi.org/10.1099/jgv.0.000339
Baumann NS, Torti N, Welten SPM, Barnstorf I, Borsa M, Pallmer K, Oduro JD, Cicin-Sain L, Ikuta K, Ludewig B, Oxenius A (2018) Tissue maintenance of CMV-specific inflationary memory T cells by IL-15. PLoS Pathog 14(4):e1006993. https://doi.org/10.1371/journal.ppat.1006993
Smith CJ, Turula H, Snyder CM (2014) Systemic hematogenous maintenance of memory inflation by MCMV infection. PLoS Pathog 10(7):e1004233. https://doi.org/10.1371/journal.ppat.1004233
Torti N, Walton SM, Brocker T, Rulicke T, Oxenius A (2011) Non-hematopoietic cells in lymph nodes drive memory CD8 T cell inflation during murine cytomegalovirus infection. PLoS Pathog 7(10):e1002313. https://doi.org/10.1371/journal.ppat.1002313
Seckert CK, Schader SI, Ebert S, Thomas D, Freitag K, Renzaho A, Podlech J, Reddehase MJ, Holtappels R (2011) Antigen-presenting cells of haematopoietic origin prime cytomegalovirus-specific CD8 T-cells but are not sufficient for driving memory inflation during viral latency. J Gen Virol 92(9):1994–2005. https://doi.org/10.1099/vir.0.031815-0
Seckert CK, Griessl M, Buttner JK, Scheller S, Simon CO, Kropp KA, Renzaho A, Kuhnapfel B, Grzimek NK, Reddehase MJ (2012) Viral latency drives 'memory inflation': a unifying hypothesis linking two hallmarks of cytomegalovirus infection. Med Microbiol Immunol 201(4):551–566. https://doi.org/10.1007/s00430-012-0273-y
Seckert CK, Renzaho A, Tervo HM, Krause C, Deegen P, Kuhnapfel B, Reddehase MJ, Grzimek NK (2009) Liver sinusoidal endothelial cells are a site of murine cytomegalovirus latency and reactivation. J Virol 83(17):8869–8884. https://doi.org/10.1128/jvi.00870-09
Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA (2003) Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101(7):2711–2720. https://doi.org/10.1182/blood-2002-07-2103
Ouyang Q, Wagner WM, Zheng W, Wikby A, Remarque EJ, Pawelec G (2004) Dysfunctional CMV-specific CD8(+) T cells accumulate in the elderly. Exp Gerontol 39(4):607–613. https://doi.org/10.1016/j.exger.2003.11.016
Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A (2004) Is immunosenescence infectious? Trends Immunol 25(8):406–410. https://doi.org/10.1016/j.it.2004.05.006
Lachmann R, Bajwa M, Vita S, Smith H, Cheek E, Akbar A, Kern F (2012) Polyfunctional T cells accumulate in large human cytomegalovirus-specific T cell responses. J Virol 86(2):1001–1009. https://doi.org/10.1128/jvi.00873-11
Karrer U, Wagner M, Sierro S, Oxenius A, Hengel H, Dumrese T, Freigang S, Koszinowski UH, Phillips RE, Klenerman P (2004) Expansion of protective CD8+ T-cell responses driven by recombinant cytomegaloviruses. J Virol 78(5):2255–2264
Dekhtiarenko I, Jarvis MA, Ruzsics Z, Cicin-Sain L (2013) The context of gene expression defines the immunodominance hierarchy of cytomegalovirus antigens. J Immunol 190(7):3399–3409. https://doi.org/10.4049/jimmunol.1203173
Borkner L, Sitnik KM, Dekhtiarenko I, Pulm AK, Tao R, Drexler I, Cicin-Sain L (2017) Immune protection by a cytomegalovirus vaccine vector expressing a single low-avidity epitope. J Immunol 199(5):1737–1747. https://doi.org/10.4049/jimmunol.1602115
Tsuda Y, Caposio P, Parkins CJ, Botto S, Messaoudi I, Cicin-Sain L, Feldmann H, Jarvis MA (2011) A replicating cytomegalovirus-based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PLoS Neglect Trop Dis 5(8):e1275. https://doi.org/10.1371/journal.pntd.0001275
Klyushnenkova EN, Kouiavskaia DV, Parkins CJ, Caposio P, Botto S, Alexander RB, Jarvis MA (2012) A cytomegalovirus-based vaccine expressing a single tumor-specific CD8+ T-cell epitope delays tumor growth in a murine model of prostate cancer. J Immunother (Hagerstown, Md: 1997) 35(5):390–399. https://doi.org/10.1097/CJI.0b013e3182585d50
