Abstract
Concerning calculation function, studies have already reported on localizing computational function in patients and volunteers by functional magnetic resonance imaging and transcranial magnetic stimulation. However, the development of accurate repetitive navigated TMS (rTMS) with a considerably higher spatial resolution opens a new field in cognitive neuroscience. This study was therefore designed to evaluate the feasibility of rTMS for locating cortical calculation function in healthy volunteers, and to establish this technique for future scientific applications as well as preoperative mapping in brain tumor patients. Twenty healthy subjects underwent rTMS calculation mapping using 5 Hz/10 pulses. Fifty-two previously determined cortical spots of the whole hemispheres were stimulated on both sides. The subjects were instructed to perform the calculation task composed of 80 simple arithmetic operations while rTMS pulses were applied. The highest error rate (80 %) for all errors of all subjects was observed in the right ventral precentral gyrus. Concerning division task, a 45 % error rate was achieved in the left middle frontal gyrus. The subtraction task showed its highest error rate (40 %) in the right angular gyrus (anG). In the addition task a 35 % error rate was observed in the left anterior superior temporal gyrus. Lastly, the multiplication task induced a maximum error rate of 30 % in the left anG. rTMS seems feasible as a way to locate cortical calculation function. Besides language function, the cortical localizations are well in accordance with the current literature for other modalities or lesion studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In neurosurgery as well as in neuroscience, the mapping of higher cortical and neuropsychological function is of high importance. Previously, there have been certain studies of the cortical calculation function reported on localizing computational function and dyscalculia in patients and healthy subjects mainly by functional magnetic resonance imaging (fMRI) and transcranial magnetic stimulation (TMS). Now, the advanced method of repetitive navigated TMS (rTMS), especially in comparison to the previously used technique of non-navigated TMS, allows the researcher a distinctive and more precise way of locating neuropsychological cortical function as well as the possibility of combining this technique with various other tasks.
A non-invasive mapping of even those neuropsychological functions would be helpful not only for preoperative planning, but also for neuropsychological research per se. Since fMRI (McGraw et al. 2001) was shown to be less reliable in the vicinity of tumoral lesions, another technique seems promising: TMS.
This method was established in 1985 to trigger motor-evoked potentials (MEP) by inducing an electric field within the motor cortex to find motor-associated areas. Because of neuronal depolarization, an action potential could be evoked, which was then measurable as an MEP (Barker et al. 1985). According to Pascual-Leone et al. (1991), language-associated areas could be detected via TMS-induced so-called ‘virtual lesions’, whereby neuronal activity is inhibited (Pascual-Leone et al. 1991). Yet, on one hand, this technique was mainly used to detect motor and language-associated areas (Epstein 1998; Pascual-Leone et al. 1991; Sollmann et al. 2013a). On the other hand, it was also used for research purposes regarding neuropsychological cortical function in general (Van Honk and Schutter 2004; Rusconi et al. 2005; Andres et al. 2011; Kadosh et al. 2007). For detection of language and other higher cortical functions, rTMS is used, which inhibits cortical function rather than eliciting MEPs. Most of these neuroimaging studies on arithmetic processing used single-step arithmetic problems (e.g., 3 + 4, 4 − 3, 4 × 3); (Arsalidou and Taylor 2011). One disadvantage of non-navigated TMS is its relatively low spatial resolution. This shortcoming can now be faced by the introduction of rTMS (Ruohonen and Ilmoniemi 1999; Sollmann et al. 2013a). It allows the researcher a more precise way to locate cortical function and was furthermore already shown to improve outcome for the resection of motor eloquently located intracranial lesions (Frey et al. 2014; Krieg et al. 2014a). Additionally, there was a good correlation with intraoperative direct cortical stimulation (DCS) revealed for motor and language function (Krieg et al. 2014a, 2012b; Picht et al. 2013; Tarapore et al. 2012).
In order to evaluate rTMS for detecting cortical regions of calculation function and to establish this technique for future preoperative mapping in brain tumor patients, this study aims at three issues:
-
1.
The feasibility of locating cortical calculation function via rTMS per se.
-
2.
Comparing the four arithmetic types of calculation in terms of their spatial distribution.
-
3.
Investigating differences in arithmetic processing between the two hemispheres and different cortical regions.
Thus, this study, with its solid amount of data concerning the calculation-mapping of both whole hemispheres, represents the first step of establishing this technique in healthy volunteers.
Materials and methods
Study subjects
Twenty healthy and purely right-handed (according to the Edinburgh Handedness Inventory) volunteers without cerebral pathology were enrolled. Eleven volunteers were female, and nine were male. The median age was 25.0 ± 1.7 years (range 22.0–29.5 years, Table 1). No volunteer was under any kind of medication. Inclusion criteria of the participants were German as mother tongue, right-handedness, written informed consent, and age above 18 years. Exclusion criteria were having a cardiac pacemaker, a cochlear implant (Rossi et al. 2009), a second mother tongue, ambidexterity or left-handedness, deep brain stimulation, any previous neurological medical history, developmental language deficits, previous seizures, pathological findings on cranial MRI, or any other neurological impairment.
Study design
The subjects underwent two rTMS mapping sessions. Both hemispheres were investigated in a randomized way with a 13–16-day interval between the mappings of each hemisphere. All mappings were conducted by the first author, who underwent rTMS training on several healthy volunteers as well as manufacturer certification prior to this study to rule out learning curve effects.
Ethics
This current study was conducted with the consent of the local ethics committee of our university (Ethics Committee Registration Number 5811/13) and in accordance with the Declaration of Helsinki. Written informed consent was provided by all volunteers prior to MRI scans.
