Abstract
The mesocorticolimbic system contains dopamine (DA)-producing neurons in the ventral tegmental area (VTA) and their projection targets, including the medial prefrontal cortex (mPFC), amygdala (AMY) and nucleus accumbens (NAc). Disruption of this system might attribute to mental illnesses. In the present study, we adopted the postweaning social isolation paradigm to model neuropsychiatric disorders and studied the functional and structural changes of the mesocorticolimbic system. After 8–9 weeks of isolation, rats exhibited hyperlocomotor activity and impaired sensorimotor gating compared to group-reared controls. However, the number of tyrosine hydroxylase-positive VTA neurons and the volume of VTA were not affected. Comparing with group-reared controls, the DA levels in the isolation-reared were not altered in the VTA, mPFC and NAc but decreased in the AMY. In the structural aspect, dendritic features of layer II/III pyramidal mPFC neurons; pyramidal neurons in the basolateral nucleus of amygdala (BLA) and medium spiny neurons in the core region of the NAc (NAcc) were examined. Interestingly, the neuronal changes were region-specific. The mPFC neurons had reduced dendritic complexity, spine density and elongated terminal branches. The BLA neurons had extensive dendritic arbors with short branches but unchanged spine density. The NAcc neurons had reduced total dendritic length but the segment length and spine density remained the same. Together, the results demonstrated the structural and functional changes in the mesocorticolimbic DA system of socially isolated rats. These changes may account for the behavioral impairments in these rats and attribute to the susceptibility to mental disorders related to schizophrenia and depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accumulating evidence supports that the mesocorticolimbic dopamine (DA) system is critical for normal brain functions (Bertolucci-D’Angio et al. 1990; Horvitz 2000; Phillips et al. 2003; Nestler and Carlezon 2006; Laviolette 2007; Young et al. 2011). In this system, dopaminergic neurons in the midbrain ventral tegmental area (VTA) project to the cortical regions like medial prefrontal cortex (mPFC) and limbic structures such as the nucleus accumbens (NAc) and amygdala (AMY) (Björklund and Dunnett 2007). Furthermore, these four brain regions are interconnected. The excitability of the projection neurons in the basolateral nucleus of amygdala (BLA) is enhanced by DA (Kröner et al. 2005). BLA neurons then send excitatory projections to the NAc and mPFC (Kelley et al. 1982; McDonald 1991; Laviolette et al. 2005) and modulate the dopaminergic innervations and functions (Phillips et al. 2003; Floresco and Tse 2007). On the other hand, the mPFC neurons regulate the activity of BLA projection neurons via inhibitory and dopaminergic mechanisms (Rosenkranz and Grace 2002). The NAc neurons receive excitatory afferents from the BLA and mPFC (Groenewegen et al. 1999) and this glutamatergic neurotransmission is regulated by DA (Floresco 2007). Functionally, the interwoven mesocorticolimbic system has been implicated as a crucial neural system in emotion- and motivation-related processing (Phillips et al. 2003). Dysfunction of this system, unfortunately, may lead to various affective and cognitive impairments, such as symptoms seen in depression and schizophrenia (Nestler and Carlezon 2006; Laviolette 2007).
The DA hypotheses for mood dysregulation and schizophrenia have long been proposed (Wise 1982; Seeman 1987). Neurochemically, altered DA levels have been reported in patients and animal models of depression and schizophrenia (Reynolds 1983; Kapur and Mann 1992; Yadid et al. 2001; Dunlop and Nemeroff 2007; Heinz and Schlagenhauf 2010; Niwa et al. 2010). Anatomically, in stress-induced depressive animals, changes of neuronal morphology in the mesocorticolimbic system are noted (Shansky and Morrison 2009; Eiland et al. 2011). In the BLA, the pyramidal neurons develop exuberant dendritic arbors in response to stress (Vyas et al. 2002). However, in the mPFC, repetitive stress causes atrophy of dendritic arbors in layer II/III pyramidal neurons (Radley et al. 2004; Cook and Wellman 2004; Liston et al. 2006; Perez-Cruz et al. 2009; Shansky et al. 2009), which is similar to the finding in a genetic schizophrenia animal model (Niwa et al. 2010). Given the mesocorticolimbic DA system plays important role in emotional and cognitive functions, we are interested in the pathogenetic alterations of this system in emotional and cognitive neuropsychiatric disorders.
Postweaning social isolation of rodents has been used as a noninvasive and nonpharmacological model that produces sequelae in brain development and functions resemble emotional and cognitive neuropsychiatric disorders such as depression and schizophrenia in human (Fone and Porkess 2008). The isolation-reared rats exhibit increased locomotor activity, social interaction and aggression, disrupted prepulse inhibition, and have altered monoamine levels in the brain (Heidbreder et al. 2000; Lapiz et al. 2003; Fone and Porkess 2008; Koike et al. 2009; Lukkes et al. 2009). In the present study, we aimed to examine the structural and functional changes of the mesocorticolimbic DA system in socially isolated rats with neurochemical and neuroanatomical means. Differential neuronal changes noted in the mPFC, BLA and NAc may account for the behavioral changes in this developmental neuropsychiatric disorder animal model.
Materials and methods
Animals
All animal handling was in accordance with a protocol approved by National Taiwan University College of Medicine and College of Public Health Institutional Animal Care and Use Committee. Male Wistar rats bred in the laboratory animal center of National Taiwan University College of Medicine were used in this study. After weaning at postnatal day (P) 28, rats were randomly assigned into group-rearing and isolation-rearing groups, respectively. For grouped animals, 3–4 rats were reared in one cage, whereas the isolated animals were reared individually. Isolation-reared rats shared the same light, temperature and humidity controlled environment with group-reared rats and they could see, hear and smell each other, however, the physical contacts with other rats were deprived. Totally, 17 group-reared and 18 isolation-reared male rats from six litters were used in this study. All animals were housed in the Laboratory Animal Center of National Taiwan University College of Medicine under 12-h light/dark cycle with free access to food and water.
Behavioral examinations
After 8–9 weeks grouped/isolated rearing, the following animal behaviors were examined.
