Abstract
Background
Navigated brain stimulation (NBS) is a newly evolving technique. In addition to its supposed purpose, e.g., preoperative mapping of the central region, little is known about its further use in neurosurgery. We evaluated the usefulness of diffusion tensor imaging fiber tracking (DTI-FT) based on NBS compared to conventional characterization of the seed region.
Methods
We examined 30 patients with tumors in or close to the corticospinal tract (CST) using NBS with the Nexstim eXimia system. NBS was performed for motor cortex mapping, and DTI-FT was performed by three different clinicians using BrainLAB iPlan® Cranial 3.0.1 at two time points. Number of fibers, tract volume, aberrant tracts, and proximity to the tumor were compared between the two methods.
Results
We recognized a higher number of fibers (1,298 ± 1,279 vs. 916 ± 986 fibers; p < 0.01), tract volume (23.0 ± 15.3 vs. 18.3 ± 14.0 cm3; p < 0.01), and aberrant tracts (0.6 ± 0.5 vs. 0.3 ± 0.5 aberrant tracts/tracked CST; p < 0.001) when the seed region was defined conventionally, while proximity of the tracts to the tumor did not differ. While NBS-based DTI-FT is independent of the planning clinician, conventional outlining of the seed region shows generally higher variability between investigators.
Conclusions
Conventional DTI-FT showed significant differences between the two modalities, most likely because of the more specific definition of the seed region when DTI-FT is based on NBS. Moreover, NBS-aided DTI fiber tracking is user-independent and, therefore, a method for further standardization of DTI fiber tracking.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The resection of tumors within motor-eloquent areas, particularly the precentral gyrus and the corticospinal tract (CST), is always a compromise between the extent of resection and preserving motor function.
Especially in gliomas, surgical tumor reduction has a significant impact on survival and, thus, has to be as extensive as possible [13, 25]. On the other hand, motor function has to be preserved to secure quality of life for the patient. To achieve both of these goals, neurosurgeons employ various modalities to examine, visualize, and monitor motor function and anatomy before and during the resection of such tumors.
For preoperative mapping of the rolandic region, navigated transcranial magnetic stimulation (nTMS), also known as navigated brain stimulation (NBS), has gained increasing influence in neurosurgery and neuroscience in the last 2 years [22]. Moreover, good concordance between preoperative NBS mapping and intraoperative DCS mapping during surgery was shown in our own but also in other series [9, 23].
Intraoperatively, several techniques allow the identification of CST, including neuronavigation, motor-evoked potentials (MEPs), and subcortical mapping. While electrophysiological monitoring and mapping via subcortical stimulation are the gold standard during the resection of tumors within or adjacent to the CST [5, 14, 19, 22, 25], MRI-based diffusion tensor imaging fiber tracking (DTI-FT) is a recent method used for presurgical planning and intraoperative localization of white matter tracts [6, 7, 11–13, 21]. Based on DTI and the concept of anisotropic water diffusion, specific white matter tracts such as the CST can be visualized; used for surgical planning, including three-dimensional (3D) reconstruction; and imported into the neuronavigation system [18]. Various studies have compared DTI-FT with intraoperative subcortical mapping during the surgical removal of tumors and attested to good accuracy [1, 2].
In reconstructing white matter fibers by DTI-FT, the definition of a functionally significant seed region is crucial for initializing the tracking process. Especially when tumors compromise the rolandic region, functional as well as structural neuroanatomy is considerably deranged, and the definition of seed areas can be severely hampered.
NBS provides specific information where the monosynaptic motor-evoked potential (MEP) is elicited in the precentral gyrus, which is supposed to be equivalent to the primary motor cortex. Thus, this study was designed to prospectively evaluate the value and feasibility of DTI fiber tracking based on NBS data as the seed region in patients with tumors within or close to the CST.
Methods
Patients
From May to December 2010, we performed preoperative mapping in 30 patients with tumors in or adjacent to the subcortical white matter motor tract using navigated brain stimulation.
The mean age was 57.2 ± 16.5 years (range 18.7–78.8 years); 16 patients (53%) were female, and 14 (47%) were male. Seventeen patients (57%) had a history of seizures, while 14 (47%) were on antiepileptic medication. Mild preoperative motor deficit occurred in 12 cases (40%), but there were no severe preoperative motor deficits. Seventeen tumors (57%) were in the dominant hemisphere.