MRI acquisition
All volunteers underwent MR imaging prior to the first rTMS mapping. MRI was performed on a 3 tesla MR scanner combined with an 8-channel phased array head coil (Achieva 3 T, Philips Medical Systems, The Netherlands B.V.). For anatomical co-registration, the scanning protocol consisted of a 3D gradient echo sequence (TR/TE 9/4 ms, 1 mm2 isovoxel covering the whole head, 6 min 58 s acquisition time) without intravenous contrast administration. Subsequently, the 3D dataset was transferred to the rTMS system using the DICOM standard.
rTMS mapping
Experimental setup
The following experimental setup was applied to all volunteers in an equal manner. Each participant underwent rTMS mapping twice with the Nexstim eXimia NBS system version 4.3 and a NexSpeech® module (Nexstim Oy, Helsinki, Finland), including a magnetic stimulator with a biphasic figure-eight TMS coil and a radius of 50 mm, which was connected to an infrared tracking system (Polaris Spectra, Waterloo, ON, Canada); (Picht et al. 2009, 2013; Krieg et al. 2013; Tarapore et al. 2013). The 3D T1-weighted MRI of the volunteer was used as an anatomical reference to visualize the analogue brain area receiving rTMS pulses by the stereotactic infrared tracking system to track the coil position (Ruohonen and Ilmoniemi 1999; Ilmoniemi et al. 1999). At first, the resting motor threshold (rMT) was determined by motor mapping of the contralateral cortical representation of the hand area on each hemisphere (abductor pollicis brevis and abductor digiti minimi muscle), as outlined in previous reports (Sollmann et al. 2013a; Krieg et al. 2012b). The rTMS calculation mapping was performed afterwards, using 100 % rMT applied with a frequency of 5 Hz and 10 pulses. Thus, each train lasted 1.8 s.
Calculation task
Twenty simple arithmetic tasks for each calculation subtype (addition, subtraction, division, and multiplication) were used, such as “5 + 6,” “6 − 2,” “1 × 4,” or “9:3,” for example. The numbers were presented in black font on a white background on a 15-inch screen, and the answers had to be given in one single number in German. All tasks were displayed in a randomized way on a screen 60 cm in front of the volunteer.
Calculation mapping procedure
Prior to each rTMS mapping, the volunteers performed a baseline, where they were instructed to solve the 80 different calculation tasks without any rTMS stimuli or sham stimulation. The answers to the randomly presented tasks on the screen had to be given accurately, as fast as possible, and without any incorrect pronunciation or stuttering. All falsely calculated, misnamed, or wrongly pronounced tasks were counted and excluded from the stimulus sequence, as outlined in Table 1. Each picture of the calculation task was displayed for 700 ms with a fixed inter-picture interval (IPI) of 3 s and 0 ms picture-to-trigger interval (Baptiste and Fehlings 2006), which is the time from displaying the task on the screen to the onset of the rTMS pulse train. Exactly the same modalities were used for the baseline performance as well as for the calculation mapping procedure. During mapping, the volunteers had to solve the arithmetic problems in German while rTMS pulses were applied. The whole mapping session, including the baseline performance without any stimulation, was video recorded for objective post hoc analysis (Lioumis et al. 2012). Thus, every answer could be directly compared in terms of accuracy and speed with the baseline for precise analysis and documentation. Local rTMS-induced pain in temporal brain regions and the remaining hemisphere (convexity) was evaluated via a visual analogue scale (VAS). The subjects were asked to rate the pain from 0 (no pain) to 10 points (maximum imaginable pain) (Table 1).
Stimulated points
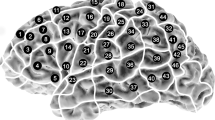
The subjects underwent rTMS mapping of each hemisphere on 52 previously determined spots, which were distributed over the whole hemispheres and anatomically identified in every subject (Fig. 1). This is a specialty regarding the fact that most TMS studies do not stimulate both hemispheres on such a large amount of cortical spots. These spots were tagged on the 3D MRI prior to the first mapping in each participant. The locations of all 52 spots were chosen based on the experience of various rTMS researchers depending on their accessibility and the maximum discomfort their stimulation could cause (Krieg et al. 2013, 2014b; Picht et al. 2013). Thus, the orbital part of the inferior frontal gyrus (orIFG), polar superior (polSTG) and middle temporal gyruses (MTG), anterior middle temporal gyrus (aMTG) and polar superior (polSFG), and polar middle (polMFG) and polar inferior frontal gyruses (polIFG) were not investigated. Because stimulation intensity decreases below 50 V/m (increasing distance between skin and brain), the inferior temporal gyrus (ITG) was excluded as well (Krieg et al. 2013). The localization of the spots was described by the cortical parcellation system (CPS) from Corina et al. (2005). Each of the 52 spots was stimulated three times. Between the visual display of two images, the stimulation coil was moved to the next spot, to reach maximum field induction. The coil was placed tangential to the skull in strict anterior–posterior field orientation (Lioumis et al. 2012; Wassermann et al. 1999; Epstein et al. 1996). Within the brain region of interest, the electric field strength ranged between 55 and 80 V/m for all volunteers.
Mapping template with cortical parcellation system. Mapping template with 52 previously determined cortical spots over the left and right hemispheres. Each spot was stimulated with 3 stimulation trains using 5 Hz/10 pulses. The left and right hemispheres were investigated in a randomized manner with an interval of 2 weeks between mappings. The cortical parcellation system (CPS) and its anatomical areas are outlined as described in Corina et al. (2005). The abbreviations are explained in Table 2
Video data analysis
The analysis of the recorded videos of all rTMS sessions was performed as described in previous publications (Sollmann et al. 2013b; Lioumis et al. 2012; Krieg et al. 2014b; Tarapore et al. 2013). In every case, the investigator was blinded to stimulated cortical spots and previous results. The baseline performance was analyzed first, followed by the task performance during stimulation. Any impairment of calculation, like incorrectly given answers, hesitations, or language deficits, was compared to the baseline. Therefore, the rTMS-induced errors were categorized into the following error types:
-
No-response errors (no answer at all during stimulation)
-
Hesitations (delayed answer during stimulation)
-
Calculation errors
If at least one out of the three stimulations per spot evoked any error, the associated cortical region was considered as error-positive for the calculation task. Therefore, the error rates were evaluated in two different ways:
-
1.
Error rates for all errors per total number of stimulations; in this case the error rate describes the actually made mistakes or errors per specified category in percentage.
-
2.