Open field test
The locomotor activity of group-reared (n = 8) and isolation-reared (n = 8) rats was examined in an open field arena (40 cm × 40 cm). Animals were habituated to the testing environment 30 min before the test. Once placed in the center of the open field, rats were videotaped for 15 min. The floor of the arena was equally divided into 25 squares. The central nine squares were denoted as the central area. The rest 16 squares were the peripheral area. The travel distance and numbers of entrances into central and peripheral areas were examined with the aid of TopScan LITE software (Ver. 2.0, Clever system, Reston, VA, USA). The arena was cleaned after each test.
Acoustic startle responses and prepulse inhibition
The acoustic startle responses of group-reared rats (n = 8) and isolation-reared rats (n = 8) were examined in a SR-Lab system (San Diego Instruments, San Diego, CA, USA). After a 5-min acclimation period, individual animals received a test consisted of 56 trials divided into 3 blocks. The background noise was set to be 70 dB. Blocks 1 and 3 consisted of four no stimulus trails and five 115 dB startle pulse alone (40 ms) trails. This design was to establish a baseline of startle response at the beginning and the end of the session. These data were not included in the calculation of prepulse inhibition (PPI). Block 2 contained 38 trails including four types randomly presented trails: no stimulus, 115 dB startle pulse alone, and trails with a prepulse (20 ms, 77 dB or 86 dB) 100 ms before the 115 dB startle stimulus. The interval between trials was 15 s in average (ranging from 10 to 20 s). The percentage of PPI was calculated as the formula: PPIn (%) = [1−(Pn/S)] × 100, where n is the magnitude of the prepulse (77 or 86 dB), P is the average startle amplitude for prepulse-pulse trails and S is the average startle amplitude of startle pulse alone trials.
Measurement of dopamine levels
Some brains from group-reared (n = 6) and isolation-reared (n = 6) rats were used for the measurement of basal DA level in the brain using high performance liquid chromatography (HPLC). In brief, tissues from the mPFC, AMY, NAc and VTA were collected and homogenized. After sonication in 0.1 N perchloric acid, the homogenates were centrifuged (14,000 rpm at 4°C for 10 min). The supernatants were used for monoamine analyses through HPLC with an electrochemical detector (Waters, Milford, MA, USA). The pellets were neutralized in 0.1 N NaOH and dissolved in RIPA buffer for protein assay. Total protein was quantified by Bradford method using a protein assay kit (Bio-Rad, Hercules, CA, USA). Finally, DA and 3,4-Dihydroxy-phenylacetic acid (DOPAC) levels were analyzed and expressed as pg/μg protein.
Quantification of tyrosine hydroxylase (TH)-positive neurons in the midbrain
Some rats from group-reared (n = 4) and isolation-reared (n = 7) rats were killed and transcardially perfused with phosphate-buffered saline (PBS) followed by fixative (4% paraformaldehyde in phosphate buffer, pH 7.4). After perfusion, the brains were taken and kept in PBS containing sodium azide (0.1%). Coronal sections of the VTA from −4.80 to −5.60 mm Bregma were cut at thickness of 50 μm with a vibrating microtome (vibratome 1000, The Vibratome Company, St. Louis, MO, USA). Sections were then reacted with 10% methanol and 0.3% H2O2 in PBS for 10 min to block the endogenous peroxidase activity. After PBS rinses, sections were transferred to a blocking solution containing 4% normal goat serum, 1% bovine serum albumin and 0.4% TritonX-100 for 1 h. After blocking, sections were incubated with the mouse anti-tyrosine hydroxylase (1: 2,000; Sigma, St. Louis, MO, USA) overnight at 4°C. After washed in PBS, sections were incubated with the biotinylated second antibody (goat anti-mouse IgG, 1:500, Vector Labs, Burlingame, CA, USA) for 2 h and avidin–biotin peroxidase complex (ABC kit, Vector Labs) for 1 h. Finally, sections were reacted with 3,3′-diaminobenzidine (with 0.01% H2O2 in PBS) for 10 min. After several rinses, sections were mounted with glycerol-based aqueous mounting medium on gelatin-coated slides and coverslipped.
The density of tyrosine hydroxylase (TH)-positive neurons in the VTA was estimated by stereological method unilaterally. In brief, sections were examined on a light microscope (Olympus, Tokyo, Japan) with computer-controlled motorized stage and the optical fractionators sampling protocol in the Stereo Investigator system (MicroBrightField Inc, Williston, USA) was used. Six sections for each brain from 1:6 series were analyzed. By using 10× objective, area of right VTA according to the visible TH-positive neurons and atlas of rat brain was outlined and measured. There was no significant shrinkage from the original 48–50 μm thickness. A guard zone distance of 8 μm and dissector height of 30 μm was set. The sampling grid size of 240 μm × 240 μm was used and the counting frame size was 120 μm × 120 μm. For quantifying TH-positive neurons, a 20× objective was used. TH-positive neurons with obvious nucleus were counted. The coefficient of error (CE) value of each brain was estimated 0.06–0.08.
Golgi-Cox impregnation and morphometric analysis
Some brains from group-reared (n = 7) and isolation-reared (n = 5) rats were collected for morphological examinations at the cellular level. In the present study, we focused on the dendritic arborization and spine density of the layer II/III pyramidal neurons in the mPFC, the pyramidal neurons in the BLA and the medium spiny cells in the core region of the NAc (NAcc). To visualize dendritic structures, the Golgi-Cox method was used (Lee 2009). In brief, 4% paraformaldehyde-fixed brains were immersed into an impregnation solution (mixture of solution A: 1.0 g potassium dichromate and 1.0 g mercuric chloride in 85 ml distilled water with solution B: 0.8 g potassium chromate and 0.5 g sodium tungstate in 20 ml distilled water) for 8–10 days at room temperature. Specimens were cut at thickness of 150–200 μm with a vibratome. Sections were collected and briefly reacted with 15% ammonium hydroxide and followed by diluted (1:5) rapid fixer solution (Ilford, Marly, Switzerland). Finally, sections were dehydrated through series of alcohols and mounted with Depex (Electron Microscopy Sciences, Washington, PA, USA).
