Abstract
The pathogenesis of Staphylococcus aureus from local infection to systemic dissemination involves a range of virulence factors including structural and secreted products. Among various control mechanisms, small noncoding RNAs are involved in the regulation of multiple pathogenicity factors in S. aureus. The sRNA SprX which is encoded in the pathogenicity island of methicillin-susceptible S. aureus strain Newman and was shown to influence antibiotic resistance previously, upregulated the expression of virulence genes, especially the cell wall-associated clumping factor B (ClfB) and delta hemolysin (Hld). Bioinformatic analysis revealed several multiple mRNAs associated with pathogenicity as targets for SprX1, one of the three copies of sprX. Both overexpression and chromosomal disruption of sprX1 supported the scheme of upregulation of clfB and hld expression. Altered expression of SprX1 altered the levels of Hld and ClfB mRNAs, hemolysis, clumping of cells, biofilm formation by plate adhesion studies and confocal microscopic analysis as well as infection pathology of modified strains in mice models. ClfB and Hld mRNAs interacted directly with SprX1 in in vitro assays. Increased level of the regulatory RNA, namely RNAIII, that comprises Hld mRNA and also regulates the biofilm formation, indicates that SprX1 may also function through RNAIII for regulating virulence factors. An immunodominant protein, antigen A, was downregulated by SprX1 in two-dimensional electrophoresis. Taken together, these results signify the role of sRNA SprX in the pathogenicity of S. aureus Newman.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus addresses its pathogenicity by the production of various virulence factors which include coagulase, leukocidin, hyaluronate lyase, staphylokinase, toxic shock syndrome toxin, hemolysins, enterotoxins and microbial surface components which recognize adhesive matrix molecules to promote colonization and tissue invasion [1, 2]. During infection, S. aureus utilizes various gene regulatory mechanisms, among which small noncoding RNAs (sRNAs) are established as gene regulators. These sRNAs are generally expressed under specific stress responses or during virulence, iron uptake, quorum sensing, biofilm formation and pathogenicity. They regulate and execute their function by binding to specific proteins or base pairing with either cis- or trans-encoded mRNAs leading to repression or activation of protein activity or target genes [3, 4].
Over 730 small RNAs have been identified in Staphylococcus aureus [5] some of which have been studied extensively. These include RNAIII [6], RsaE [7], SprD [8], SprA1/SprA1AS [9], SSR42 [10], SprX [11], RsaA [12], ArtR [13], SprG1/SprF1 [14], SprC [15], all of which are known for their role in the pathogen physiology of this organism. Another sRNA SSR42 in S. aureus MSSA and MRSA strains regulates the expression of approximately 80 mRNAs of which several virulence factors are affected in a strain-dependent manner [10]. This may be because of the influence of SSR42 on staphylococcal accessory regulator (Sar) homologues that control the production of virulence factors in a strain-dependent manner [16]. Unlike in several gram-negative organisms, Hfq is not required in target regulation of S. aureus, notably RN6390, COL and Newman [17], although Hfq binding is reported in RNAIII—spa [18], ArtR—sarT/hla [13].
Staphylococcus aureus strain Newman [19] has been extensively used in studying the staphylococcal disease in animal models due to its virulence phenotypes. The present study is aimed at investigating the functional role of a small regulatory RNA, SprX, expressed from staphylococcal pathogenicity island (SaPI) in the clinical isolate methicillin-susceptible Staphylococcus aureus Newman (MSSA) strain. SprX was recently shown to influence vancomycin and teicoplanin antibiotic resistance in Staphylococcus aureus HG001 strain by downregulating the spoVG expression [11]. This work describes the overexpression and disruption of sprX1 in the clinical isolate S. aureus Newman and the subsequent effect of its altered expression on candidate target virulent genes of clumping factor B (ClfB), delta hemolysin (Hld), immunoglobulin binding protein G (Sbi), staphylocoagulase (Coa), immunodominant antigen A (IsaA) by molecular, physiological assays and their corroboration with animal infection studies. Pathogenicity studies in an animal model of infection have further confirmed the influence of small noncoding RNA in S. aureus.
Experimental procedures
Bacterial strains and growth conditions
Staphylococcus aureus strains and plasmids used in this study are listed in Table 1. All E. coli and S. aureus strains were grown in Luria–Bertani medium at 37 °C. Antibiotics were supplemented at the following concentrations: erythromycin and chloramphenicol each 10 µg/ml for plasmid selection, kanamycin—15 µg/ml for chromosomal marker in S. aureus and ampicillin 100 µg/ml for plasmid selection in E. coli, methicillin—0.5 µg/ml for induction of PblaZ.
Bioinformatic analyses
Sequences of sRNAs which have been earlier identified in S. aureus N315 [7, 11, 20] were used to find the homologues in the clinical isolate S. aureus strain Newman. The secondary structure of SprX1 RNA was predicted with the program mfold, based on the algorithm of Zuker and Stiegler [21]. Online target prediction tools TargetRNA [22], RNA predator [23], RNAup [24] and IntaRNA [25] programs were used to predict the mRNA targets for the sRNAs. Virulent target mRNAs with potential base pairing of 9–25 nt having significant P value ≤0.01 were selected.