Dekhtiarenko I, Ratts RB, Blatnik R, Lee LN, Fischer S, Borkner L, Oduro JD, Marandu TF, Hoppe S, Ruzsics Z, Sonnemann JK, Mansouri M, Meyer C, Lemmermann NA, Holtappels R, Arens R, Klenerman P, Fruh K, Reddehase MJ, Riemer AB, Cicin-Sain L (2016) Peptide processing is critical for T-cell memory inflation and may be optimized to improve immune protection by CMV-based vaccine vectors. PLoS Pathog 12(12):e1006072. https://doi.org/10.1371/journal.ppat.1006072
Nikolich-Zugich J, Goodrum F, Knox K, Smithey MJ (2017) Known unknowns: how might the persistent herpesvirome shape immunity and aging? Curr Opin Immunol 48:23–30. https://doi.org/10.1016/j.coi.2017.07.011
Riddell NE, Griffiths SJ, Rivino L, King DCB, Teo GH, Henson SM, Cantisan S, Solana R, Kemeny DM, MacAry PA, Larbi A, Akbar AN (2015) Multifunctional cytomegalovirus (CMV)-specific CD8(+) T cells are not restricted by telomere-related senescence in young or old adults. Immunology 144(4):549–560. https://doi.org/10.1111/imm.12409
Fruh K, Picker L (2017) CD8+ T cell programming by cytomegalovirus vectors: applications in prophylactic and therapeutic vaccination. Curr Opin Immunol 47:52–56. https://doi.org/10.1016/j.coi.2017.06.010
Humphreys IR, Sebastian S (2018) Novel viral vectors in infectious diseases. Immunology 153(1):1–9. https://doi.org/10.1111/imm.12829
Quinn M, Erkes DA, Snyder CM (2016) Cytomegalovirus and immunotherapy: opportunistic pathogen, novel target for cancer and a promising vaccine vector. Immunotherapy 8(2):211–221. https://doi.org/10.2217/imt.15.110
Kurz SK, Rapp M, Steffens HP, Grzimek NK, Schmalz S, Reddehase MJ (1999) Focal transcriptional activity of murine cytomegalovirus during latency in the lungs. J Virol 73(1):482–494
Grzimek NK, Dreis D, Schmalz S, Reddehase MJ (2001) Random, asynchronous, and asymmetric transcriptional activity of enhancer-flanking major immediate-early genes ie1/3 and ie2 during murine cytomegalovirus latency in the lungs. J Virol 75(6):2692–2705. https://doi.org/10.1128/jvi.75.6.2692-2705.2001
Simon CO, Kühnapfel B, Reddehase MJ, Grzimek NKA (2007) Murine cytomegalovirus major immediate-early enhancer region operating as a genetic switch in bidirectional gene pair transcription. J Virol 81(14):7805–7810. https://doi.org/10.1128/jvi.02388-06
Simon CO, Seckert CK, Dreis D, Reddehase MJ, Grzimek NK (2005) Role for tumor necrosis factor alpha in murine cytomegalovirus transcriptional reactivation in latently infected lungs. J Virol 79(1):326–340. https://doi.org/10.1128/jvi.79.1.326-340.2005
Simon CO, Holtappels R, Tervo HM, Bohm V, Daubner T, Oehrlein-Karpi SA, Kuhnapfel B, Renzaho A, Strand D, Podlech J, Reddehase MJ, Grzimek NK (2006) CD8 T cells control cytomegalovirus latency by epitope-specific sensing of transcriptional reactivation. J Virol 80(21):10436–10456. https://doi.org/10.1128/jvi.01248-06
Presti RM, Pollock JL, Dal Canto AJ, O'Guin AK, Virgin HWT (1998) Interferon gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J Exp Med 188(3):577–588
Dag F, Dolken L, Holzki J, Drabig A, Weingartner A, Schwerk J, Lienenklaus S, Conte I, Geffers R, Davenport C, Rand U, Koster M, Weiss S, Adler B, Wirth D, Messerle M, Hauser H, Cicin-Sain L (2014) Reversible silencing of cytomegalovirus genomes by type I interferon governs virus latency. PLoS pathogens 10(2):e1003962. https://doi.org/10.1371/journal.ppat.1003962
Kropp KA, Robertson KA, Sing G, Rodriguez-Martin S, Blanc M, Lacaze P, Hassim MFBN, Khondoker MR, Busche A, Dickinson P, Forster T, Strobl B, Mueller M, Jonjic S, Angulo A, Ghazal P (2011) Reversible inhibition of murine cytomegalovirus replication by gamma interferon (IFN-γ) in primary macrophages involves a primed type I IFN-signaling subnetwork for full establishment of an immediate-early antiviral state. J Virol 85(19):10286–10299. https://doi.org/10.1128/JVI.00373-11
