Abstract
The expression of glycine receptors in the retina of clawed frog, Xenopus laevis was studied immunocytochemically. Glycine receptors (GlyRs), as revealed by means of several different antibodies, were mainly distributed in the inner (IPL) and the outer plexiform layers. Their composition was determined to include α2 and α3 subunits. Typical punctate appearance and specific lamination in the IPL were seen with each of the antibodies directed against the different GlyRs’ subunits. A notion for diversity of the glycine receptors was put forward, according to which the α2 and α3 subunits are located in different subtypes of glycine synapses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The inhibitory neurotransmission in the retina is mediated mainly by two major neurotransmitters: γ-amino butyric acid (GABA) and glycine. While GABA and its receptors are well studied immunocytochemically, the investigation of glycine and glycine receptors has begun only recently. It is known that the glycine receptor (GlyR) is a heteropentameric receptor forming chloride channel. It is composed of three alpha (α) and two beta (β) subunits (reviewed by Legendre 2001; Laube et al. 2002). Several isoforms of α subunit are known: α1, α2, α3 and α4. They are coded by separate genes and are differently expressed during the ontogenesis in the different species. β subunit is coded by a single gene and no data about different isoforms exist until now (Matzenbach et al. 1994). It is a common belief that the α subunits are responsible for the GlyR sensitivity to glycine and its agonists and antagonists, while the β subunit possesses affinity to gephyrin, a protein which determines the clustering of receptors at the postsynaptic sites.
By means of immunocytochemical methods the GlyRs and their subunits’ composition are well documented in the retinae of several mammalian species: monkey, rabbit, rat and mouse (Grünert and Wässle 1993, 1996; Sassoè-Pognetto et al. 1994; Wässle et al. 1998; Grünert 2000; Haverkamp and Wässle 2000; Lin et al. 2000; Haverkamp et al. 2003, 2004). However, relatively little is known about the GlyRs in the retinae of lower vertebrates (Yazulla and Studholme 1991a, b, 2001; Zucker and Ehinger 1993). In most of the aforementioned studies the distribution of gephyrin is mainly described. Gephyrin is a protein, which is known to participate in the formation of the GlyR complex. It acts as scaffold protein that binds GlyRβ subunit to the tubulin of the cytoskeleton. So, gephyrin is essential for the clustering of the GlyRs at the postsynaptic densities. However, recent data showed that it is also essential for GABAA receptors’ clustering (Sassoè-Pognetto et al. 1995; Fischer 2000). That is why gephyrin cannot be used as a reliable indicator for the GlyR location. The aim of the present investigation was to study the GlyRs in the retina of clawed frog Xenopus laevis, applying the two additional monoclonal antibodies, mAb4a (against all subunits of the receptor) and mAb2b (against the α1 subunit of the receptor), besides the monoclonal antibody against gephyrin (mAb7a). Two novel polyclonal antibodies recognizing the α2 and the α3 subunits of the receptor were also applied (Haverkamp et al. 2003, 2004). All of the aforementioned antibodies were recently used by us to study the distribution of GlyRs in the retina of the frog Rana ridibunda (Vitanova et al. 2004). A question of interest arose: if the GlyRs in these two kindred types of amphibian retinae differ or not. The results presented here give answer to this question allowing comparison of the GlyRs in the retina of X. laevis with those in the retina of R. ridibunda, two species widely used in electrophysiological experiments (Stone and Schütte 1991; Popova et al. 1997, 2000).
Materials and methods
Eyecups of an adult clawed frog, X. laevis were used in the experiments. The animal was anesthetized in water containing Tricaine-Methansulfonate (500 mg/l, MS-222, Sigma) and decapitated afterwards. The eyes were opened along the ora serrata and fixed for 15–30 min in 4% paraformaldehyde in 0.1 M phosphate buffer (pH=7.4). Afterwards, the retinae were isolated and cryoprotected in graded sucrose solutions (10, 20 and 30%w/v respectively.). Cryostat sections were cut at 14 μm, collected on gelatin-coated slides and stored at −20°. For the spinal cord preparations a small portion from the lumbar part was dissected, fixed for 10 min by immersion and cryoprotected; so, it was treated like the retina. Cryostat sections of 12 μm were used.
Immunocytochemical labeling was obtained by indirect immunofluorescence. First, the sections were incubated overnight in one of the following primary antibodies:
-
1.