Transformation in S. aureus
Electrocompetent cells were prepared according to manufacturer’s protocol (BTX Harvard apparatus) with some modifications [26]. S. aureus cells of OD600 0.4 were washed twice with 0.5 M sucrose, and 200 µl cells were electroporated with 3–5 µg of plasmid at 2.3 kV, 50 μF, 100 ohms. The cells were then immediately suspended in 500 μl of LB containing 0.5 M sucrose with shaking for 2 h at 37 °C. The transformants were selected on respective antibiotic-containing medium and incubated for 24–48 h at 37 °C.
Expression of SprX1 in multicopy plasmid
Recombinant DNA protocols were as described in Sambrook and Russel [27]. SprX1 sequence along with endogenous promoter was amplified using SprX primers (Table 2) and cloned in the E. coli–S. aureus shuttle vector pCN40 at SalI/BamHI sites and introduced into S. aureus strain Newman after passing through intermediate host S. aureus RN4220 by electroporation.
Construction of disruption sprX1::kan and complementation
A 1.5-kb DNA flanking 700 and 800 bp upstream and downstream respectively, of SprX1 gene was amplified from S. aureus Newman using the primers FSprX (Table 2) and cloned in pBSKS to generate pFsprX. The sprX1 gene in the above construct was inactivated by inserting the kanamycin gene at +10 position of SprX1 using NsiI to generate pFsprXKan. The disrupted sprX1::kan fragment was cloned into the BamHI site of the temperature-sensitive plasmid pIMAY to generate the disruption plasmid psprX1kan and was passed through E. coli DC10B strain before electroporation into S. aureus. sprX1 chromosomal gene disruption in S. aureus Newman was achieved by homologous recombination at the flanking sequence at nonpermissive temperature [26] and confirmed by PCR, northern and southern analyses. Further, the disruption strain was complemented with SprX1 overexpression plasmid for restoration.
Analysis of RNA expression
Total RNA (15–20 µg) extracted using acid guanidinium thiocyanate [28] was separated on denaturing 6 % urea-PAGE [27] and hybridized with strand-specific digoxigenin-labeled RNA probe as per manufacturer’s protocol (Roche Applied science, USA). Quantitative real-time PCR was performed with gene-specific primers using SYBR Green Master Mix in Step One Thermal Cycler (Applied Biosystems). 5S rRNA was used as internal control. The primers used are listed in Table 2.
Measurement of biofilm formation
Biofilm production was assessed using a semiquantitative adherence assay in 96-well microtiter plates [29] with some modifications. Overnight grown cultures were diluted ~1:100 in LB with 2 % glucose. Diluted cells (200 µl) having similar OD were seeded onto microtiter plates and incubated at 37 °C for 24–48 h. Adherent cells were stained with 1 % crystal violet for 45 min, washed with PBS and dissolved in 200 μl of 33 % glacial acetic acid. The absorbance of each well was measured at OD570. Each strain was tested in four replicates.
Biofilm formation and clumping of cells were also examined by confocal laser scanning microscope (CLSM). Cells grown in 2 % glucose were allowed to adhere on glass cover slide, fixed, labeled with FITC (0.001 %) and imaged [30]. Clumping of cells was assessed by labeling the cell suspension with FITC (0.001 %).
Assay of alpha and delta hemolysis
Delta hemolysis was assayed by spectrophotometer using the modified protocol [31]. Culture supernatants (400 µl) collected from the late exponential phase of S. aureus were subjected to heat treatment at 100 °C for 45 s to inactivate other hemolysins [32] and incubated with 106 human RBCs at 37 °C for 1 h. Samples were then centrifuged at 10,000 rpm for 5 min. The released hemoglobin in the supernatant was measured at OD541 with PBS as blank. Alpha hemolysis was assayed on rabbit RBCs in PBS (pH 7.2) containing 0.1 % bovine serum albumin (Sigma) without heat treatment of the enzyme source [33, 34] and measured at OD416.
Protein precipitation and 2D gel electrophoresis
Extracellular protein from the stationary phase culture (OD600 13.0) grown in Todd Hewitt broth was precipitated using chloroform–methanol, dissolved in 6 M urea and quantified using Folin–Lowry method. Isoelectric focusing was performed on 7-cm-long ready strips of pI 4–7 (Bio-Rad), with 25 µg protein samples for a total of 15 kVh at 20 °C. Further the IPG strips were equilibrated and run on SDS-PAGE. Protein spots were cut and analyzed using LC–mass spectrometry (C-CAMP, Bangalore, India).