Shnayder M, Nachshon A, Krishna B, Poole E, Boshkov A, Binyamin A, Maza I, Sinclair J, Schwartz M, Stern-Ginossar N (2018) Defining the transcriptional landscape during cytomegalovirus latency with single-cell RNA sequencing. mBio 9(2):e00013–e00018. https://doi.org/10.1128/mBio.00013-18
Cheng S, Caviness K, Buehler J, Smithey M, Nikolich-Zugich J, Goodrum F (2017) Transcriptome-wide characterization of human cytomegalovirus in natural infection and experimental latency. Proc Natl Acad Sci USA 114(49):E10586–e10595. https://doi.org/10.1073/pnas.1710522114
Reeves MB, Coleman H, Chadderton J, Goddard M, Sissons JG, Sinclair JH (2004) Vascular endothelial and smooth muscle cells are unlikely to be major sites of latency of human cytomegalovirus in vivo. J Gen Virol 85(Pt 11):3337–3341. https://doi.org/10.1099/vir.0.80285-0
Reddehase MJ, Balthesen M, Rapp M, Jonjic S, Pavic I, Koszinowski UH (1994) The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J Exp Med 179(1):185–193
Bohm V, Seckert CK, Simon CO, Thomas D, Renzaho A, Gendig D, Holtappels R, Reddehase MJ (2009) Immune evasion proteins enhance cytomegalovirus latency in the lungs. J Virol 83(19):10293–10298. https://doi.org/10.1128/jvi.01143-09
Oduro JD, Redeker A, Lemmermann NA, Ebermann L, Marandu TF, Dekhtiarenko I, Holzki JK, Busch D, Arens R, Cicin-Sain L (2015) Murine cytomegalovirus infection via the intranasal route offers a robust model of immunity upon mucosal CMV infection. J Gen Virol. https://doi.org/10.1099/jgv.0.000339
Del Val M, Munch K, Reddehase MJ, Koszinowski UH (1989) Presentation of CMV immediate-early antigen to cytolytic T lymphocytes is selectively prevented by viral genes expressed in the early phase. Cell 58(2):305–315
Ziegler H, Thale R, Lucin P, Muranyi W, Flohr T, Hengel H, Farrell H, Rawlinson W, Koszinowski UH (1997) A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity 6(1):57–66
Bohm V, Simon CO, Podlech J, Seckert CK, Gendig D, Deegen P, Gillert-Marien D, Lemmermann NA, Holtappels R, Reddehase MJ (2008) The immune evasion paradox: immunoevasins of murine cytomegalovirus enhance priming of CD8 T cells by preventing negative feedback regulation. J Virol 82(23):11637–11650. https://doi.org/10.1128/jvi.01510-08
Munks MW, Pinto AK, Doom CM, Hill AB (2007) Viral interference with antigen presentation does not alter acute or chronic CD8 T cell immunodominance in murine cytomegalovirus infection. J Immunol 178(11):7235–7241
Hutchinson S, Sims S, O'Hara G, Silk J, Gileadi U, Cerundolo V, Klenerman P (2011) A dominant role for the immunoproteasome in CD8+ T cell responses to murine cytomegalovirus. PloS One 6(2):e14646. https://doi.org/10.1371/journal.pone.0014646
Cicin-Sain L, Brien JD, Uhrlaub JL, Drabig A, Marandu TF, Nikolich-Zugich J (2012) Cytomegalovirus infection impairs immune responses and accentuates T-cell pool changes observed in mice with aging. PLoS pathogens 8(8):e1002849. https://doi.org/10.1371/journal.ppat.1002849
Farrington LA, Smith TA, Grey F, Hill AB, Snyder CM (2013) Competition for antigen at the level of the APC is a major determinant of immunodominance during memory inflation in murine cytomegalovirus infection. J Immunol 190(7):3410–3416. https://doi.org/10.4049/jimmunol.1203151
Reusser P, Riddell SR, Meyers JD, Greenberg PD (1991) Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood 78(5):1373–1380
Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Loffler J, Grigoleit U, Moris A, Rammensee HG, Kanz L, Kleihauer A, Frank F, Jahn G, Hebart H (2002) Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 99(11):3916–3922