Golgi-Cox impregnated neurons were examined under a light microscope (Olympus) with a 40× objective. Only neurons with no obvious truncation in the dendritic profiles were selected for qualitative and quantitative analyses. Serial stacks of images with 0.5 μm interval were taken from top to bottom of the impregnated neuron and exported as a series of TIFF images to a PC workstation. The somatodendritic structures of neurons were traced and 3-D reconstructed with Neurolucida software (MicroBrightField). Numerous morphometric parameters including soma area, dendritic length, number of dendritic branch nodes and terminals, number and length of dendritic segments were measured with Neurolucida explorer (MicroBrightField). The spine density was determined from the first order (primary dendrites that directly derived from the soma), second order (segments that derived from the primary dendrites) to at least the 4th order and expressed as number of spines per μm dendrite length.
Data analysis
All data were analyzed with Excel (Microsoft, Redmond, WA, USA). Data were expressed as mean ± SEM. Statistical analysis was performed between groups using two-tailed unpaired student’s t test. Asterisks were used to indicate significant differences (*P < 0.05; **P < 0.01; ***P < 0.001).
Results
Abnormal behaviors in social isolation-reared rats
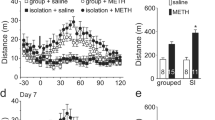
After 8–9 weeks of grouped/isolated rearing, rats were first examined behaviorally in a novel open field. The distance a rat traveled during 15 min was measured (Fig. 1). The isolation-reared rats traveled greater distance (1,652.84 ± 178.7 cm, n = 8 rats) than the group-reared rats (1,158.48 ± 127.79 cm, n = 8 rats, P < 0.05), particularly during the first 5-minute session (Fig. 1a). We also counted the number of entrances into central and peripheral areas of the open field. Isolation-reared rats exhibited greater number of entrances into the peripheral areas (Fig. 1b). In many psychiatric disorders, sensorimotor gating is impaired. We then examined this gating capacity of isolation-reared rats. The acoustic startle responses were measured and the percentage of PPI was calculated (Fig. 1c). Compared with group-reared rats, the isolation-reared rats exhibited reduced PPI, revealing their deficit in sensorimotor gating. The body weight of the isolation-reared was slightly lighter than the isolation-reared rats (411.3 ± 13.3 g in grouped rats and 401.3 ± 12.1 g in isolated rats, respectively). However, the statistical significance was not reached (P = 0.586).
Increased locomotor activity and impaired prepulse inhibition in social isolation-reared rats. a Locomotor activities of rats were examined in an open field for 15 min. b The number of entrances into central and peripheral regions was counted. c Prepulse inhibition was examined using a brief (20 ms) acoustic prepulse (77 or 86 dB) 100 ms prior to the startle pulse of 115 dB. Results are mean ± SEM. Asterisks are used to indicate significant differences between group-reared (grouped, n = 8) and isolation-reared (isolated, n = 8) rats (**P < 0.01)
Dopamine neurons in the midbrain
In order to study the structural and functional changes of the mesocorticolimbic DA system in socially isolated rats, the DA neurons of the midbrain were first examined (Fig. 2). DA neurons in the VTA were revealed by tyrosine hydroxylase (TH) immunohistochemistry (Fig. 2a–c) and the density of TH-positive neurons was estimated. The area of VTA according to the visible TH-positive neurons and rat brain atlas was outlined in series sections and the volume of VTA was then calculated. There was no significant change in the volume of VTA in isolated rats (n = 7) compared to that of grouped ones (n = 4) (Fig. 2d). The number of DA (TH-positive) neurons was estimated by optical fractionators sampling method. The number of TH-positive neurons in unilateral VTA was 19,730 ± 2,442.7 in group-reared rats and 16,787 ± 1,611.6 in isolation-reared rats, respectively (Fig. 2e). The estimated DA neuron number in the present study was close to previous reports (Nair-Roberts et al. 2008; Johnson et al. 2010). However, the number was comparable between group-reared and isolation-reared rats (P = 0.36). The density of VTA DA neurons in rats was therefore not affected by postweaning social isolation (Fig. 2f).
Tyrosine hydroxylase (TH)-positive neurons in the midbrain. a Schematic representation of the ventral tegmental area (VTA) in the coronal section of a rat brain. Number shows distance from Bregma according to the atlas of Paxinos and Watson (1998). b Low magnification of a 50-μm-thick coronal section with TH immunoreactivity in the substantia nigra pars compacta (SNc) and VTA. c Delimited area of VTA in b is shown at higher magnification for better illustration of TH-positive neurons. The d volume of VTA, e the estimated population and f the density of TH-positive neurons in VTA were compared in group- and isolation-reared rats. Bar is 250 μm in b and 25 μm in c
Basal dopamine levels in the mesocorticolimbic system
Since VTA neurons send dopaminergic afferents to numerous cortical and limbic brain structures, we then examined the basal levels of DA and its major metabolite, 3,4-Dihydroxy-phenylacetic acid (DOPAC), in the mesocorticolimbic regions. The basal level of DA was not significantly altered in the VTA, mPFC and the NAcc of isolated rats except for the AMY. In the AMY, the DA level is lower in the isolation-reared rats than the group-reared rats (Fig. 3a). However, the DA turnover, as estimated by DA/DOAPC ratio, in isolation-reared rats was not significantly altered in these four mesocorticolimbic regions (Fig. 3b, c).
Basal dopamine levels in the brain. a Levels of dopamine (DA) in the ventral tegmental area (VTA), medial prefrontal cortex (mPFC), amygdala (AMY) and the core region of NAc (NAcc). b Levels of a major DA metabolite 3, 4-Dihydroxyphenylacetic acid (DOPAC), in VTA, mPFC, AMY and NAcc, respectively. c the DA turnover estimated as DA/DOAPC ratio in various regions. Results are mean ± SEM. Asterisks indicate significant differences between group-reared (grouped, n = 6) and isolation-reared (isolated, n = 6) rats (*P < 0.05)
Effects of social isolation on the dendritic morphology of neurons in the mPFC
The mPFC plays important roles in sensorimotor gating (Zhang et al. 2005) which is disrupted in isolation-reared rats (Geyer et al. 1993; Heidbreder et al. 2000; Cilia et al. 2001; Day-Wilson et al. 2006; Roncada et al. 2009). Since the basal DA level in their mPFC was not changed, we wondered if the impaired mPFC function is resulted from the structural changes. Golgi-Cox impregnated layer II/III pyramidal neurons in the mPFC were collected from group-reared rats (n = 13 neurons) and isolation-reared rats (n = 16 neurons) (Fig. 4a, b). In order to compare the morphometric parameters of both apical and basilar dendrites, neurons were chosen according to the shape (pyramidal type with both one apical and several basilar dendrites) and location (roughly 300 μm from the surface, the layer II/III). For the apical dendrites, reduction in dendritic complexity (e.g. number of bifurcation nodes and terminal endings) and total length was observed in the apical dendrites of mPFC neurons from the isolated animals (Table 1). The numbers of intersections (Fig. 4c), bifurcation nodes (Fig. 4d) as well as terminal endings (Fig. 4e) were reduced particularly in the proximal regions (<100 μm). For the basilar dendrites of mPFC neurons, similar dendritic reduction (Fig. 4c–e; Table 1), like that in the apical dendrites, but to a lesser extent, was observed in the isolated rats.