In vitro transcription and gel mobility shift assay
DNA template of SprX1 carrying T3 promoter was generated by PCR amplification from plasmid clones. The mRNA targets Hld−48 to +130 and ClfB2603 to 2758 and the nonspecific RNA gene PhrD gene were PCR-amplified and cloned in pBSKS+. DIG-labeled sRNA SprX1, unlabeled mRNA Hld, ClfB and PhrD transcripts were generated from the above templates using T3/T7 RNA polymerase (Roche, Germany) yielded 150, 255, 231 and 182 nts, respectively, and used for interaction studies in gel mobility shift assay. The transcripts were denatured at 65 °C for 10 min before using for binding assays. DIG-labeled sRNA SprX1 was incubated with increasing amounts of unlabeled mRNAs in 1× TMN RNA binding buffer [20 mM tris acetate (pH 7.6), 5 mM magnesium acetate, 100 mM sodium acetate]. RNA–RNA duplex formation was allowed at 37 °C for 30 min. Samples were run on native 6 % polyacrylamide gel, electroblotted and detected by anti-digoxigenin according to manufacturer’s instructions (Roche, Germany). Nonspecific, unrelated PhrD RNA from Pseudomonas aeruginosa was used for competition assays.
Animal model of infection
Groups of three female BALB/c mice of eight weeks old were infected with different constructs of S. aureus Newman by intravenous injection of 109 cells for sepsis model of infection [8]. Uninfected mice, injected with phosphate-buffered saline (PBS), served as negative controls. Mice were killed after 7 days of infection and assessed by clinical signs, measurement of body weight and biochemical markers such as blood urea nitrogen (BUN), creatinine, serum glutamic oxaloacetic acid transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT). Kidneys of the infected mice were excised, homogenized with sterile PBS with 0.1 % Triton X-100, and bacterial loads were enumerated by plating serial dilutions on mannitol salt agar. The results are represented as dot blots, and statistical analysis was done using two-way ANOVA to interpret the data.
Microbial growth curve analysis with altered level of SprX1
Growth rates were measured at OD600. All experimental data were normalized for mg/ml cell protein to account for the difference in growth rates. 5S rRNA was used as an internal control in real-time PCRs. Growth curves for all construct strains are shown in the supplementary file Fig. S3.
Results
In silico characterization of SprX
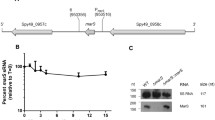
The sRNA SprX, originally identified in methicillin-resistant Staphylococcus aureus N315 strain [7] is conserved among several staphylococcal strains and phages. The SprX in most of the strains varies in length (149–157 nt) with 1–2 copies, except S. aureus Newman which contains three chromosomal copies [11]. Comparative multiple sequence alignment of SprX in Newman with other S. aureus strains is given in the supplementary file (Fig. S1A). The 156 nt long SprX1 in Newman differs from the 154 nt SprX2 and SprX3 by 6 nt (Fig. S1B). SprX1 is flanked by genes encoding staphylokinase and truncated amidase, while both SprX2 and SprX3 show 100 % similarity and are flanked by phage amidase and hypothetical protein (Fig. 1a). The secondary structure of SprX1 is characterized by four stems and U-rich loops. Prediction by TargetRNA and RNA predator programs revealed possible interaction at stem loop 2 (Fig. 1b) with multiple mRNA targets (Fig. 1c). A list of putative targets of SprX1 is given in the supplementary file (Table S1). The targets selected for further analysis in this study include delta hemolysin (Hld), clumping factor B (ClfB), immunoglobulin binding protein (Sbi) and staphylocoagulase (Coa).
Location, structure and target base pairing of SprX1 RNA. a Schematic representation of the genetic organization of sprX loci in S. aureus Newman. The size, orientation, ORF genes flanking the sRNA and its respective coordinates are indicated in bold. HP1 and HP2 are different hypothetical proteins, PA phage amidase, TA truncated amidase and sak Staphylokinase. b Secondary structure of SprX1 using mfold program. Individual stem loops are marked. c Potential base pairings of target mRNAs with SprX1
SprX1 overexpression and disruption
SprX1 in S. aureus Newman was cloned along with its endogenous promoter and overexpressed in a multicopy vector bearing a methicillin-inducible PblaZ [35]. However, poor expression was seen from PblaZ promoter under methicillin induction and maximum expression of SprX1 was detected as a 146 nt RNA from the endogenous promoter in stationary phase cultures (Fig. 2a). Weak expression from PblaZ was seen as a 415-nt transcript. Strain bearing vector pCN40 served as the control.
Expression analysis of SprX. a Overexpression of SprX1 in S. aureus Newman in northern blot hybridized with strand-specific DIG-labeled SprX RNA probe. pMNSprX, pCN40: overexpression and control vector. −/+ indicates uninduced and induced with 0.5 µg/ml of methicillin. b sprX1::kan, Newman: disruption and wild-type strain. Loading control shown are 23S and 16S rRNA
Chromosomal sprX1 was disrupted by introducing kanamycin-resistant gene close to the promoter (as in materials and methods) and subsequent homologous recombination. Southern blot and PCR analysis confirmed the disruption in the few mutants selected, by an increase in 1.4 kb in the sprX1 (Fig. S2A and S2B). The absence of sprX1 expression was confirmed in northern blot analysis, although both the wild-type and disruption strains expressed an additional 100-nt transcript which could have arisen from the additional gene copies of sprX (Fig. 2b).