Feuchtinger T, Opherk K, Bethge WA, Topp MS, Schuster FR, Weissinger EM, Mohty M, Or R, Maschan M, Schumm M, Hamprecht K, Handgretinger R, Lang P, Einsele H (2010) Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood 116(20):4360–4367. https://doi.org/10.1182/blood-2010-01-262089
Reddehase MJ, Weiland F, Munch K, Jonjic S, Luske A, Koszinowski UH (1985) Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol 55(2):264–273
Bunde T, Kirchner A, Hoffmeister B, Habedank D, Hetzer R, Cherepnev G, Proesch S, Reinke P, Volk HD, Lehmkuhl H, Kern F (2005) Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med 201(7):1031–1036. https://doi.org/10.1084/jem.20042384
Pahl-Seibert MF, Juelch M, Podlech J, Thomas D, Deegen P, Reddehase MJ, Holtappels R (2005) Highly protective in vivo function of cytomegalovirus IE1 epitope-specific memory CD8 T cells purified by T-cell receptor-based cell sorting. J Virol 79(9):5400–5413. https://doi.org/10.1128/jvi.79.9.5400-5413.2005
Holtappels R, Podlech J, Pahl-Seibert M-F, Jülch M, Thomas D, Simon CO, Wagner M, Reddehase MJ (2004) Cytomegalovirus misleads its host by priming of CD8 T cells specific for an epitope not presented in infected tissues. J Exp Med 199(1):131–136. https://doi.org/10.1084/jem.20031582
Holtappels R, Thomas D, Reddehase MJ (2009) The efficacy of antigen processing is critical for protection against cytomegalovirus disease in the presence of viral immune evasion proteins. J Virol 83(18):9611–9615. https://doi.org/10.1128/jvi.00936-09
Fink A, Lemmermann NA, Gillert-Marien D, Thomas D, Freitag K, Bohm V, Wilhelmi V, Reifenberg K, Reddehase MJ, Holtappels R (2012) Antigen presentation under the influence of 'immune evasion' proteins and its modulation by interferon-gamma: implications for immunotherapy of cytomegalovirus infection with antiviral CD8 T cells. Med Microbiol Immunol 201(4):513–525. https://doi.org/10.1007/s00430-012-0256-z
Ebert S, Lemmermann NA, Thomas D, Renzaho A, Reddehase MJ, Holtappels R (2012) Immune control in the absence of immunodominant epitopes: implications for immunotherapy of cytomegalovirus infection with antiviral CD8 T cells. Med Microbiol Immunol 201(4):541–550. https://doi.org/10.1007/s00430-012-0268-8
Polic B, Hengel H, Krmpotic A, Trgovcich J, Pavic I, Luccaronin P, Jonjic S, Koszinowski UH (1998) Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J Exp Med 188(6):1047–1054
Acknowledgments
I gratefully acknowledge Iryna Dekhtiarenko for her contribution to the development of the project and data shown in Fig. 2. Furthermore, this project was supported by the Helmholtz Association through the Helmholtz-EU Partnership consortium MCMVaccine, the German Scientific Foundation through the project CRC900, project B2 and the Excellence cluster RESIST, as well as the German Ministry of Education and Science through DZIF funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Human and animal rights
Data shown in this article were published previously, with the exception of Fig. 2, which was an in vitro study of murine cell lines. CTLs used in the experiment were generated from primary T cells acquired from mice in accordance with institutional and state guidelines and approved as an animal protocol under the running number 33.19-42502-05-10A039 by the Lower Saxony Office for Consumer Protection. No clinical data are shown and no human-derived biological samples were used in this study.
Additional information
Edited by: Matthias J. Reddehase.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Special Issue on Immunological Imprinting during Chronic Viral Infection.
Rights and permissions
About this article
Cite this article
Cicin-Sain, L. Cytomegalovirus memory inflation and immune protection. Med Microbiol Immunol 208, 339–347 (2019). https://doi.org/10.1007/s00430-019-00607-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-019-00607-8