Error rates for subjects who generated errors per all stimulated subjects.
Statistical analysis
The error rate was defined as the quotient of the number of rTMS-induced calculation errors divided by the number of calculation tasks and rTMS stimulation. For testing the distribution of various attributes, we performed a Chi square test. We compared all errors of all stimulations in the entire calculation mapping in the left versus the right hemisphere. Differences in error rates between the two hemispheres were tested using the Mann–Whitney–Wilcoxon test for multiple comparisons on ranks for independent samples for non-parametric distributions. Therefore we compared the error rates for all errors of all subjects separated for each type of arithmetic operations (addition, subtraction, multiplication, division) in the left versus the right hemisphere. Furthermore, we tested the differences in error rates regarding the four basic types of arithmetic problems, using the ANOVA Kruskal–Wallis test by comparing these error rates for all errors of all subjects within one hemisphere. All results are presented as odds ratios (OR) with 95 % confidence intervals (CI) (GraphPad Prism 6.04, La Jolla, CA, USA). The level of significance was 0.05 (two-sided) for each statistical test.
Results
The mapping procedure was generally well tolerated by all volunteers. The mean levels of discomfort during stimulation according to the VAS were 2.5 ± 1.7 (range 0–7) for the convexity and 4.9 ± 1.7 (range 1–8) for the temporal region due to temporal muscle activation (Table 1). The mean rMT for the mapping sessions was 33.2 ± 4.9 % (range 25–42 %) of the maximum stimulator output. Errors during baseline performance ranged from 0 to 7 out of 80 presented tasks, with a median of 77 (CI 0.95–1.0) for correctly given answers. Table 1 provides an overview of subject and mapping characteristics.
All calculation tasks
Table 3 provides an overview of the different types of errors observed in all subjects including no-response, hesitation, division, subtraction, addition, and multiplication errors and error rates. Additionally, Table 4 illustrates all generated errors regarding the 52 stimulation spots. Table 5 shows the median error rates for all errors of all subjects regarding the CPS regions and lobes. Moreover, Table 6 shows the median error rates for all errors of all stimulations concerning the CPS regions.
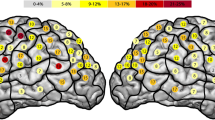
Comparing all error rates of the calculation tasks and the four types of arithmetic problems, we observed the highest error rate (35 %) for all errors of the stimulations in the ventral precentral gyrus (vPrG; stimulation point 23) of the right hemisphere, and in the left hemisphere’s middle middle frontal gyrus (mMFG; stimulation point 13) and angular gyrus (anG; stimulation point 46) with a 37 % error rate each (Table 4).
Concerning the number of subjects with actual errors at each specific spot, Figs. 2, 7a and Table 3 illustrate all errors for the total number of subjects with a high error rate of 80 % of participants in the right vPrG (stimulation point 23) and 65 % in the left mMFG (stimulation points 12 and 13) and anG (stimulation point 45). In total, the whole right hemisphere generated an error rate of 44 % for all errors per subjects, and concerning the entire left hemisphere we observed a 43 % error rate. Regarding all generated errors of all stimulations in the left versus the right hemisphere, we could not show any statistical significance (p = 0.570). Nevertheless, Fig. 7a graphically illustrates the slightly higher error frequency in the right hemisphere.
Error rate in entire calculation task. This is the error rate for all errors of all subjects generated during the entire calculation task. Gray represents the lowest observed error rate, and dark red represents the highest. The highest error rates were observed in the right ventral precentral gyrus (vPrG; 80 %) and in the left middle middle frontal gyrus (mMFG; 65 %) and angular gyrus (anG; 65 %)
Division task
Errors per stimulations
The highest error rate for all errors of all stimulations was located in the right hemisphere’s vPrG (13 %; stimulation point 23) and in the left hemisphere’s posterior superior temporal gyrus (pSTG; 17 %; stimulation point 38) (Table 4). Table 6 moreover provides an overview of the highest median error rate of 11 % concerning the CPS regions and all errors of all subjects in the left triangular inferior frontal gyrus (Tables 2, 6).
Subjects with errors per stimulated subjects
Out of all stimulations during division, error rates of 40 % occurred in the right vPrG (stimulation point 23) and 45 % in the left mMFG (stimulation point 12) for all errors of all subjects (Figs. 3, 7b; Table 3). The whole right hemisphere generated an error rate of 18 % for all errors per subjects, and the total error rate for the left hemisphere was 18 % as well. Comparing all errors in the left and right hemispheres concerning the error rate of all subjects, there was no statistical significance observed (p = 0.867).
Subtraction task
Errors per stimulations
During this task, we located the highest error rate for all errors of all stimulations in the right anG (13 %; stimulation point 43), after this the left mMFG (stimulation point 13) and the left anG (10 %; stimulation point 42) (Table 4). We furthermore detected the highest median error rates regarding the CPS regions of 11 % for all errors of all stimulations summarized in the right posterior supramarginal gyrus (pSMG) (Tables 2, 6).
Subjects with errors per stimulated subjects
Comparing all errors of all subjects, the highest rate occurred in the right anG of 40 % (stimulation point 43), and in the left mMFG (stimulation point 13) and anG (stimulation point 42) of 30 % each (Figs. 4, 7c; Table 3). Concerning the whole hemispheres, we observed an error rate of 16 % for all errors of all subjects in the right hemisphere, as graphically displayed in Fig. 7c, and a 13 % error rate for the left hemisphere. Comparing the error rates of all errors per subjects in total, we found no statistical significance (p = 0.111).
Addition task
Errors per stimulations
We detected the highest error rate for all errors per stimulations in the right posterior middle frontal gyrus (pMFG; 8 %; stimulation point 18), the right pSTG (8 %; stimulation point 38), and the anG (8 %; stimulation point 40) (Table 4). Furthermore, the left opercular inferior frontal gyrus (opIFG; stimulation point 9) and the left anterior superior temporal gyrus (aSTG; stimulation point 24) showed error rates of 12 % each. With regard to the highest median error rates of the CPS regions, we located the highest error rates of 35 % in the left anterior superior temporal gyrus (aSTG) (Tables 2, 5, 6).