SprX1 influences increased production of hemolysins and biofilm
In silico analysis indicated that SprX1 interacts with 26 nucleotides in the 5′ untranslated region (UTR), Shine-Dalgarno (SD) sequence and AUG codon of the mRNA encoding delta hemolysin and a 14-nucleotide stretch within the 3′ coding region of clumping factor B mRNA (Fig. 1c). Delta hemolysin, an extracellular cytotoxin produced at the late exponential phase [36], is transcribed within another regulatory small RNA, RNAIII serving as a marker for its activity [37].
Overexpression of SprX1 resulted in a 1.5-fold increase in Hld mRNA in real-time PCRs, whereas it was downregulated in the disruption strain (Fig. 3a). The increase in Hld transcripts was also paralleled by a 72 % increase in hemolysis of human RBCs (Fig. 3b). A primer pair corresponding to Hld mRNA and 3′ end of RNAIII (Table 2) was used for their simultaneous and coupled detection. This increase in RNAIII was also reflected by a 32 % increase in the activity of alpha hemolysin (Fig. 3c) which is one of its well-studied targets [38]. The increased alpha hemolysis on rabbit RBCs is an indirect evidence for the stability of RNAIII transcripts in the presence of SprX1. Complementation of overexpression plasmid pMNSprX in the sprX1 disruption mutant strain resulted in restoration of delta and alpha hemolysis activities (Fig. 3b, c).
Expression of predicted targets under altered levels of SprX1 in S. aureus Newman. a qRT-PCR analysis of expression of target genes in control (pCN40), SprX1 overexpression (pMNSprX) and disruption (sprX1::kan) expression. clfB—clumping factor B, hld—delta hemolysin, coa—staphylocoagulase and sbi—immunoglobulin binding protein. Asterisks represent significant statistical differences determined for each of the genes compared with the vector control by using two-way ANOVA ***(P < 0.001), *(P < 0.05) and **(P < 0.01). b Spectrophotometric assay of delta hemolysis from culture supernatants on human RBCs. c Spectrophotometric assay of alpha hemolysis from culture supernatants on rabbit RBCs. Asterisks represent the significant statistical differences of P ≤ 0.0001 and P ≤ 0.0014, respectively, for b and c when each construct was compared with the vector control as determined by one-way ANOVA. pCN40, pMNSprX, sprX1::kan, sprX1::kan + pMNSprX:, control vector, overexpression, disruption and the complemented sprX1 constructs
Several functionally diverse proteins such as ClfB, glucosaminidase, IsdA, IsaA, SACOL0688 and nuclease have been detected in biofilms of S. aureus strains [39]. Clumping factor B (ClfB) mRNA which was predicted as one of the targets of SprX1 in this study exhibited 2.24-fold increased levels under the overexpression of SprX1 while it was 0.7-fold of the control when the chromosomal sprX1 was disrupted, as quantified by real-time PCR (Fig. 3a). Further, the increase in clumping factor B was in co-relation by a 40 % enhancement in the levels of biofilm formation by microtiter plate assay (Fig. 4). Complementation by SprX1 partly restored the biofilm in the sprX1 disruption strain. This relative increase in ClfB which is required in the early phase of staphylococcal dissemination in the host [40, 41] suggests that SprX1 may support colonization of S. aureus during infection.
Influence of SprX1 on biofilm formation. Microtiter plate assay of biofilm formation in S. aureus Newman bearing different constructs of SprX: control (pCN40), SprX1 overexpression (pMNSprX), disruption (sprX1::kan) and complemented sprX1 (sprX1::kan + pMNSprX) constructs. Statistical difference *(P ≤ 0.0001) between the constructs was determined by using one-way ANOVA in comparison with the vector control
The cells overexpressing SprX1 also exhibited an increased adherence resulting in a dramatic increase in the biofilm formation, when tested on the glass cover slip, in comparison with the non homogenous film formed by the control strain (Fig. 5a1, b1, c1). Further, confocal microscopy of planktonic culture grown in 2 % glucose demonstrated aggregates of each 4–8 cells as compared to the strain bearing disruption or the control, both of which had uniformly dispersed single cells (Fig. 5a2, b2, c2). Collectively, these results indicate that increased dose of SprX1 contributes the cell to cell interaction leading to intercellular aggregation and biofilm phenotype.
Confocal laser scanning microscope (CLSM) images of biofilms and clumped cells. Series 1 in upper panels show the biofilm formation on the cover glass slip. Series 2 in lower panels illustrate the clumping of cells in culture suspension of different constructs a control (pCN40), b SprX1 overexpression (pMNSprX) and c disruption (sprX1::kan) of S. aureus Newman, respectively
In vitro interactions of SprX1 and target mRNAs
The interaction of computationally predicted specific target sequences in Hld and ClfB mRNAs with SprX1 was tested by in vitro gel mobility shift experiments. Target RNA sequences corresponding to the interaction region were produced by in vitro transcription using T3/T7 polymerase from cloned and amplified genes. Higher molecular weight complex was observed, with DIG-labeled 0.5 pmol of SprX1 and unlabeled mRNAs in the range of 0.5–2.5 pmol. With the 5′ UTR and initial stretch of the coding sequence of the Hld mRNA interaction was best at the maximum concentration of 2.5 pmol tested (Fig. 6a), which is in agreement with the bioinformatic analysis. However, weak interaction with ClfB was observed at the molar ratio of 1:3 pmol (Fig. 6b). The addition of excess of unlabeled nonspecific competitor PhrD RNA of Pseudomonas origin did not affect the duplex formation of either SprX1/Hld or SprX1/ClfB mRNA pairs in mobility shift assays.