Dendritic arborization in layer II/III pyramidal neurons of the mPFC. a The mPFC is marked grey in the coronal sections of a rat brain. Numbers show distance from Bregma according to the atlas of Paxinos and Watson (1998). b Examples of Golgi-Cox impregnated pyramidal neurons were obtained from the layer II/III of the mPFC in socially reared (grouped) and isolated rats. Spines are omitted in this illustration. The complexity of apical and basilar dendrites was estimated by the concentric-rings method of Sholl. The number of c intersections, d bifurcating nodes and e terminal endings was counted in relation to the distance from the soma center. Bar is 100 μm in b. Results are mean ± SEM. Asterisks are used to indicate significant difference (*P < 0.05)
Next, we quantified the number and length of segments into each dendritic order. A total of 753 apical and 1,971 basilar dendritic segments from the first to the tenth orders of dendrites in mPFC neurons of grouped and isolated rats were collected and measured. For the apical dendrites of mPFC neurons, the number of segments was reduced in the isolation-reared rats especially from the fourth to eighth orders (Fig. 5a; Table 1), indicating that the dendritic arborization was affected. The length of internodal segments (between two bifurcation nodes) was comparable in the two groups whereas the mPFC neurons in the isolated rats seemed to have longer terminal segments (Fig. 5b, c). For the basilar dendrites of mPFC neurons in the isolation-reared rats, reduced arborization was revealed by the reduction of segment number especially in the third and fourth orders (Fig. 5d), in line with a previous report (Pascual et al. 2007). Like the apical dendrites, the length of terminal segments of basilar dendrites also altered in the isolated rats (Fig. 5e, f).
Dendritic segments in layer II/III pyramidal neurons of the mPFC. The number of segments was quantified in relation to the dendritic order (a and d are for apical and basilar dendrites, respectively). Dendritic segments were divided into internodal and terminal segments and the length of segments was measured and plotted in relation to the dendritic order (b and e are for apical and basilar dendrites, respectively) or expressed as averaged values (c and f are for apical and basilar dendrites, respectively). Results are mean ± SEM. Asterisks are used to indicate significant difference (*P < 0.05; **P < 0.01; ***P < 0.001)
Altered dendritic branching and segment length in BLA neurons of socially deprived rats
The BLA controls the activity of mPFC and NAc (Phillips et al. 2003). The structure of BLA projection neurons, pyramidal neurons, was then examined (Fig. 6a). Neurons from group-reared rats (n = 14 neurons) and isolation-reared rats (n = 15 neurons) had comparable soma size (Fig. 6b). However, the dendritic complexity was greatly affected by early social isolation as revealed by Sholl analyses. The number of intersections (Fig. 6c), bifurcation nodes (Fig. 6d) and terminal endings (Fig. 6e) were all increased in the isolated rats principally in the proximal regions (<100 μm). The number of segments was increased in all dendritic orders of BLA neurons in the isolation-reared rats (Fig. 6f). Furthermore, the total counts of nodes, endings, segments as well as the highest order of dendrites were increased in the BLA neurons of isolated rats; however, the increase of total dendritic length was not significant (Table 2). Together, these data suggested that the dendritic arborization of BLA pyramidal neurons was augmented by early social isolation. To our knowledge, this is the first report of exuberant dendritic arbors in the pyramidal BLA neurons induced by early social isolation. However, similar morphological changes of BLA neurons had been observed in rats after chronic (10 days) immobilization stress (Vyas et al. 2002).
Increased dendritic branches in pyramidal BLA neurons of socially deprived rats. a Sketches of the amygdala in the coronal sections of a rat brain. The basolateral nucleus of amygdala (BLA) is marked grey. Numbers show distance from Bregma according to the atlas of Paxinos and Watson (1998). b Examples of Golgi-Cox impregnated pyramidal cells were obtained from the BLA of socially reared (grouped) and isolated rats. The neurons from isolated rats had greater dendritic complexity revealed as more c intersections, d bifurcating nodes and e terminal endings than that in the grouped rats especially in the proximal regions. f The number of segments in the BLA neurons was quantified by each dendritic order and more dendritic segments were counted in the isolated rats in all orders than that in the grouped rats. In the isolates, both internodal and terminal segments were shorter than those in segments in the grouped rats (g and h). Bar is 100 μm in B. Results are mean ± SEM. Asterisks are used to indicate significant difference (*P < 0.05; **P < 0.01; ***P < 0.001)
We then further measured the length of each dendritic segment in order to examine if the elongation of dendrites was also affected by early social deprivation. A total of 2,158 dendritic segments from the first to the tenth orders of dendrites in BLA pyramidal neurons of grouped and isolated rats were collected and measured. The internodal and terminal segments of BLA neurons were significantly shorter in the isolates than that in group-reared rats (Fig. 6g, h). The BLA neurons in the isolates had more segments but the length of segments was shorter; this explained the comparable total dendritic length of BLA neurons in two groups (Table 2). Collectively, the data demonstrated that both arborization and elongation of dendrites in the BLA pyramidal neurons were altered in isolation-reared rats.