Subjects with errors per stimulated subjects
Concerning all errors of all subjects, rTMS was able to elicit an error rate of 25 % in the right pMFG (stimulation point 18), pSTG (stimulation point 38), and anG (stimulation point 40), as well as an error rate of 35 % in the left aSTG (stimulation point 24) (Figs. 5, 7d; Table 3). Comparing the hemispheres, we observed a total error rate for all errors of all subjects of 11 % for the whole right hemisphere, and 12 % for the left hemisphere. Comparing the two hemispheres and all observed errors for all subjects, there was no statistically significant difference revealed (p = 0.595).
Error rate in addition task. This is the error rate for all errors of all subjects generated in the addition task. The highest error rates are located in the right posterior middle frontal gyrus (pMFG), posterior superior temporal gyrus (pSTG) and angular gyrus (anG) (25 % each), as well as in the left anterior superior temporal gyrus (aSTG, 35 %)
Multiplication task
Errors per stimulations
The highest error rate for all errors of all stimulations was observed in the right mMFG (8 %; stimulation points 7 and 8), the right pSTG (8 %; stimulation point 38) and the right polar lateral occipital gyrus (polLOG; 8 %; stimulation point 52). In addition, the left anG (stimulation point 46) was characterized by an error rate of 15 % (Tables 2, 4, 6).
Subjects with errors per stimulated subjects
The highest error rate for all errors of all subjects was located in the right mMFG (25 %; stimulation points 7 and 8) and poILOG (25 %; stimulation point 52) as well as in the left anG (30 %; stimulation point 46) (Figs. 6, 7e; Table 3). When further comparing both hemispheres, a corresponding error rate of 10 % for each of both hemispheres was observed due to rTMS (p = 0.803).
Error rate in multiplication task. This is the error rate for all errors per subjects generated in the multiplication task. We observed the highest error rates in the right middle middle frontal gyrus (mMFG; 25 %) and polar lateral occipital gyrus (poILOG; 25 %), as well as in the left angular gyrus (anG; 30 %)
Hemispheric comparison. This is the hemispheric comparison for all calculation tasks and separated in each different task for all subjects. Right hemisphere: black, left hemisphere: gray. The anatomical areas were described by Corina et al. (2005). a All errors of all subjects. b Division errors of all subjects. c Subtraction errors of all subjects. d Addition errors of all subjects. e Multiplication errors of all subjects
Differences between calculation subtypes
Overall, we could not show any statistical significance when comparing the entire observed error rates for all stimulations per stimulation point in the left versus the right hemisphere (p = 0.570). However, Fig. 7a–e graphically illustrates visual differences between the left and the right hemisphere regarding the highest observed error rates for all errors of all stimulations, although not reaching statistical significance. In summary, we observed the highest error rates for all errors (35 % per stimulations and 80 % per subjects in the vPrG, and for subtraction (13 % per stimulations and 40 % per subjects in the anG) within the right hemisphere. In contrast, the highest error rates for the division task were found in the left hemisphere (17 % per stimulations in the pSTG and 45 % per subjects in the mMFG), which is also true for the addition task (12 % per stimulations and 35 % per subjects in the aSTG) and the multiplication task (15 % per stimulations and 30 % per subjects in the anG).
In summary, the highest error rates concerning the four different arithmetic operations were observed during the division task (45 % per subjects), followed by the subtraction task (40 %), the addition task (35 %), and the multiplication task (30 %). Differences among the error rates per subjects for each type of arithmetic operations for the left and right hemisphere were not statistically significant. However, the subtraction task showed a trend (left vs. right: subtraction: p = 0.111, addition: p = 0.595, multiplication: p = 0.804, division: p = 0.867). Comparing the different types of arithmetic operations, we were able to point out a statistically significant difference within one hemisphere of more division and fewer multiplication mistakes for all division and multiplication errors of all subjects (left: p = 0.009, right: p = 0.011), and additionally more division and fewer addition mistakes for all division and addition errors of all subjects (right: p = 0.017, left: p = 0.022).
Discussion
Besides our intention to investigate the feasibility of navigated rTMS mapping for preoperative mapping of calculation function, the computational function and dyscalculia were examined earlier by the preceding method of non-navigated TMS (Kadosh et al. 2007; Rusconi et al. 2005; Andres et al. 2011), intraoperative functional mapping (Della Puppa et al. 2013; Pu et al. 2011; Duffau et al. 2002), and fMRI studies (Cohen et al. 2000; Arsalidou and Taylor 2011; Benn et al. 2012; Fehr et al. 2007). Nonetheless, the new technique of rTMS has not been used to detect cortical regions of calculation until now.
Feasibility of locating cortical regions of calculation via rTMS
The central finding of the present study is that rTMS seems feasible to detect calculation as a cortical function and to distinguish between different cortical regions. The detected cortical localizations in the left parietal lobe and adjacent frontal areas are confirmed by current literature using other modalities/lesion studies (Sanai et al. 2012; Arsalidou and Taylor 2011; Kadosh et al. 2007; Pu et al. 2011). However, the examination of distinctive neuropsychological and cognitive models still remains debatable because of the multiple ways to complete the same task since more than one neural pathway is activated (Price and Friston 2002). A meta-analysis of fMRI studies showed that regions such as the middle and superior frontal gyri were activated during calculation tasks, as well (Arsalidou and Taylor 2011). Another publication presented findings on healthy subjects of bilateral intraparietal sulcus (IPS) activation during fMRI but only unilateral behavioral TMS effects in the right IPS while performing calculation tasks (Kadosh et al. 2007). Pu et al. (2011) detected brain areas within the left parietal lobe via intraoperative electrostimulation to be specific for calculation, but no specific brain sites were found within the right parietal lobe (Pu et al. 2011). Similarly, Duffau et al. (2002) showed the paramount importance of the left anG for arithmetic processing by using intraoperative electrostimulation in a patient with a left parietal lobe glioma (Duffau et al. 2002). According to Rusconi et al. (2005), rTMS over the left anG disrupted number magnitude processing in healthy volunteers (Rusconi et al. 2005). In general, the anG seems to be responsible for frequently repeated and complicated arithmetic operations. On the other hand, complicated but untrained arithmetic operations are being solved in the left intraparietal sulcus (IPS) and adjacent dorsolateral frontal areas (Delazer et al. 2003).