Interaction of SprX1 with Hld/ClfB mRNAs. In vitro-transcribed DIG-labeled SprX1 (0.5 pmol) incubated with unlabeled Hld/ClfB RNA at 37 °C for 30 min. a1 Unlabeled SprX1 and Hld run on 6 % native PAGE gel, a2 lanes 1–5 complex formation of SprX1 with increasing concentrations of Hld mRNA: 0.5, 1.0, 1.5, 2.0, 2.5 pmol, respectively, lane 6 SprX1 alone, a3 Hld (2.0, 2.5 pmol) and SprX1 (0.5 pmol) interaction under ten fold excess of nonspecific unlabeled competitor PhrD RNA with no reduction in complex formation. b1 Unlabeled SprX and ClfB run on 6 % native PAGE, b2 lane 1 SprX1 alone, lanes 2–6 complex formation of SprX1 with increasing concentrations of ClfB mRNA: 0.5, 1.0, 1.5, 2.0, 2.5 pmol, respectively, b3 ClfB (1.5, 2.0 pmol) and SprX1 (0.5 pmol) interaction under tenfold excess of nonspecific unlabeled competitor PhrD RNA with no reduction in complex formation. −/+ indicates the presence and absence of PhrD
SprX1 downregulates the expression of immunodominant antigen A
Altered regulation of SprX1 resulted in differential expression of several proteins in 2D electrophoresis. Out of more than 10 differentially regulated proteins, spots marked 1–7 in Fig. 7 were found to be upregulated and spots number 8–12 were downregulated in strains overexpressing SprX1. The downregulated protein spots 8–11 were identified by nano-LC–MS/MS as pI variant isomers (pI 4.5, 4.8, 5.12 and 5.7) of immunodominant antigen A (IsaA), an extracellular protein of 29 kDa.
Two-dimensional gel electrophoresis of proteins from S. aureus Newman strains with altered expression of SprX1. a SprX1 overexpression (pMNSprX) and b control (pCN40). Spots 1–7 and 8–12 indicate the upregulated and downregulated proteins respectively, under SprX1 overexpression. The downregulated protein spots 8–11 were identified as pI variant isomers (pI 4.5, 4.8, 5.12 and 5.7) of 29-kDa immunodominant antigen A (IsaA)
SprX1 enhances the virulence of S. aureus in mice model of infection
SprX1 enhanced the virulence of S. aureus in mice model of infection. When mice were challenged with strains expressing altered SprX1 levels, the overexpression strain was found to be more pathogenic in comparison with the strains bearing control pCN40 or the disruption. Animals infected with overexpression strain exhibited multiple abscesses and discoloration in the kidneys, lungs, heart, liver and spleen (Fig. 8) and also showed increased levels of BUN (Fig. 9a), creatinine kinase (Fig. 9b), SGOT (Fig. 9c), SGPT (Fig. 9d) which serve as pathophysiological markers. Bacterial load was markedly high in the kidney of mice infected with the overexpression than any other strains (Fig. 9e). These results indicate that SprX1 is essential for the virulence of S. aureus Newman.
Morphological features of infected organs of mice with altered strains of S. aureus Newman. Organs of mice infected with overexpression of SprX1 shows multiple abscess formation in kidney and discoloration of lungs, liver, heart and spleen. a Uninfected, b vector control (pCN40), c SprX1 overexpression (pMNSprX), d disruption (sprX1::kan)
Virulence studies in mice model of infection of S. aureus Newman with altered levels of SprX1. Biochemical parameters: a blood urea nitrogen (BUN), b creatinine kinase, c serum glutamic oxaloacetic acid transaminase (SGOT) and d serum glutamic pyruvic acid transaminase (SGPT). e bacterial counts from kidneys. Mice were infected with S. aureus Newman strain bearing vector control (pCN40), overexpression (pMNSprX) and disruption (sprX1::kan). Asterisks represent significant statistical differences determined for each of the parameters compared with the vector control by using two-way ANOVA ***(P < 0.001), **(P < 0.01) and *(P < 0.05)
Discussion
The small RNAs SprD [8], SprA1/SprA1AS (antisense of SprA1) [9], SprG/F [14] and SprC [15] which are expressed from the pathogenicity island of S. aureus have significant impact on virulence by modulating the expression level of target mRNAs through various networks. The sRNA SprX which is also encoded in the pathogenicity island of S. aureus Newman was characterized in this work for its functional significance. Previously, SprX has been reported to affect the expression of DNA binding protein spoVG that regulates bacterial resistance in the strain S. aureus HG001 [11]. The evidence presented in this work is the first direct demonstration of its involvement in the regulation of pathogenicity factors as targets in the clinical isolate S. aureus Newman.