Of 30 cases, there were 15 GBMs, 2 anaplastic astrocytomas, 3 diffuse WHO grade II astrocytomas, 1 WHO grade I DNET, 1 grade I meningioma, 1 AVM, and 7 metastases.
Preoperative magnetic resonance imaging
All patients underwent a preoperative MRI scan, which assessed the topographic association between the tumor and corticospinal tract or rolandic cortex, and eligible patients were recruited for this study. Informed consent was obtained.
A whole-body 3-T imaging system (Philips Achieva, Philips Electronics, Amsterdam, The Netherlands) with an eight-channel head coil was used, and 3D imaging was performed by continuous sagittal images using a T1-weighted 3D gradient echo sequence with isotropic voxels of 1 mm in length after intravenous administration of 0.1 mmol/kg of gadopentetate dimeglumine. Moreover, diffusion tensor imaging (DTI) sequences were performed with a single-shot spin echo EPI (TR/TE = 7,571/55 ms) with b values of 0 and 800 and six orthogonal diffusion directions. Using parallel imaging (sensitivity encoding factor 2), two averages of 73 contiguous 2-mm slices with a matrix of 112°-112 mm covering the whole brain were acquired within 2 min 15 s. The DTI data were then interpolated to a matrix of 224°-224, resulting in a voxel size of 0.88°-0.88°-2 mm3. In addition, data were corrected for motion artifacts by using the software installed on the scanner. For navigation, a 3D fastfield echo sequence was chosen with TR/TE = 9/4 ms and a flip angle of 8°. A sense factor of 1.5 and a turbo factor of 164 allowed the covering of the whole head in an isotropic resolution of 1 mm3.
The 3D data set was then transferred to the NBS system (Nexstim eXimia, Helsinki, Finland) and to a BrainLAB iPlan® Net server (BrainLab, Feldkirchen, Germany) using the DICOM standard.
Navigated brain stimulation
The NBS system (Nexstim eXimia 3.2, Helsinki, Finland) includes a magnetic stimulator with two biphasic figure-of-eight TMS coils with a mean radius of 50 mm. The device also includes a navigation system that orients individual 3D MR images to the patient’s head by infrared tracking (Polaris Spectra, Waterloo, Ontario, Canada), as previously reported [9, 23].
While performing stimulation with TMS, EMG was monitored continuously with four channels for the upper extremities and two channels for the lower extremities (Nexstim eXimia, Helsinki, Finland). For mapping of the upper extremities, we used electrodes on the abductor pollicis brevis (APB), abductor digit minimi (ADM), flexor carpi radialis (FCR), and biceps brachii (BCS) muscle. For mapping of the lower extremities, we used electrodes over the skin of the tibialis anterior (TA) and gastrocnemius (GCN) muscle. The reference electrode was placed at the elbow above the tendon of the BCS. Mapping of the primary motor cortex, including determination of the resting motor threshold (rMT), was performed as shown by our group and others [9, 23]. For final mapping, a stimulator output of 110% rMT was used. It started at the hot spot and proceeded over the complete precentral gyrus, the tumor, and adjacent gyri until no CMAP was detected anymore. For mapping of the lower extremities, stimulation intensity was increased until CMAP was recorded at up to 130% of the stimulator output.
In the upper as well as in the lower extremities, CMAP above 50 μV was considered significant if latency was within the commonly known latency range of MEPs for each muscle.
After finishing the mapping, every stimulation point was assessed for its correctness, and data were exported via the DICOM standard and imported into the neuronavigation planning system (BrainLAB iPlan® Net, BrainLAB AG, Feldkirchen, Germany).
Neuronavigation and fiber tracking
NBS mapping was imported to the neuronavigation planning system (BrainLAB iPlan® Cranial 3.0.1, BrainLAB AG, Feldkirchen, Germany) and fused with continuous sagittal images of the T1-weighted 3D gradient echo sequence, T2 FLAIR, and DTI data. The white matter tracts were computed from the DTI data set as previously described using BrainLAB iPlan® Cranial 3.0.1 [20]. Seeding was performed using two regions of interest (ROI) for each fiber tracking. One ROI was set in the ipsilateral brain stem at the level of the tentorium; the second ROI was placed over the ipsilateral primary motor cortex.