When further comparing the error rates of the present study, they were mostly generated outside of typical language areas, except in one case where we observed the highest error rates for all errors of all subjects in the right vPrG. This can principally be regarded as further evidence of rTMS being able to specifically detect the calculation function. During the video analysis of the calculation mapping, we tried to precisely determine whether the mistakes made were generated because of language or speech impairment or due to calculation function impairment. Subsequent to every mapping, we asked the volunteers whether they were able to calculate or speak properly during stimulation. However, we are not able to assure with certainty that the confirmed localizations (especially the vPrG) are attributable to calculation function rather than due to impairment of language function.
Comparison of the arithmetic types of calculation and their localization
Division
Our confirmed localizations are comparable to the results of Gruber et al. (2011), who detected blood oxygenation level dependent (BOLD) activation in the fMRI also in the left frontal lobe (in the left IFG and in the left ventrolateral prefrontal cortex) during division processing (Gruber et al. 2001). Additionally, it has long been known that division-related areas can become damaged such that solely division processing becomes insufficient, while addition-, subtraction-, and multiplication-related areas are spared from any impairment (Berger 1926; Lewadovsky and Stadelmann 1908).
Subtraction
In an fMRI-guided TMS study, Andres et al. (2011) identified an increased bilateral activation in the horizontal segment of the IPS (hIPS) and the posterior superior parietal lobule (PSPL) during subtraction operations (Andres et al. 2011), comparable to our results in the parietal lobe. A meta-analysis of various fMRI studies also showed subtraction-relevant areas within parietal regions, as well as in occipito-temporal visual regions and frontal and prefrontal regions (Arsalidou and Taylor 2011). According to Cohen et al. (2000), subtraction-related areas are also predominantly localized in right intraparietal regions (Cohen et al. 2000). In summary, these findings are in good accordance with the results of our study, so we can underline the importance of the right parietal lobe in arithmetic and subtraction processing. In general, subtraction-related areas of the cortex are known for being robust toward brain lesions or aphasia, in contrast to multiplication- or division-related cortical areas (Lampl et al. 1994; Pesenti et al. 1994). In particular, verbal associations and language function seem to be less important for solving subtraction tasks than for solving multiplication tasks.
Addition
Kawashima et al. (2004) showed in an fMRI study activation in the right frontal cortex as well as in bilateral inferior temporal cortex areas during addition tasks (Kawashima et al. 2004), whereas Arsalidou and Taylor (2011) described relevant cortical regions in visual areas, parietal areas, frontal, and prefrontal areas, and they also defined the left hemisphere as the dominant one for solving addition problems (Arsalidou and Taylor 2011). Consequently, the dominance of the left hemisphere for solving addition problems is well in accordance with our results. On the other hand, there are controversies about the exact localization for addition-related cortical areas.
Multiplication
An fMRI meta-analysis also reported on important regions for multiplication in the parietal lobe (Arsalidou and Taylor 2011). According to Kawashima et al. (2004), the right frontal cortex is also important for multiplication processing (Kawashima et al. 2004). As already mentioned, a recent work by Andres et al. (2011) presented an increased bilaterally activation in the hIPS and the PSPL during multiplication tasks (Andres et al. 2011). They furthermore proceeded to say that “under the assumption that multiplication problems are retrieved from memory without accessing the magnitude of the numbers, they should remain unaffected when TMS is applied over the hIPS (Andres et al. 2011). Similarly, Campbell and Graham (1985), who claimed that there is an associative network evolved in which multiplication problem operands became linked to specific sets of candidate answers, mostly compatible with memory function (Campbell and Graham 1985). However, in summary, the current literature as well as our results confirm, on the one hand, the localization of multiplication-related cortical areas in the left parietal lobe and, on the other hand, their capability to be influenced by rTMS. Another theory is about the bilateral involvement of multiplication processing (Andres et al. 2011; Arsalidou and Taylor 2011). We also detected bilateral processing in this case, which was more exact in the left parietal lobe but less in the right parietal lobe than in the right frontal and occipital lobe. It should, however, be noted in the context that we examined only healthy volunteers without any lesions. One possibility is that these lesions were the reason for a shift of calculation-related areas.
Differences in arithmetic processing between the two hemispheres and different cortical regions
According to Dehaene et al. (2003), intraparietal areas on both hemispheres are associated with core quantity organization, and the left anG is associated with verbal processing of numbers (Dehaene et al. 2003). This theory would be in accordance with our confirmed localizations in the left parietal lobe regarding calculation function, especially subtraction and multiplication processing. The author furthermore suggests that the left hemisphere’s frontal gyrus and anG are underlying language-dependent coding of exact arithmetic facts with verbal associations, although they do not include classical language areas (Dehaene et al. 1999). This is also in the line with our located highest error rates concerning exact addition processing in the left hemisphere´s frontal gyrus and anterior superior temporal gyrus. This might be another evidence for the spatial localization and coexistence of arithmetic processing and language function in the left frontal lobe. Yet Warrington (1982) observed impairment of arithmetic processing after left parietal lesions without aphasia (Warrington 1982). This fact underlines the possibility of independent calculation areas in the left parietal lobe without interaction with speech-related areas but contradicts the theses of Dehaene (1999, 2003). Arsalidou and Taylor (2011) suggested that the left hemisphere is dominant for solving addition problems (Arsalidou and Taylor 2011), which might again underline the theory that the right hemisphere operates in simple arithmetic problems and estimations, whereas the left hemisphere precisely solves complicated arithmetic problems. In fact, the highest error rates for all errors of all subjects for the division, addition, and multiplication tasks were observed in the left hemisphere in the present study. For subtraction processing, Arsalidou and Taylor (2011) suggested a mixed hemispheric dominance, which again corresponds well with our results (Arsalidou and Taylor 2011). Multiplication processing, on the other hand, was almost and unexpectedly right-lateralized, in contrast to our observed localizations. Kawashima et al. (2004) also showed activation of the left middle frontal, bilateral inferior temporal, and bilateral lateral occipital cortices during addition, subtraction, and multiplication tasks (Kawashima et al. 2004).