The poor expression of SprX1 from blaZ promoter in the overexpression construct was not anticipated. However, high expression of the intact RNA from the endogenous promoter in the multi-copy clone resulted in sufficient fold increase to make comparisons.
The identification of clumping factor B and delta hemolysin as new targets and the subsequent observation of their upregulation by SprX1 in real-time PCRs and physiological assays leading to increased pathogenicity have ascertained the functional significance of this RNA. The presence of hld gene within the other regulatory RNA, RNAIII makes it serve as a biomarker of RNAIII [37], which contributes to regulation of bacterial adhesion and invasion of epithelial cells [42]. SprX1 may exert its effect in influencing both Hld and RNAIII levels. It is reported that intramolecular base pairing of 5′ and 3′ end of RNAIII blocks the ribosomal binding site of Hld mRNA [43]. The interaction of SprX1 with the RNAIII in this region possibly may release this intramolecular base pairing leading to changes in the secondary structure and allowing the ribosome to bind for effective translation.
One of the major defense mechanism of Staphylococcus aureus is the capacity to form biofilms. Staphylococcal biofilm formation often occurs on medical implants [44] which are in direct contact with blood. SprX1 reported here is the third sRNA to be involved in biofilm formation in addition to RsaA [12] and RNAIII [45–47] in S. aureus. Clumping factor B is one of the biofilm-associated genes required for the early stage of biofilm formation [39, 48, 49]. The marked increase in biofilm formation under the overexpression of SprX1 could be partly attributed to increased levels of ClfB. In addition, SprX might mediate its effect on biofilm by increasing the stability of RNAIII which has been reported to influence biofilm structuring and dissemination [46, 47]. Several small RNAs such as OmrA/B, Qrr1-4, ArcZ and PhrS have been reported to be involved in biofilm formation and mediating the switching of planktonic cells to surface-mediated growth in other bacteria such as E. coli, Vibrio cholera, Salmonella typhimurium, Pseudomonas aeruginosa, respectively [45]. Biofilm formation is known to help the bacterium to circumvent host immune response and protect it from phagocytosis [50]. It is really interesting to look how SprX influences the regulation of another noncoding RNA such as RNAIII which is involved in the expression of several virulent targets.
The differential expression of several proteins was observed in two-dimensional gel electrophoresis which indicates that SprX1 may also function as an indirect regulator. One among them is the immunodominant antigen A (IsaA), which was found to be downregulated under the overexpression of SprX1. IsaA is highly antigenic and act as a better vaccine against disease [51, 52]. It also has lytic trans-glycosylase activity which promotes autolysis and regulates clumping of cells, thereby playing an important role in staphylococcal growth and survival. IsaA reduces the biofilm formation [53]. It is also reported that inactivation of isaA resulted in increased colonization of S. aureus [52, 54]. Thus, the downregulation of immunodominant antigen A by SprX1 reinforces the involvement in clumping and colonization of S. aureus.
Further, the reduced expression of virulent targets as well as the decreased virulence in mice was observed in the single sprX1 disruption mutant. Variation of SprX2 and SprX3 from SprX1 in six nucleotides resulted in poor interaction with the same targets in bioinformatic analysis (Fig. S4), thereby signifying the role of SprX1 among the three copies in regulating the pathogenicity. Although the immunoglobulin binding protein (Sbi) and staphylocoagulase (Coa) were among the bioinformatically predicted targets, there was no correlation in their expression with respect to SprX1 levels. The RNA interaction studies reinforce delta hemolysin and clumping factor B as direct targets of SprX1. Taken together all these results, sRNA SprX1 in our study appears to be of great importance in maintaining the virulence of S. aureus Newman.