Positioning of the ROI within the primary motor cortex was performed in two different ways. Primarily, the seed region was traditionally outlined by the investigator according to anatomical landmarks, e.g., the hand knob, on multiple axial slices, creating a 3D volume of the precentral gyrus as ROI. Second, the seed region of the primary motor cortex was generated from the NBS points of positive eliciting of MEPs as described above. To do so, an additional rim of two millimeters was calculated for each positive stimulation point, and the whole volume was defined as ROI.
The fractional anisotropy (FA) threshold and minimum fiber length were individually defined for each patient, remained constant for each patient, and were all below 0.2 and around 100 mm, respectively. The iteration was started and continued until certain stop criteria, such as FA value and fiber angulation (>30°), were reached. Finally, tracked white matter fibers were included as an object in the 3D T1 anatomic MR data set; i.e., fibers were enveloped within one contour.
Fiber tracking using the free hand and NBS seed regions was performed by three different investigators using BrainLAB iPlan® Cranial 3.0.1 (BrainLAB AG, Feldkirchen, Germany) at two different time points. The number of tracked fibers, CST volume, aberrant tracts, and proximity of the tracts to the tumor were compared for the two different seed regions and three observers, and intra- and interobserver differences were evaluated. Proximity of the tracts to the tumor was evaluated on axial MRI slices by BrainLAB iPlan® Cranial 3.0.1 (BrainLAB AG, Feldkirchen, Germany). Depending on the kind of lesion, gadolinium-enhanced T1-weighted or contrast-free T2-weighted sequences were used.
Ethical standard
The study was conducted in accordance with ethical standards of the Technical University of Munich, the local ethics committee (registration no.: 2793/10), and the Declaration of Helsinki.
Statistical analysis
Differences between groups were tested by the Kruskall-Wallis test for nonparametric one-way analysis of variance (ANOVA), followed by Dunn's test or the Student-Newman-Keuls test as a post hoc test. Differences between two groups were tested using the Mann-Whitney-Wilcoxon test for multiple comparisons on the ranks for independent samples. All results are presented as mean ± standard deviation (SD) (SigmaStat 3.5, Jandel Scientific, Erkrath, Germany); p < 0.05 was considered significant. The variability of the different methods was evaluated and visualized by a Bland-Altman plot. The limits of agreement during Bland-Altman analysis were the average difference ± 1.96 standard deviation of the difference [3].
Results
From May to December 2010, 30 patients with tumors in or adjacent to the precentral gyrus as well as in the subcortical white matter motor tract were examined by NBS.
NBS mapping
NBS was performed successfully in all cases. One patient experienced NBS as unpleasant, but none as painful. All patients underwent DTI studies during preoperative MRI for neuronavigation. Therefore, DTI-FT was possible in all patients.
Differences from standard fiber tracking
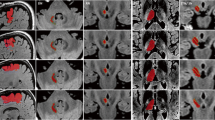
When comparing DTI-FT based on NBS with conventional tracking, it was much easier to recognize the precentral gyrus, and the calculated fiber tracts seemed more compact (Figs. 1 and 2). Therefore, we tried to find parameters that can be quantified to find measureable differences.
When using NBS as the seed region for DTI-FT, we observed a significantly smaller number of fibers within the tracked CST compared to standard tracking (NBS: 916 ± 986 fibers; standard: 1,298 ± 1,279 fibers; p < 0.01; Fig. 3).
By using navigated brain stimulation (NBS) as the seed region for diffusion tensor imaging fiber tracking (DTI-FT), we observe a significantly smaller number of fibers within the tracked corticospinal tract (CST) compared to standard tracking (NBS: 916.0 ± 986.0 fibers; standard: 1,297.9 ± 1,278.7 fibers; p < 0.01)
Furthermore, we were not able to detect any differences in the proximity of the calculated CST to the tumor (NBS: 5.0 ± 5.7 mm; standard: 6.0 ± 6.9 mm). However, there was a greater variability when using the standard definition of seed regions.