Reduced dendritic arborization in NAcc neurons of isolation-reared rats
Sensorimotor gating deficits in isolation-reared rats have been linked with altered protein expression in the NAc (Roncada et al. 2009). The dendritic structure might as well altered, to test this hypothesis, the morphology of medium spiny neurons obtained from the NAcc was examined (Fig. 7a). Neurons from group-eared rats (n = 14 neurons) and isolated rats (n = 18 neurons) had comparable soma size (Fig. 7b). However, the dendritic complexity was reduced by early social isolation as revealed by Sholl analyses. The number of intersections in the isolated rats was reduced (Fig. 7c) which is comparable to the finding of Alquicer et al. (2008). The overall distributions of bifurcation nodes and terminal endings in the two groups of animals were similar (Fig. 7d, e). However, when the counts were accumulated, the NAcc neurons in the isolated rats had significantly fewer nodes and endings than those of grouped rats (Table 3). Furthermore, we quantified the number of segments in each dendritic order and found reduced segment number in NAcc neurons of isolated rats (Fig. 7f). In sum, these data suggested that the arborization of dendrites in the NAcc neurons was constrained by postweaning social isolation.
Morphometric features of neurons in the NAcc. a Sketches of nucleus accumbens (NAc) in the coronal sections of a rat brain. The core region of NAc (NAcc) is marked grey. Numbers show distance from Bregma according to the atlas of Paxinos and Watson (1998). b Examples of Golgi-Cox impregnated medium spiny cells were obtained from the NAcc of socially reared (grouped) and isolated rats. Spines are omitted in this illustration. Dendritic complexity was estimated by the Sholl method. The neurons from grouped rats had more complicated dendritic arbors than that in the isolated rats. The number of c intersections, d bifurcating nodes and e terminal endings was counted in relation to the distance from the soma. The number of segments was quantified by each f dendritic order. Dendritic segments were further divided into internodal and terminal segments and the length was then quantified by g dendritic order and h in average. Bar is 100 μm in b. Results are mean ± SEM. Asterisks are used to indicate significant difference (*P < 0.05)
We next examined if the elongation of NAcc neuron dendrites was affected by early social deprivation. A total of 1,601 dendritic segments were collected and measured. There was no difference in either length in relation to the dendritic order (Fig. 7g) or averaged segment length (Fig. 7h) between the two groups. We therefore concluded that the elongation of dendritic segment in NAcc neurons was not affected by early social isolation. The decrease of total dendritic length in the NAcc neurons of isolated rats was primarily due to the reduction of segments (Table 3).
Spine density in neurons of the mPFC, BLA and NAcc after postweaning social isolation
Dendritic spines are important structure for synaptic transmission and plasticity (Calabrese et al. 2006; Alvarez and Sabatini 2007). Dendritic spine abnormalities have been reported in various neurological disorders (Kaufmann and Moser 2000; Fiala et al. 2002; Lin and Koleske 2010; Penzes et al. 2011). We therefore checked the density of dendritic spine in the neurons in mPFC (a total of 84 segments from group-reared rats and 137 segments from isolation-reared rats were collected from the apical dendrites and 154 segments from group-reared rats and 250 segments from isolation-reared rats were collected from the basilar dendrites, respectively), BLA (419 segments from group-reared rats and 392 segments from isolation-reared rats were collected) and NAcc (270 segments from group-reared rats and 260 segments from isolation-reared rats were collected, respectively). In the isolation-reared rats, the spine density was changed in the layer II/III mPFC pyramidal neurons (Fig. 8a) but neither in the BLA (Fig. 8b) nor the NAcc (Fig. 8c) neurons. Interestingly, the spine density in the mPFC neurons of isolation-reared rats was decreased in the higher (≧4th) order branches in the apical dendrites but not in the basilar dendrites (Fig. 8a).
Dendritic spine of neurons in the mPFC, BLA and NAcc. The density of dendritic spine in layer II/III pyramidal neurons of mPFC, pyramidal BLA neurons and medium spiny neurons in the NAcc was measured in relation to the dendritic order. a In isolated rats, the density of dendritic spine in mPFC neuron was less than that in group-reared rats in higher (≥4th) order branches of the apical dendrites. Isolation-reared rats did not significantly affect the number of dendritic spine in neurons in the b BLA and c NAcc. Results are mean ± SEM. Asterisks are used to indicate significant difference (*P < 0.05)
Discussion
The mesocorticolimbic DA system consists DA-producing cells in the VTA and their projection targets such as the mPFC, AMY and NAc. The system has profound impacts on various brain functions including emotion and cognition and dysregulation of its DA function might attribute to some neuropsychiatric disorders like depression and schizophrenia. In the present study, we adopted the animal model of postweaning social isolation which exhibits abnormal neurochemical and behavioral consequences similar to those seen in patients of depression and schizophrenia (Fone and Porkess 2008) and studied the functional and structural changes of the mesocorticolimbic DA system.
After 8–9 weeks of social deprivation, isolation-reared rats exhibited hyperactivity in a novel open field and it was in line with previous studies (Heidbreder et al. 2000; Silva-Gómez et al. 2003). Furthermore, isolation-reared rats in the present study exhibited greater number of entrances into the peripheral areas implying that these rats have altered anxiety-related behavior. The sensorimotor gating, indicated by PPI, was also impaired in isolated rats, which was also comparable to previous reports (Geyer et al. 1993; Heidbreder et al. 2000; Cilia et al. 2001; Day-Wilson et al. 2006; Roncada et al. 2009). Together, the behavioral performances of isolation-reared rats in our study are consistent with others.
The number of tyrosine hydroxylase (TH)-positive DA neurons was estimated using stereological method of unbiased optical fractionators sampling protocol. To our knowledge, it is the first report of TH-positive neuron number in the midbrain VTA of adult male Wistar rats. The number (19,730 ± 2,442.7 TH-positive VTA neurons, unilateral count) is close to previous findings in adult male Sprague-Dawley (SD) rats (Nair-Roberts et al. 2008; Johnson et al. 2010). Some DA-related transcription factors and number of TH-positive neurons are sensitive to prenatal stress and maternal malnutrition (Katunar et al. 2009; Katunar et al. 2010; Vucetic et al. 2010), indicating the critical time window for the development of midbrain DA neuron is in the prenatal period (Meyer and Feldon 2009). The number and density of VTA TH-positive neurons are therefore not responsive to postweaning life events.