References
Foster TJ, Geoghegan JA, Ganesh VK, Hook M (2014) Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12:49–62
Powers ME, Wardenburg JB (2014) Igniting the fire: Staphylococcus aureus virulence factors in the pathogenesis of sepsis. PLoS Pathog 10:e1003871
Waters LS, Storz G (2009) Regulatory RNAs in bacteria. Cell 136:615–628
Storz G, Altuvia S, Wassarman KM (2005) An abundance of RNA regulators. Annu Rev Biochem 74:199–217
Sassi M, Augagneur Y, Mauro T, Ivain L, Chabelskaya S, Hallier M et al (2015) SRD: a Staphylococcus regulatory RNA database. RNA 21:1005–1017
Boisset S, Geissmann T, Huntzinger E, Fechter P, Bendridi N, Possedko M et al (2007) Staphylococcus aureus RNAIII coordinately represses the synthesis of virulence factors and the transcriptional regulator Rot by an antisense mechanism. Genes Dev 21:1353–1366
Bohn C, Rigoulay C, Chabelskaya S, Sharma CM, Marchais A, Skorski P et al (2010) Experimental discovery of small RNAs in Staphylococcus aureus reveals a riboregulator of central metabolism. Nucleic Acids Res 38:6620–6636
Chabelskaya S, Gaillot O, Felden B (2010) A Staphylococcus aureus small RNA is required for bacterial virulence and regulates the expression of an immune evasion molecule. PLoS Pathog 6:e1000927
Sayed N, Jousselin A, Felden B (2011) A cis-antisense RNA acts in trans in Staphylococcus aureus to control translation of a human cytolytic peptide. Nat Struct Mol Biol 19:105–112
Morrison JM, Miller EW, Benson MA, Alonzo FIII, Yoong P, Torres VJ et al (2012) Characterization of SSR42, a novel virulence factor regulatory RNA that contributes to the pathogenesis of a Staphylococcus aureus USA300 representative. J Bacteriol 194:2924–2938
Eyraud A, Tattevin P, Chabelskaya S, Felden B (2014) A small RNA controls a protein regulator involved in antibiotic resistance in Staphylococcus aureus. Nucleic Acids Res 42:4892–4905
Romilly C, Lays C, Tomasini A, Caldelari I, Benito Y, Hammann P et al (2014) A noncoding RNA promotes bacterial persistence and decreases virulence by regulating a regulator in Staphylococcus aureus. PLoS Pathog 10:e1003979
Xue T, Zhang X, Sun H, Sun B (2014) ArtR, a novel sRNA of Staphylococcus aureus, regulates α-toxin expression by targeting the 5′ UTR of sarT mRNA. Med Microbiol Immunol 203:1–12
Pinel-Marie ML, Brielle R, Felden B (2014) Dual toxic peptide coding Staphylococcus aureus RNA under antisense regulation targets host cells and bacterial rivals unequally. Cell Rep 7:424–435
Le Pabic H, Germain-Amiot N, Bordeau V, Felden B (2015) A bacterial regulatory RNA attenuates virulence, spread and human host cell phagocytosis. Nucleic Acids Res 43:9232–9248
Blevins JS, Beenken KE, Elasri MO, Hurlburt BK, Smeltzer MS (2002) Strain dependent differences in the regulatory roles of sarA and agr in Staphylococcus aureus. Infect Immun 70:470–480
Bohn C, Rigoulay C, Bouloc P (2007) No detectable effect of RNA-binding protein Hfq absence in Staphylococcus aureus. BMC Microbiol 7:10
Huntzinger E, Boisset S, Saveanu C, Benito Y, Geissmann T, Namane A et al (2005) Staphylococcus aureus RNAIII and the endoribonuclease III coordinately regulate spa gene expression. EMBO J 24:824–835
Duthie ES, Lorenz LL (1952) Staphylococcal coagulase: mode of action and antigenicity. J Gen Microbiol 6:95–107
Pichon C, Felden B (2005) Small RNA genes expressed from Staphylococcus aureus genomic and pathogenicity islands with specific expression among pathogenic strains. Proc Natl Acad Sci USA 102:14249–14254
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415
Tjaden B (2008) TargetRNA: a tool for predicting targets of small RNA action in bacteria. Nucleic Acids Res 36:109–113
Eggenhofer F, Tafer H, Stadler PF, Hofacker IL (2011) RNApredator: fast accessibility based prediction of sRNA targets. Nucleic Acids Res 39:W149–W154
Muckstein U, Tafer H, Hackermuller J, Bernhart SH, Stadler PF, Hofacker IL (2006) Thermodynamics of RNA-RNA Binding. Bioinformatics 22:1177–1182
Busch A, Richter AS, Backofen R (2008) IntaRNA: efficient prediction of bacterial sRNA targets incorporating target site accessibility and seed regions. Bioinformatics 24:2849–2856
Monk IR, Shah IM, Xu M, Tan MW, Foster TJ (2012) Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 3:e00277-11
Sambrook J, Russel DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Lab Press, New York
Chomczynski P, Sacchi N (2006) The single step method of RNA isolation by acid guanidinium thiocyanate phenol chloroform extraction: twenty something years on. Nat Protoc 1:581–585
Zmantar T, Kouidhi B, Miladi H, Mahdouani K, Bakhrouf A (2010) A microtiter plate assay for Staphylococcus aureus biofilm quantification at various pH levels and hydrogen peroxide supplementation. New Microbiol 33:137–145
Hochbaum AI, Kolodkin-Gal I, Foulston L, Kolter R, Aizenber J, Losick R (2011) Inhibitory effects of d-amino acids on Staphylococcus aureus biofilm development. J Bacteriol 193:5616–5622
Ravi MS, Vijay R, Kumari S, Panchasara C (2013) Cytotoxic and genotoxic effects of orthodontic adhesives on human lymphocyte—an in vitro study. Med Sci 2:820–829
Wiseman GM (1975) The hemolysins of Staphylococcus aureus. Bacteriol Rev 39:317–344
Kernodle DS, McGraw PA, Barg NL, Menzies BE, Voladri RK, Harshman S (1995) Growth of Staphylococcus aureus with nafcillin in vitro induces alpha-toxin production and increases the lethal activity of sterile broth filtrates in a murine model. J Infect Dis 172:410–419