Feasibility of preoperative calculation mapping in brain tumor patients
According to Della Puppa et al. (2013), the right parietal cortex, especially the inferior lobule, was detected as being specifically related to multiplication or addition processing, and the right IPS was functionally specific for multiplication in patients with right-sided parietal lobule gliomas (Della Puppa et al. 2013). Sanai et al. (2012) published a paper on 119 patients with parietal lobe gliomas in both hemispheres who underwent aggressive tumor resection, and the postoperative impairment also included dyscalculia (Sanai et al. 2012).
With this in mind, the technique presented in our study might not only be feasible for neuroscientific researchers but also for neurosurgeons for preoperative mapping in brain tumor patients in order to reduce postoperative impairment like Gerstmann’s syndrome. Whether our investigated protocol is feasible in brain tumor patients has to be evaluated as an upcoming step.
Limitations
One limitation of mapping cortical calculation function via rTMS is that the modality itself is limited to the cortex. Thus, other calculation-related areas for arithmetic processing, such as the thalamus, cerebellum, or insula, remain unexamined with this technique. Yet the combination of rTMS with diffusion tensor imaging fiber tracking—as already published for motor function—might be a seminal approach (Krieg et al. 2012a; Frey et al. 2014). Moreover, although we can examine the majority of cortical regions via advanced rTMS, it cannot be applied at every cortical region due to rTMS-induced pain. Furthermore, adjacent cortical areas might also be influenced by rTMS because of their functional connectivity, although they are not directly stimulated. In addition, one confounder of this technique is that we cannot completely differentiate whether the calculation mistakes were made because of language impairment, such as within the vPrG, or impairment of visual function, or due to calculation function impairment per se. At first visual function starts processing after approximately 120 ms. The pathway of arithmetic processing then ends with language/speech function starting after 400–600 ms. In the meantime the brain among other functions solves the arithmetic problems during the stimulation. In conclusion, we cannot differentiate all these subfunctions of processing arithmetic problems properly.
As a possibility to differentiate between errors in terms of language function and actual calculation errors, we might be able to use a language control task in future studies. In this way, the volunteers would not only be examined for calculation function but also with regard to their language related areas. The difference between both tasks might correspond much better with actually calculation-relevant areas.
Further on, hesitation errors were only compared to baseline testing, so there was no exact reaction time measurement to evaluate these errors. Yet these last two limitations might be reduced during further refinement of this technique.
Moreover, the presented technique of rTMS mapping of calculation has not been validated like motor or language mapping. In other words, there is no gold standard technique to compare or correlate this study. Ideally, direct electrical stimulation of the cortex could be used to further validate the technique. Yet, since this study is the first to investigate the feasibility of this new technique, we neither examined patients nor did we verify our non-invasive findings via intraoperative mapping in this current stage.
Another limitation of this pilot study is the number of examined subjects. An objective for future studies is to increase the number of subjects in order to potentially reach a higher statistical significance and to implement a test–retest analysis.
It is also not clear yet, whether surgery aimed at some of these areas would actually result in a detectable and persistent clinical deficit. For example, surgery is commonly performed for tumors in the left middle frontal gyrus, and patients do not seem to exhibit significant deficits.
Conclusion
The present study demonstrates the feasibility of locating the calculation function non-invasively via rTMS because the observed cortical localizations are well in accordance with the current literature based on other modalities or lesion studies.
Overall, the high importance of the parietal lobe, especially the anG, and adjacent frontal areas for arithmetic processing were confirmed with this technique.
Abbreviations
- aMTG:
-
Anterior middle temporal gyrus
- anG:
-
Angular gyrus
- ANOVA:
-
Analysis of variance
- aSTG:
-
Anterior superior temporal gyrus
- BOLD-fMRI:
-
Blood oxygen level dependent functional magnetic resonance imaging
- CI:
-
Confidence intervals
- CPS:
-
Cortical parcellation system
- DCS:
-
Direct cortical stimulation
- EMG:
-
Electromyogram
- fMRI:
-
Functional magnetic resonance imaging
- hIPS:
-
Horizontal segment of the IPS
- IPI:
-
Inter-picture interval
- IPS:
-
Intraparietal sulcus
- ITG:
-
Inferior temporal gyrus
- MEP:
-
Motor evoked potential
- MFG:
-
Middle frontal gyrus
- mMFG:
-
Middle middle frontal gyrus
- nTMS:
-
Navigated transcranial magnetic stimulation
- oplFG:
-
Opercular inferior frontal gyrus
- OR:
-
Odds ratio
- orIFG:
-
Orbital part of the inferior frontal gyrus
- pMFG:
-
Posterior middle frontal gyrus
- pMTG:
-
Posterior middle temporal gyrus
- polIFG:
-
Polar inferior frontal gyrus
- polMFG:
-
Polar middle frontal gyrus
- polSFG:
-