Subsets of VTA DA neurons project to mPFC, BLA and NAc (Lammel et al. 2008) respectively, and the DA levels are region-specifically regulated in socially isolated rats (Fone and Porkess 2008; Lukkes et al. 2009). However, owing to the diverse rat strains, length of isolation, experimental manipulations and quantification methods, the results are not consistent. For example, compared to group-reared controls, the basal DA level is increased in the mPFC of male DS rats socially isolated from P21 to P85 (Han et al. 2011), whereas it is not changed in male Lister-hooded rats isolated from P28 to P56 (Dalley et al. 2002) and is decreased in male Lister-hooded rats isolated from P25 to P109 (Fabricius et al. 2011). In the NAc, increased basal DA level has been reported in male SD rats isolated from P21 to P85 (Han et al. 2011); however, the basal DA level is not changed in male SD rats isolated from P28 to P100 (Brenes and Fornaguera 2009) and male Lister-hooded rats isolated from P25 to P109 (Fabricius et al. 2011) compared to their group-reared controls. In the present study, the basal DA levels in male Wistar rats isolated from P28 to P85 are not different from age-matched group-reared controls.
Unlike a great number of studies focusing on DA levels in the mPFC and NAc, relatively fewer works address the change of DA level in the AMY in social isolation model (Heidbreder et al. 2000; Miura et al. 2002). In isolation-reared male Wistar rats (isolated from P21 to P105), the DA level in the AMY is reduced, yet the DA turnover is increased (Heidbreder et al. 2000). However, in male F344 rats isolated from P49 to P77, the AMY DA level is increased, but the DA turnover is unchanged (Miura et al. 2002). In the present study, similar to that of F344 rats, decreased DA level in the AMY is found in isolation-reared rats; however, the DA turnover is not changed. BLA pyramidal neurons are steroidogenic (Agís-Balboa et al. 2006), producing neurosteroids like allopregnanolone (Akwa et al. 1999). The expression of a rate-limiting enzyme for producing allopregnanolone, 5α-reductase, in the BLA is declined in socially isolated mice (Agís-Balboa et al. 2007). It has been shown that the extracellular DA level is regulated by allopregnanolone (Dazzi et al. 2002; Rougé-Pont et al. 2002). The reduced DA level in the AMY of isolated rats might be resulted from decreased 5α-reductase expression (Bortolato et al. 2011). Further study is warranted to confirm this notion.
Dendritic features in mesocorticolimbic structures are very sensitive to psychological conditions. Stress, for example, causes dendritic changes in neurons in the mPFC, NAc and BLA (Vyas et al. 2002; Radley et al. 2004; Cook and Wellman 2004; Liston et al. 2006; Martínez-Téllez et al. 2009; Perez-Cruz et al. 2009; Shansky et al. 2009; Shansky and Morrison 2009; Eiland et al. 2011). In postweaning socially isolated rats, dendritic features have been studied in neurons of the mPFC and NAc. In layer II/III mPFC pyramidal neurons, the number of dendritic segments and dendritic complexity are reduced in male SD rats isolated from P18 to P32 (Pascual et al. 2006) and the spine density is decreased in male SD rats isolated from P21 to P77 (Silva-Gómez et al. 2003; Alquicer et al. 2008). In the present study, we quantified dendritic features in both apical and basilar dendrites of layer II/III pyramidal mPFC neurons. To our knowledge, it is the first report of dendritic changes in the apical dendrites of layer II/III pyramidal mPFC neurons in socially isolated rats. In our postweaning social isolation paradigm (P28–P85), decreased total dendritic length, segment number and dendritic complexity with prolonged terminal segments are found in both apical and basilar dendrites of isolation-reared rats. These changes implied the altered dendritic elongation or pruning or both of the apical dendrites. However, reduced spine density is only significant in high (≥4) order segments of the apical dendrite in the mPFC neurons of isolated rats. Given the mPFC plays important roles in many brain functions, reduced dendritic arbor and spine density in layer II/III mPFC neurons of the isolated rats may attribute to their impaired emotional and cognitive status (Fone and Porkess 2008; Lukkes et al. 2009). The morphology of NAc neurons, especially the medium spiny neurons in the NAcc, has been examined in male SD rats isolated from P21 to P77. The total dendritic length is reduced in isolation-reared rats while the spine density is not changed (Alquicer et al. 2008), same with our present findings in male Wistar rats.
The pyramidal neurons in the BLA are the major output neurons that regulate the activities of the NAc and mPFC (Kelley et al. 1982; McDonald 1991; Laviolette et al. 2005). However, to our knowledge, the morphology of these neurons has not been examined in any social isolation model. The exuberant dendritic arbor of BLA pyramidal neurons in the isolates of present study largely resembles the findings in chronic stress model (Vyas et al. 2002), suggesting the postweaning social deprivation might be considered as a chronic stress burden (Serra et al. 2007). This notion is supported by our observations of the mPFC neurons in the isolated rats. More dramatic changes are noted in the apical than the basilar dendrites of layer II/III pyramidal neurons, similar to the findings in stressed animals (Cook and Wellman 2004; Radley et al. 2004, 2008; Liston et al. 2006). However, the postweaning social isolation starts from juvenile age, when the brain structures are more “plastic” then the start point of chronic stress models, thus even the basilar dendrites in layer II/III mPFC pyramidal neurons are affected (Silva-Gómez et al. 2003; Alquicer et al. 2008 and present study).
In normal condition, the prefrontal cortex mediates thought, emotion and executive functions by organizing cortical and sub-cortical information. However, under conditions like chronic stress, the prefrontal cortex becomes hypoactive and AMY is hyperactive (Arnsten 2009; Roozendaal et al. 2009). It might also be the case in our postweaning isolation model. Reduced dendritic arbor and spine in the mPFC neurons implies the decline of synaptic efficacy and neuronal excitability (Mainen and Sejnowski 1996; Komendantov and Ascoli 2009). With reduced activity in mPFC neurons, the inhibitory effect of mPFC on BLA neurons might thus be attenuated. In addition, the extensive dendritic profile in BLA neurons of isolation-reared rats augments the synaptic connections which may further contribute to the increase of neural activity in the BLA. As the integration station of the mesocorticolimbic system, the NAc neurons receive afferents from the mPFC and AMY (Groenewegen et al. 1999; Goto and Grace 2008). The dendritic arbors of NAcc neurons are affected by early social isolation, implying that the integration capacity of NAc is impaired in the isolated rats. Together, in social isolation-reared rats, structural changes in the mPFC, BLA and NAcc neurons and reduced DA level in the AMY may cause functional consequences that eventually lead to the altered behavioral performances.