Moreover, the mean volume of the calculated CST was significantly greater for standard tracking (NBS: 18.3 ± 14.0 cm3; standard: 23.0 ± 15.3 cm3; p < 0.01), and it had greater variability and spread than DTI-FT based on NBS (Fig. 4).
We also detected significantly fewer aberrant tracts differing from the CST when using NBS data as the seed region (NBS: 0.3 ± 0.5 aberrant tracts/tracked CST; standard: 0.6 ± 0.5 aberrant tracts/tracked CST; p < 0.001).
Intraobserver differences
In both modalities, we were not able to show any significant differences between the two measurements of each observer for any examined item (data not shown). This comparison between the different observers proves the good validity of the method in repeated testing.
Interobserver differences
Figure 5 shows the interobserver variability of both methods by presenting the difference of each measurement in the mean value against the measured values themselves using a Bland-Altman plot [3]. When comparing NBS and the conventional definition of the ROI, we observed less interobserver variability in the number of tracked fibers (Fig. 5 a + b), distance to the tumor (Fig. 5 c + d), and CST volume (Fig. 5 e + f) when DTI-FT was based on NBS data.
Variability of both methods evaluated and visualized by a Bland-Altman plot. The graph shows the difference between standard diffusion tensor imaging fiber tracking (DTI-FT) and NBS-based DTI-FT between observers in correlation to the number of fibers (a: NBS; b: standard), distance to tumor (c: NBS; d: standard), and tract volume (e: NBS; f: standard)
Moreover, the standard definition of cortical seed regions led to a greater variability of aberrant tracts between observers that reached statistical significance (*p < 0.01), while NBS-based DTI-FT not only produced fewer aberrant tracts but also failed to show statistical significance between observers (Fig. 6).
Discussion
This study assessed the influence of NBS data on the DTI-FT of the CST for motor-eloquent tumors of the brain. The improvement of DTI-FT offers the chance to verify the course of subcortical structures of the CST in relation to brain lesions [10, 18].
In earlier evaluations, the localization of DTI fiber tracks was related to the results of subcortical mapping with a comparatively good concordance to detect CST fibers [1, 2].
From a technical point of view, the use of NBS motor mapping data for the determination of functionally crucial seed areas was easy to apply, and compatibility between the Nexstim eXimia 3.2 and iPlan® Cranial 3.0.1 using iPlan® Net was given by the DICOM standard and remained trouble-free when changing to iPlan® Cranial Unlimited (BrainLAB AG, Feldkirchen, Germany).
More fibers, larger tracts, and more aberrant fibers were calculated when using traditional definition of the cortical seed region. When tumors affect the rolandic region, traditional outlining of the primary motor cortex can be quite challenging because of mass effects and edema. This leads to a broader and, therefore, more unspecific definition of the cortical seed region when it is outlined conventionally. Thus, even tracts from outlined non-eloquent regions are included and cause a wider defined and, therefore, less specific definition of the CST.
Furthermore, tumors within the CST or the precentral gyrus can facilitate cerebral plasticity so that functionally important motor areas do not have to coincide with standard anatomical landmarks, which are also frequently hard to identify [8, 15, 17, 24]. Due to this fact, we should be aware that only NBS data and not anatomical landmarks can reliably detect the location of functionally crucial motor regions prior to the operation. As our described technique is based on functional anatomy, it is supposed to provide more precise white matter fiber reconstruction as well.
The course of fiber tracking from cortex to midbrain or vice versa has an influence on the detection of fibers [2]. Thus, this study used the same protocol for every mapping.
Concerning confounding factors such as the problem of crossing fibers [2], fibers of the superior longitudinal fasciculus cross the CST fibers and, therefore, hamper the identification of a primary eigenvector of a voxel and thus the tracking of fibers. Fibers adjacent to tumor margins or tumor edema are also vulnerable to false-negative results due to low anisotropy [2]. This can make DTI-FT inadequate and thus unreliable.
Therefore, the selectivity and specificity of DTI fiber tracking are weakened, and the significance of the tracked fibers in the vicinity of the future resection borders remains unclear. DTI-FT can, therefore, only provide additional information about intraoperative neuromonitoring, such as by defining the initial position for subcortical mapping, and thus might reduce the time required for surgery, as reported previously [26].