Bayer AS, Ramos MD, Menzies BE, Yeaman MR, Shen AJ, Cheung AL (1997) Hyper production of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: a host defense role for platelet microbicidal proteins. Infect Immun 65:4652–4660
Zhang L, Gray L, Novick RP, Ji G (2002) Transmembrane topology of AgrB, the protein involved in the post-translational modification of AgrD in Staphylococcus aureus. J Biol Chem 277:34736–34742
Schmitz FJ, Veldkamp KE, Van Kessel KPM, Verhoef J, Van Strijp JA (1997) Delta toxin from Staphylococcus aureus as a costimulator of human neutrophil oxidative burst. J Infect Dis 176:1531–1537
Sakoulas G, Moellering RC Jr, Eliopoulos GM (2006) Adaptation of methicillin resistant Staphylococcus aureus in the face of vancomycin therapy. Clin Infect Dis 42:S40–S50
Morfeldt E, Taylor D, von Gabain A, Arvidson S (1995) Activation of alpha-toxin translation in Staphylococcus aureus by the trans-encoded antisense RNA, RNAIII. EMBO J 14:4569–4577
Den Reijer PM, Haisma EM, Lemmens-den Toom NA, Willemse J, Koning RA et al (2016) Detection of alpha-toxin and other virulence factors in biofilms of Staphylococcus aureus on polystyrene and a human epidermal model. PLoS ONE 24:e0152544
Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM (2009) Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEBJ 23:3393–3404
Wertheim HFL, Walsh E, Choudhurry R, Melles DC, Boelens HAM, Miajlovic H et al (2008) Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med 5:e17
Iyer VR, Sharma R, Pathania R, Navani NK (2012) Small RNAs of pathogenic bacteria: not small enough to be overlooked for therapeutics. Mol Cell Pharmacol 4:17–30
Benito Y, Kolb FA, Romby P, Lina G, Etienne J, Vandensch F (2000) Probing the structure of RNAIII, the Staphylococcus aureus agr regulatory RNA and identification of the RNA domain involved in repression of protein A expression. RNA 6:668–679
Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME (2011) Staphylococcus aureus biofilms properties, regulation and roles in human disease. Virulence 2:445–459
Chambers JR, Sauer K (2013) Small RNAs and their role in biofilm formation. Trends Microbiol 21:39–41
Coelho LR, Souza RR, Ferreira FA, Guimaraes MA, Ferreira-Carvalho BT, Figueiredo AM (2008) agr RNAIII divergently regulates glucose-induced biofilm formation in clinical isolates of Staphylococcus aureus. Microbiology 154:3480–3490
Le KY, Otto M (2015) Quorum sensing regulation in staphylococci- an overview. Front Microbiol 6:1174
Resch A, Rosenstein R, Nerz C, Gotz F (2005) Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl Environ Microbiol 71:2663–2676
Abraham NM, Jefferson KK (2012) Staphylococcus aureus clumping factor B mediates biofilm formation in the absence of calcium. Microbiology 158:1504–1512
Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH et al (2011) Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 186:6585–6596
Lorenz U, Ohlsen K, Karch H, Hecker M, Thiede A, Hacker J (2000) Human antibody response during sepsis against targets expressed by methicillin resistant Staphylococcus aureus. FEMS Immunol Med Microbiol 29:145–153
Stapleton MR, Horsburgh MJ, Hayhurst EJ, Wright L, Jonsson IM, Tarkowski A et al (2007) Characterization of IsaA and SceD, two putative lytic transglycosylases of Staphylococcus aureus. J Bacteriol 189:7316–7325
Payne DE, Martin NR, Parzych KR, Rickard AH, Underwood A, Boles BR (2013) Tannic acid inhibits Staphylococcus aureus surface colonization in an IsaA-dependent manner. Infect Immun 81:496–504
Sakata N, Terakubo S, Mukai T (2005) Subcellular location of the soluble lytic transglycosylase homologue in Staphylococcus aureus. Curr Microbiol 50:47–51
Nair D, Memmi G, Hernandez D, Bard J, Beaume M, Gill S et al (2011) Whole genome sequencing of Staphylococcus aureus strain RN4220, a key laboratory strain used in virulence research, identifies mutations that affect not only virulence factors but also the fitness of the strain. J Bacteriol 193:2332–2335
Acknowledgments
We thank Professors Timothy Foster and Ian. R. Monk, Trinity College, Dublin, Abraham. L. Sonenshein, Tufts University, Boston, for providing shuttle plasmids, S. aureus and E. coli strains. The pCN40 vector was obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) program supported under NIAD, NIH Contract No. HHSN272200700055C. MK was supported by a research fellowship (F.4-1/2006(BSR)/7-128/2007) by University Grant Commission, Government of India. The initial phase of this work was partially funded by a grant from the Department of Biotechnology, India (BT/PR/P0056/AGR/36/29/2007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics statement
The study protocols for the experimental use of mice were carried out in strict accordance with and approved by Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA), Government of India (Approval number 938/PO/a/06/CPCSEA).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kathirvel, M., Buchad, H. & Nair, M. Enhancement of the pathogenicity of Staphylococcus aureus strain Newman by a small noncoding RNA SprX1. Med Microbiol Immunol 205, 563–574 (2016). https://doi.org/10.1007/s00430-016-0467-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-016-0467-9