In conclusion, postweaning social isolation deprives peer play experience and place stress burden on young rats. The time period, from juvenile or pre-adolescence (P28) to young adult (P85), is critical for functional development of the brain especially in the cognitive, emotional and social aspects (Romeo and McEwen 2006; Paus et al. 2008). Our results demonstrated that postweaning social isolation paradigm affects neurons in the mesocorticolimbic system in a region-specific manner. Neurochemical and structural abnormalities in the brains of isolation-reared rats might influence their behaviors later in life and increase the vulnerability to mental disorders related to schizophrenia and depression (Fone and Porkess 2008; Lukkes et al. 2009).
Abbreviations
- AMY:
-
Amygdala
- BLA:
-
Basolateral nucleus of amygdala
- CE:
-
Coefficient of error
- DA:
-
Dopamine
- DOPAC:
-
3,4-Dihydroxy-phenylacetic acid
- HPLC:
-
High performance liquid chromatography
- mPFC:
-
Medial prefrontal cortex
- NAc:
-
Nucleus accumbens
- NAcc:
-
Core region of the nucleus accumbens
- P:
-
Postnatal day
- PBS:
-
Phosphate-buffered saline
- PPI:
-
Prepulse inhibition
- SD:
-
Sprague-Dawley
- TH:
-
Tyrosine hydroxylase
- VTA:
-
Ventral tegmental area
References
Agís-Balboa RC, Pinna G, Zhubi A et al (2006) Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci USA 103:14602–14607
Agís-Balboa RC, Pinna G, Pibiri F et al (2007) Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci USA 104:18736–18741
Akwa Y, Purdy RH, Koob GF et al (1999) The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res 106:119–125
Alquicer G, Morales-Medina JC, Quirion R et al (2008) Postweaning social isolation enhances morphological changes in the neonatal ventral hippocampal lesion rat model of psychosis. J Chem Neuroanat 35:179–187
Alvarez VA, Sabatini BL (2007) Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci 30:79–97
Arnsten AF (2009) Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10:410–422
Bertolucci-D’Angio M, Serrano A, Driscoll P et al (1990) Involvement of mesocorticolimbic dopaminergic systems in emotional states. Prog Brain Res 85:405–416
Björklund A, Dunnett SB (2007) Dopamine neuron systems in the brain: an update. Trends Neurosci 30:194–202
Bortolato M, Devoto P, Roncada P et al (2011) Isolation rearing-induced reduction of brain 5α-reductase expression: relevance to dopaminergic impairments. Neuropharmacology 60:1301–1318
Brenes JC, Fornaguera J (2009) The effect of chronic fluoxetine on social isolation-induced changes on sucrose consumption, immobility behavior, and on serotonin and dopamine function in hippocampus and ventral striatum. Behav Brain Res 198:199–205
Calabrese B, Wilson MS, Halpain S (2006) Development and regulation of dendritic spine synapses. Physiology (Bethesda) 21:38–47
Cilia J, Reavill C, Hagan JJ et al (2001) Long-term evaluation of isolation-rearing induced prepulse inhibition deficits in rats. Psychopharmacology 156:327–337
Cook SC, Wellman CL (2004) Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol 60:236–248
Dalley JW, Theobald DE, Pereira EA et al (2002) Specific abnormalities in serotonin release in the prefrontal cortex of isolation-reared rats measured during behavioural performance of a task assessing visuospatial attention and impulsivity. Psychopharmacology (Berl) 164:329–340
Day-Wilson KM, Jones DN, Southam E et al (2006) Medial prefrontal cortex volume loss in rats with isolation rearing-induced deficits in prepulse inhibition of acoustic startle. Neuroscience 141:1113–1121
Dazzi L, Serra M, Vacca G et al (2002) Depletion of cortical allopregnanolone potentiates stress-induced increase in cortical dopamine output. Brain Res 932:135–139
Dunlop BW, Nemeroff CB (2007) The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64:327–337
Eiland L, Ramroop J, Hill MN et al. (2011) Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology doi:10.1016/j.psyneuen.2011.04.015
Fabricius K, Steiniger-Brach B, Helboe L et al (2011) Socially isolated rats exhibit changes in dopamine homeostasis pertinent to schizophrenia. Int J Dev Neurosci 29:347–350
Fiala JC, Spacek J, Harris KM (2002) Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Rev 39:29–54
Floresco SB (2007) Dopaminergic regulation of limbic-striatal interplay. J Psychiatry Neurosci 32:400–411
Floresco SB, Tse MT (2007) Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala-prefrontal cortical pathway. J Neurosci 27:2045–2057
Fone KC, Porkess MV (2008) Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev 32:1087–1102
Geyer MA, Wilkinson LS, Humby T et al (1993) Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiatry 34:361–372
Goto Y, Grace AA (2008) Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci 31:552–558
Groenewegen HJ, Wright CI, Beijer AV et al (1999) Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci 877:49–63
Han X, Wang W, Shao F et al (2011) Isolation rearing alters social behaviors and monoamine neurotransmission in the medial prefrontal cortex and nucleus accumbens of adult rats. Brain Res 1385:175–181
Heidbreder CA, Weiss IC, Domeney AM et al (2000) Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience 100:749–768
Heinz A, Schlagenhauf F (2010) Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull 36:472–485
Horvitz JC (2000) Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience 96:651–656
Johnson ML, Day AE, Ho CC et al (2010) Androgen decreases dopamine neurone survival in rat midbrain. J Neuroendocrinol 22:238–247
Kapur S, Mann JJ (1992) Role of the dopaminergic system in depression. Biol Psychiatry 32:1–17
Katunar MR, Saez T, Brusco A et al (2009) Immunocytochemical expression of dopamine-related transcription factors Pitx3 and Nurr1 in prenatally stressed adult rats. J Neurosci Res 87:1014–1022
Katunar MR, Saez T, Brusco A et al (2010) Ontogenetic expression of dopamine-related transcription factors and tyrosine hydroxylase in prenatally stressed rats. Neurotox Res 18:69–81
Kaufmann WE, Moser HW (2000) Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex 10:981–991
Kelley AE, Domesick VB, Nauta WJ (1982) The amygdalostriatal projection in the rat: an anatomical study by anterograde and retrograde tracing methods. Neuroscience 7:615–630
Koike H, Ibi D, Mizoguchi H et al (2009) Behavioral abnormality and pharmacologic response in social isolation-reared mice. Behav Brain Res 202:114–121