Image-guided resection was not able to show a positive influence on the extent of resection or outcome in the past [16, 26], but it was shown to be helpful for the surgeon from a personal point of view in 74% of the cases and is regarded as an essential modality in selected cases [4]. Similarly, DTI-FT might increase the safety of operations in eloquent areas, which is not quantifiable as the extent of resection or neurological outcome. Therefore, it should be used in combination with intraoperative neuromonitoring as part of a multimodal approach that also includes preoperative NBS.
However, we have to be aware that in case of large volume lesions or in largely infiltrating tumors, NBS might not be able to stimulate all the fibers that form the CST because of infiltration or distortion of these fibers by tumor or edema. Therefore, the tract might appear more compact than observed with the usual tractography. These are the fibers, which are located around the tumors in standard tractography in the upper part of the tract, which seem to be missing in the NBS designed tracts.
As DTI-FT for visualization of the CST has several vulnerable points and, therefore, offers a wide range for potentially improving measures, such as increased standardization. This study demonstrates that precise and reliable NBS data can facilitate a more standardized tractography of the CST. The combination of these two techniques is promising for the preoperative evaluation of functionally essential white matter networks, and there is a high potential to broaden its application to further functional systems within the brain, such as the sensory system and speech, as well as for research on brain plasticity or development far beyond neurosurgical purposes. Nevertheless, NBS data have to be available, and we have to question whether the exclusive use of DTI-FT justifies the costs and amount of time required for NBS mapping within a clinical setup in neurosurgery. However, in combination with preoperative assessment of the rolandic region, it will be a promising expansion for the application of NBS in neurosurgery.
Conclusion
The use of NBS data for tractography of the CST makes DTI-FT almost independent of the experience of the investigator and of distortion of the central region. As already investigated for traditional CST tractography, we have to evaluate the precision of NBS-aided DTI-FT versus subcortical mapping during tumor resection. However, this study is only capable of showing the feasibility of this new method.
References
Bello L, Gambini A, Castellano A, Carrabba G, Acerbi F, Fava E, Giussani C, Cadioli M, Blasi V, Casarotti A, Papagno C, Gupta AK, Gaini S, Scotti G, Falini A (2008) Motor and language DTI Fiber Tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage 39:369–382
Berman JI, Berger MS, Chung SW, Nagarajan SS, Henry RG (2007) Accuracy of diffusion tensor magnetic resonance imaging tractography assessed using intraoperative subcortical stimulation mapping and magnetic source imaging. J Neurosurg 107:488–494
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8:135–160
Buchmann N, Gempt J, Stoffel M, Foerschler A, Meyer B, Ringel F (2011) Utility of diffusion tensor-imaged (DTI) motor fiber tracking for the resection of intracranial tumors near the corticospinal tract. Acta Neurochir (Wien) 153:68–74; discussion 74
Cedzich C, Taniguchi M, Schafer S, Schramm J (1996) Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery 38:962–970
Clark CA, Barrick TR, Murphy MM, Bell BA (2003) White matter fiber tracking in patients with space-occupying lesions of the brain: a new technique for neurosurgical planning? Neuroimage 20:1601–1608
Coenen VA, Krings T, Mayfrank L, Polin RS, Reinges MH, Thron A, Gilsbach JM (2001) Three-dimensional visualization of the pyramidal tract in a neuronavigation system during brain tumor surgery: first experiences and technical note. Neurosurgery 49:86–92; discussion 92–83
Duffau H (2006) New concepts in surgery of WHO grade II gliomas: functional brain mapping, connectionism and plasticity–a review. J Neurooncol 79:77–115
Forster MT, Hattingen E, Senft C, Gasser T, Seifert V, Szelenyi A (2011) Navigated transcranial magnetic stimulation and functional magnetic resonance imaging: advanced adjuncts in preoperative planning for central region tumors. Neurosurgery 68:1317–1324; discussion 1324–1315