Polar superior frontal gyrus
- polSTG:
-
Polar superior temporal gyrus
- PSPL:
-
Posterior superior parietal lobule
- pSTG:
-
Posterior superior temporal gyrus
- PTI:
-
Picture-to-trigger interval
- rMT:
-
Resting motor threshold
- rTMS:
-
Repetitive navigated transcranial magnetic stimulation
- TMS:
-
Transcranial magnetic stimulation
- VAS:
-
Visual analogue scale
- vPrG:
-
Ventral precentral gyrus
References
Andres M, Pelgrims B, Michaux N, Olivier E, Pesenti M (2011) Role of distinct parietal areas in arithmetic: an fMRI-guided TMS study. Neuroimage 54(4):3048–3056. doi:10.1016/j.neuroimage.2010.11.009
Arsalidou M, Taylor MJ (2011) Is 2 + 2 = 4? Meta-analyses of brain areas needed for numbers and calculations. Neuroimage 54(3):2382–2393. doi:10.1016/j.neuroimage.2010.10.009 S1053-8119(10)01301-7 [pii]
Baptiste DC, Fehlings MG (2006) Pathophysiology of cervical myelopathy. Spine J 6(6 Suppl):190S–197S. doi:10.1016/j.spinee.2006.04.024 S1529-9430(06)00222-1 [pii]
Barker AT, Jalinous R, Freeston IL (1985) Non-invasive magnetic stimulation of human motor cortex. Lancet 1(8437):1106–1107 S0140-6736(85)92413-4 [pii]
Benn Y, Zheng Y, Wilkinson ID, Siegal M, Varley R (2012) Language in calculation: a core mechanism? Neuropsychologia 50(1):1–10. doi:10.1016/j.neuropsychologia.2011.09.045
Berger H (1926) Über Rechenstörungen bei Herderkrankungen des Großhirns. Archiv f Psychiatrie 78(1):238–263. doi:10.1007/BF01996620
Campbell JI, Graham DJ (1985) Mental multiplication skill: structure, process, and acquisition. Can J Psychol/Revue canadienne de psychologie 39(2):338–366. doi:10.1037/h0080065
Cohen L, Dehaene S, Chochon F, Lehéricy S, Naccache L (2000) Language and calculation within the parietal lobe: a combined cognitive, anatomical and fMRI study. Neuropsychologia 38(10):1426–1440. doi:10.1016/S0028-3932(00)00038-5
Corina DP, Gibson EK, Martin R, Poliakov A, Brinkley J, Ojemann GA (2005) Dissociation of action and object naming: evidence from cortical stimulation mapping. Hum Brain Mapp 24(1):1–10. doi:10.1002/hbm.20063
Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S (1999) Sources of mathematical thinking: behavioral and brain-imaging evidence. Science 284(5416):970–974
Dehaene S, Piazza M, Pinel P, Cohen L (2003) Three parietal circuits for number processing. Cognit Neuropsychol 20:487–506
Delazer M, Girelli M, Grana´ A, Domahs F (2003) Number processing and calculation—normative data from healthy adults. Clin Neuropsychol 17:331–350
Della Puppa A, De Pellegrin S, d’Avella E, Gioffre G, Munari M, Saladini M, Salillas E, Scienza R, Semenza C (2013) Right parietal cortex and calculation processing: intraoperative functional mapping of multiplication and addition in patients affected by a brain tumor. J Neurosurg 119(5):1107–1111. doi:10.3171/2013.6.JNS122445
Duffau H, Denvil D, Lopes M, Gasparini F, Capelle L, Van Effenterre R (2002) Intraoperative mapping of the cortical areas involved in multiplication and subtraction: an electrostimulation study in a patient with left parietal glioma. J Neurol Neurosurg Psychiatry 73:733–738. doi:10.1136/jnnp.73.6.733
Epstein CM (1998) Transcranial magnetic stimulation: language function. J Clin Neurophysiol 15(4):325–332
Epstein CM, Lah JJ, Meador K, Weissman JD, Gaitan LE, Dihenia B (1996) Optimum stimulus parameters for lateralized suppression of speech with magnetic brain stimulation. Neurology 47(6):1590–1593
Fehr T, Code C, Herrmann M (2007) Common brain regions underlying different arithmetic operations as revealed by conjunct fMRI-BOLD activation. Brain Res 1172:93–102. doi:10.1016/j.brainres.2007.07.043 S0006-8993(07)01745-3 [pii]
Frey D, Schilt S, Strack V, Zdunczyk A, Rosler J, Niraula B, Vajkoczy P, Picht T (2014) Navigated transcranial magnetic stimulation improves the treatment outcome in patients with brain tumors in motor eloquent locations. Neuro Oncol 16(10):1365–1372. doi:10.1093/neuonc/nou110
Gruber O, Indefrey P, Steinmetz H, Kleinschmidt A (2001) Dissociating neural correlates of cognitive components in mental calculation. Cereb Cortex 11(4):350–359. doi:10.1093/cercor/11.4.350
Ilmoniemi RJ, Ruohonen J, Karhu J (1999) Transcranial magnetic stimulation-a new tool for functional imaging of the brain. Crit Rev Biomed Eng 27(3–5):241–284
Kadosh RC, Kadosh KC, Schuhmann T, Kaas TA, Goebel R, Henik A, Sack AT (2007) Virtual dyscalculia induced by parietal-lobe TMS impairs automatic magnitude processing. Curr Biol 17(8):689–693. doi:10.1016/j.cub.2007.02.056
Kawashima R, Taira M, Okita K, Inoue K, Tajima N, Yoshida H, Sasaki T, Sugiura M, Watanabe J, Fukuda H (2004) A functional MRI study of simple arithmetic—a comparison between children and adults. Brain Res Cognit Brain Res 18(3):227–233
Krieg SM, Buchmann NH, Gempt J, Shiban E, Meyer B, Ringel F (2012a) Diffusion tensor imaging fiber tracking using navigated brain stimulation—a feasibility study. Acta Neurochir (Wien) 154(3):555–563. doi:10.1007/s00701-011-1255-3
Krieg SM, Shiban E, Buchmann N, Gempt J, Foerschler A, Meyer B, Ringel F (2012b) Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas. J Neurosurg 116(5):994–1001. doi:10.3171/2011.12.JNS111524
Krieg SM, Sollmann N, Hauck T, Ille S, Foerschler A, Meyer B, Ringel F (2013) Functional language shift to the right hemisphere in patients with language-eloquent brain tumors. PLoS One 8(9):e75403. doi:10.1371/journal.pone.0075403
Krieg SM, Sabih J, Bulubasova L, Obermueller T, Negwer C, Janssen I, Shiban E, Meyer B, Ringel F (2014a) Preoperative motor mapping by navigated transcranial magnetic brain stimulation improves outcome for motor eloquent lesions. Neuro-oncology. doi:10.1093/neuonc/nou007