Komendantov AO, Ascoli GA (2009) Dendritic excitability and neuronal morphology as determinants of synaptic efficacy. J Neurophysiol 101:1847–1866
Kröner S, Rosenkranz JA, Grace AA (2005) Dopamine modulates excitability of basolateral amygdala neurons in vitro. J Neurophysiol 93:1598–1610
Lammel S, Hetzel A, Häckel O et al (2008) Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57:760–773
Lapiz MD, Fulford A, Muchimapura S et al (2003) Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol 33:13–29
Laviolette SR (2007) Dopamine modulation of emotional processing in cortical and subcortical neural circuits: evidence for a final common pathway in schizophrenia? Schizophr Bull 33:971–981
Laviolette SR, Lipski WJ, Grace AA (2005) A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci 25:6066–6075
Lee LJ (2009) Neonatal fluoxetine exposure affects the neuronal structure in the somatosensory cortex and somatosensory-related behaviors in adolescent rats. Neurotox Res 15:212–223
Lin YC, Koleske AJ (2010) Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu Rev Neurosci 33:349–378
Liston C, Miller MM, Goldwater DS et al (2006) Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 26:7870–7874
Lukkes JL, Watt MJ, Lowry CA et al (2009) Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front Behav Neurosci 3:18
Mainen ZF, Sejnowski TJ (1996) Influence of dendritic structure on firing pattern in model neocortical neurons. Nature 382:363–366
Martínez-Téllez RI, Hernández-Torres E, Gamboa C et al (2009) Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse 63:794–804
McDonald AJ (1991) Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience 44:1–14
Meyer U, Feldon J (2009) Prenatal exposure to infection: a primary mechanism for abnormal dopaminergic development in schizophrenia. Psychopharmacology (Berl) 206:587–602
Miura H, Qiao H, Ohta T (2002) Influence of aging and social isolation on changes in brain monoamine turnover and biosynthesis of rats elicited by novelty stress. Synapse 46:116–124
Nair-Roberts RG, Chatelain-Badie SD, Benson E et al (2008) Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 152:1024–1031
Nestler EJ, Carlezon WA Jr (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–1159
Niwa M, Kamiya A, Murai R et al (2010) Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron 65:480–489
Pascual R, Zamora-León SP, Valero-Cabré A (2006) Effects of postweaning social isolation and re-socialization on the expression of vasoactive intestinal peptide (VIP) and dendritic development in the medial prefrontal cortex of the rat. Acta Neurobiol Exp (Wars) 66:7–14
Pascual R, Zamora-León P, Catalán-Ahumada M et al (2007) Early social isolation decreases the expression of calbindin D-28 k and dendritic branching in the medial prefrontal cortex of the rat. Int J Neurosci 117:465–476
Paus T, Keshavan M, Giedd JN (2008) Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9:947–957
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, New York
Penzes P, Cahill ME, Jones KA et al (2011) Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14:285–293
Perez-Cruz C, Simon M, Czéh B et al (2009) Hemispheric differences in basilar dendrites and spines of pyramidal neurons in the rat prelimbic cortex: activity- and stress-induced changes. Eur J Neurosci 29:738–747
Phillips AG, Ahn S, Howland JG (2003) Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior. Neurosci Biobehav Rev 27:543–554
Radley JJ, Sisti HM, Hao J et al (2004) Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125:1–6
Radley JJ, Rocher AB, Rodriguez A et al (2008) Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol 507:1141–1150
Reynolds GP (1983) Increased concentrations and lateral asymmetry of amygdala dopamine in schizophrenia. Nature 305:527–529
Romeo RD, McEwen BS (2006) Stress and the adolescent brain. Ann N Y Acad Sci 1094:202–214
Roncada P, Bortolato M, Frau R et al (2009) Gating deficits in isolation-reared rats are correlated with alterations in protein expression in nucleus accumbens. J Neurochem 108:611–620
Roozendaal B, McEwen BS, Chattarji S (2009) Stress, memory and the amygdala. Nat Rev Neurosci 10:423–433
Rosenkranz JA, Grace AA (2002) Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci 22:324–337
Rougé-Pont F, Mayo W, Marinelli M et al (2002) The neurosteroid allopregnanolone increases dopamine release and dopaminergic response to morphine in the rat nucleus accumbens. Eur J Neurosci 16:169–173
Seeman P (1987) Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse 1:133–152
Serra M, Sanna E, Mostallino MC et al (2007) Social isolation stress and neuroactive steroids. Eur Neuropsychopharmacol 17:1–11
Shansky RM, Morrison JH (2009) Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res 1293:108–113
Shansky RM, Hamo C, Hof PR et al (2009) Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex 19:2479–2484
Silva-Gómez AB, Rojas D, Juárez I et al (2003) Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res 983:128–136
Vucetic Z, Totoki K, Schoch H et al (2010) Early life protein restriction alters dopamine circuitry. Neuroscience 168:359–370
Vyas A, Mitra R, Shankaranarayana Rao BS et al (2002) Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22:6810–6818
Wise RA (1982) Neuroleptics and operant behavior: the anhedonia hypothesis. Behav Brain Sci 5:39–87
Yadid G, Overstreet DH, Zangen A (2001) Limbic dopaminergic adaptation to a stressful stimulus in a rat model of depression. Brain Res 896:43–47
Young KA, Gobrogge KL, Wang Z (2011) The role of mesocorticolimbic dopamine in regulating interactions between drugs of abuse and social behavior. Neurosci Biobehav Rev 35:498–515
Zhang TY, Chrétien P, Meaney MJ et al (2005) Influence of naturally occurring variations in maternal care on prepulse inhibition of acoustic startle and the medial prefrontal cortical dopamine response to stress in adult rats. J Neurosci 25:1493–1502
Acknowledgments
This work was supported by National Science Council of the Republic of China (Grant numbers: NSC 98-2410-H-002-033-, NSC 96-2628-B-002-053-MY3 and NSC 99-2628-B-002-052-MY3) and National Taiwan University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y.-C. Wang and U.-C. Ho contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, YC., Ho, UC., Ko, MC. et al. Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct Funct 217, 337–351 (2012). https://doi.org/10.1007/s00429-011-0355-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-011-0355-4