Hendler T, Pianka P, Sigal M, Kafri M, Ben-Bashat D, Constantini S, Graif M, Fried I, Assaf Y (2003) Delineating gray and white matter involvement in brain lesions: three-dimensional alignment of functional magnetic resonance and diffusion-tensor imaging. J Neurosurg 99:1018–1027
Kamada K, Houkin K, Iwasaki Y, Takeuchi F, Kuriki S, Mitsumori K, Sawamura Y (2002) Rapid identification of the primary motor area by using magnetic resonance axonography. J Neurosurg 97:558–567
Kamada K, Sawamura Y, Takeuchi F, Kawaguchi H, Kuriki S, Todo T, Morita A, Masutani Y, Aoki S, Kirino T (2005) Functional identification of the primary motor area by corticospinal tractography. Neurosurgery 56:98–109; discussion 198–109
Kamada K, Sawamura Y, Takeuchi F, Kawaguchi H, Kuriki S, Todo T, Morita A, Masutani Y, Aoki S, Kirino T (2007) Functional identification of the primary motor area by corticospinal tractography. Neurosurgery 61:166–176; discussion 176–167
Kombos T, Suess O, Ciklatekerlio O, Brock M (2001) Monitoring of intraoperative motor evoked potentials to increase the safety of surgery in and around the motor cortex. J Neurosurg 95:608–614
Lehericy S, Duffau H, Cornu P, Capelle L, Pidoux B, Carpentier A, Auliac S, Clemenceau S, Sichez JP, Bitar A, Valery CA, Van Effenterre R, Faillot T, Srour A, Fohanno D, Philippon J, Le Bihan D, Marsault C (2000) Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: comparison with intraoperative stimulation in patients with brain tumors. J Neurosurg 92:589–598
Litofsky NS, Bauer AM, Kasper RS, Sullivan CM, Dabbous OH (2006) Image-guided resection of high-grade glioma: patient selection factors and outcome. Neurosurg Focus 20:E16
Martino J, Taillandier L, Moritz-Gasser S, Gatignol P, Duffau H (2009) Re-operation is a safe and effective therapeutic strategy in recurrent WHO grade II gliomas within eloquent areas. Acta Neurochir (Wien) 151:427–436
Mori S, van Zijl PC (2002) Fiber tracking: principles and strategies—a technical review. NMR Biomed 15:468–480
Neuloh G, Pechstein U, Cedzich C, Schramm J (2004) Motor evoked potential monitoring with supratentorial surgery. Neurosurgery 54:1061–1070
Nimsky C, Ganslandt O, Fahlbusch R (2006) Implementation of fiber tract navigation. Neurosurgery 58:ONS-292-303; discussion ONS-303-294
Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG, Fahlbusch R (2005) Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery 56:130–137; discussion 138
Pechstein U, Cedzich C, Nadstawek J, Schramm J (1996) Transcranial high-frequency repetitive electrical stimulation for recording myogenic motor evoked potentials with the patient under general anesthesia. Neurosurgery 39:335–343
Picht T, Schmidt S, Brandt S, Frey D, Hannula H, Neuvonen T, Karhu J, Vajkoczy P, Suess O (2011) Preoperative functional mapping for rolandic brain tumor surgery: comparison of navigated transcranial magnetic stimulation to direct cortical stimulation. Neurosurgery 69:581–588; discussion 588
Robles SG, Gatignol P, Lehericy S, Duffau H (2008) Long-term brain plasticity allowing a multistage surgical approach to World Health Organization grade II gliomas in eloquent areas. J Neurosurg 109:615–624
Taniguchi M, Cedzich C, Schramm J (1993) Modification of cortical stimulation for motor evoked potentials under general anesthesia: technical description. Neurosurgery 32:219–226
Willems PW, Taphoorn MJ, Burger H, Berkelbach van der Sprenkel JW, Tulleken CA (2006) Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J Neurosurg 104:360–368
Acknowledgement
The authors want to thank Maria Becker for her continous effort in performing all MRI studies with outstanding quality and motivation parallel to her daily routine and far beyond her duty.
Disclosure
The study was completely financed by institutional grants of the Department of Neurosurgery. The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krieg, S.M., Buchmann, N.H., Gempt, J. et al. Diffusion tensor imaging fiber tracking using navigated brain stimulation—a feasibility study. Acta Neurochir 154, 555–563 (2012). https://doi.org/10.1007/s00701-011-1255-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-011-1255-3