Krieg SM, Tarapore PE, Picht T, Tanigawa N, Houde J, Sollmann N, Meyer B, Vajkoczy P, Berger MS, Ringel F, Nagarajan S (2014b) Optimal timing of pulse onset for language mapping with navigated repetitive transcranial magnetic stimulation. Neuroimage 100:219–236. doi:10.1016/j.neuroimage.2014.06.016
Lampl Y, Eshel Y, Gilad R, Sarova-Pinhas I (1994) Selective acalculia with sparing of the subtraction process in a patient with left parieto-temporal hemorrhage. Neurology 44:1759–1761
Lewadovsky M, Stadelmann E (1908) Über einen bemerkenswerten Fall von Hirnblutung und über Rechenstörungen bei Herderkrankungen des Gehirns. Zeitschrift für Neurologie und Psychiatrie 2:249–265
Lioumis P, Zhdanov A, Makela N, Lehtinen H, Wilenius J, Neuvonen T, Hannula H, Deletis V, Picht T, Makela JP (2012) A novel approach for documenting naming errors induced by navigated transcranial magnetic stimulation. J Neurosci Methods 204(2):349–354. doi:10.1016/j.jneumeth.2011.11.003
McGraw P, Mathews VP, Wang Y, Phillips MD (2001) Approach to functional magnetic resonance imaging of language based on models of language organization. Neuroimag Clin N Am 11(2):343–353
Pascual-Leone A, Gates JR, Dhuna A (1991) Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology 41(5):697–702
Pesenti M, Seron X, Van der Linden M (1994) Selective impairment as evidence for mental organisation of arithmetic facts: BB, a case of preserved subtraction? Cortex 30:661–671
Picht T, Mularski S, Kuehn B, Vajkoczy P, Kombos T, Suess O (2009) Navigated transcranial magnetic stimulation for preoperative functional diagnostics in brain tumor surgery. Neurosurgery 65(6 Suppl):93–98
Picht T, Krieg SM, Sollmann N, Rosler J, Niraula B, Neuvonen T, Savolainen P, Lioumis P, Makela JP, Deletis V, Meyer B, Vajkoczy P, Ringel F (2013) A comparison of language mapping by preoperative navigated transcranial magnetic stimulation and direct cortical stimulation during awake surgery. Neurosurgery 72(5):808–819. doi:10.1227/NEU.0b013e3182889e01
Price CJ, Friston KJ (2002) Degeneracy and cognitive anatomy. Trends Cognit Sci 6(10):416–421. doi:10.1016/S1364-6613(02)01976-9
Pu S, Li YN, Wu CX, Wang YZ, Zhou XL, Jiang T (2011) Cortical areas involved in numerical processing: an intraoperative electrostimulation study. Stereotact Funct Neurosurg 89(1):42–47. doi:10.1159/000321186
Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2009) Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120(12):2008–2039. doi:10.1016/j.clinph.2009.08.016 S1388-2457(09)00519-7 [pii]
Ruohonen J, Ilmoniemi RJ (1999) Modeling of the stimulating field generation in TMS. Electroencephalogr Clin Neurophysiol Suppl 51:30–40
Rusconi E, Walsh V, Butterworth B (2005) Dexterity with numbers: rTMS over left angular gyrus disrupts finger gnosis and number processing. Neuropsychologia 43(11):1609–1624. doi:10.1016/j.neuropsychologia.2005.01.009
Sanai N, Martino J, Berger MS (2012) Morbidity profile following aggressive resection of parietal lobe gliomas. J Neurosurg 116(6):1182–1186. doi:10.3171/2012.2.JNS111228
Sollmann N, Hauck T, Obermuller T, Hapfelmeier A, Meyer B, Ringel F, Krieg SM (2013a) Inter- and intraobserver variability in motor mapping of the hotspot for the abductor policis brevis muscle. BMC Neurosci 14(1):94. doi:10.1186/1471-2202-14-94 1471-2202-14-94 [pii]
Sollmann N, Picht T, Makela JP, Meyer B, Ringel F, Krieg SM (2013b) Navigated transcranial magnetic stimulation for preoperative language mapping in a patient with a left frontoopercular glioblastoma. J Neurosurg 118(1):175–179. doi:10.3171/2012.9.JNS121053
Tarapore PE, Tate MC, Findlay AM, Honma SM, Mizuiri D, Berger MS, Nagarajan SS (2012) Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. J Neurosurg 117(2):354–362. doi:10.3171/2012.5.JNS112124
Tarapore PE, Findlay AM, Honma SM, Mizuiri D, Houde JF, Berger MS, Nagarajan SS (2013) Language mapping with navigated repetitive TMS: proof of technique and validation. Neuroimage 82:260–272. doi:10.1016/j.neuroimage.2013.05.018 S1053-8119(13)00512-0 [pii]
Van Honk J, Schutter DJLG (2004) Transcranial magnetic stimulation and processing of facial threats. Am J Psychiatry 161(5):928. doi:10.1176/appi.ajp.161.5.928
Warrington EK (1982) The fractionation of arithmetical skills: a single case study. Q J Exp Psychol 34(Pt 1):31–51
Wassermann EM, Blaxton TA, Hoffman EA, Berry CD, Oletsky H, Pascual-Leone A, Theodore WH (1999) Repetitive transcranial magnetic stimulation of the dominant hemisphere can disrupt visual naming in temporal lobe epilepsy patients. Neuropsychologia 37(5):537–544 S002839329800102X [pii]
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.K. is a consultant for BrainLAB AG (Feldkirchen, Germany). All other authors declare that they have no conflict of interest concerning this current study. The study was completely financed by institutional grants of the Department of Neurosurgery and the Section of Neuroradiology. The authors report no conflict of interest affecting the materials or methods used in this study or the findings specified in this paper.
Rights and permissions
About this article
Cite this article
Maurer, S., Tanigawa, N., Sollmann, N. et al. Non-invasive mapping of calculation function by repetitive navigated transcranial magnetic stimulation. Brain Struct Funct 221, 3927–3947 (2016). https://doi.org/10.1007/s00429-015-1136-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-015-1136-2