A monoclonal mouse antibody against the GlyR mAb4a (GlyR “all”, 1:100). MAb4a recognizes an epitope between positions 96 and 105 of α1 subunit (Schröder et al. 1991), which is highly conserved in all α subunits and in the β subunit (Harvey et al. 2000). It is assumed that mAb4a is able to reveal all possible isoforms of the glycine receptor, no matter what its subunits’ composition is. The antibody was kindly provided by H. Betz (Frankfurt/M, Germany).
-
2.
A monoclonal mouse antibody mAb2b which recognizes the α1 subunit of the receptor (GlyRα1, 1:100, 1:50) (Pfeiffer et al. 1984). The antibody was kindly provided by H. Betz (Frankfurt/M, Germany).
-
3.
A polyclonal rabbit antiserum against α2 subunit of the receptor (GlyRα2, 1:1000) (Haverkamp et al. 2004); kind gift of R. Harvey (London).
-
4.
A polyclonal rabbit antiserum against α3 subunit of the receptor (GlyRα3, 1:1000) (Haverkamp et al. 2003); kind gift of R. Harvey (London).
-
5.
A monoclonal mouse antibody against gephyrin mAb7a (Geph, 1:100). It binds to 93 kDa protein gephyrine (Pfeiffer et al. 1984), which interacts with the cytoskeletal component tubulin and is responsible for clustering of the glycine receptors at postsynaptic sites. mAb7a does not cross react with any of the glycine receptor polypeptides (Schmitt et al.1987). The antibody was kindly provided by H. Betz (Frankfurt/M, Germany).
After the incubation in primary antibody, 1 h incubation in one of the following secondary antibodies was achieved: goat anti-mouse or goat anti-rabbit, coupled to either AlexaTM 488 or AlexaTM 594 and causing green or red fluorescence, respectively (1:500, Molecular Probes, Eugene, USA). The control sections were processed the same way, but the primary antibodies were omitted. No specific staining was encountered in these sections.
In the double-labeling experiments the sections were incubated in a mixture of primary antibodies first, and in a mixture of secondary antibodies afterwards.
The sections were examined and photographed using Zeiss Axiophot fluorescence microscope (Oberkochen, Germany) and cooled CCD camera (Universal Imaging, West Chester, PA). All the images were processed with Adobe Photoshop 6.0.
Results
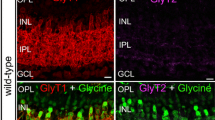
A low-power micrograph of vertical section through the retina of X. laevis immunostained for all subunits of the glycine receptor by the monoclonal antibody mAb4a (GlyR “all”) is represented in Fig. 1a. On the Nomarski micrograph (Fig. 1b) the retinal layers of the section are denoted. It can be seen (Fig. 1a) that the immunoreactivity is mainly located in both plexiform layers of the retina: the inner plexiform layer (IPL) and the outer plexiform layer (OPL).
Fluorescent micrographs of vertical sections through the retina of Xenopus laevis immunolabeled for all subunits of the glycine receptor (mAb4a, GlyRs “all”). a Low-power micrograph showing the distributions of GlyR “all” labels in both plexiform layers, OPL and IPL. b Nomarski micrograph showing the retinal layers: IS photoreceptors inner segments; ONL outer nuclear layer; OPL outer plexiform layer; INL inner nuclear layer; IPL inner plexiform layer; GCL ganglion cell layer. Scale bar 55 μm. c High-power micrograph showing the punctate labeling in the IPL. d Nomarski micrograph, showing the retinal layers: the indications are as denoted earlier; scale bar 20 μm
The immunolabeling in the IPL has a punctate appearance which reflects the clustering of the receptor subunits in the postsynaptic densities, as was demonstrated previously for GlyRs and for other membrane receptors by electron microscopy (Brandstätter et al. 1998; Wässle et al. 1998). The distribution of the puncta across the layer is uneven. It can be noticed that the magnitude and the density of the puncta increase in the direction of the distal to the proximal part of the layer. The punctate appearance of the label in the IPL is better seen at higher power resolution in Fig. 1c.
The immunostaining in the OPL is forming a single discontinuous band (Fig. 1a). Again the punctate appearance, typical for all kinds of membrane receptors, can be seen. The size and the density of the puncta are smaller than in the IPL.
In order to establish the subunits’ composition of the glycine receptors, in addition to the mAb4a three other antibodies directed against the α1, α2 and α3 subunits, respectively, were tested. The monoclonal antibody against the α1 subunit of the glycine receptor mAb2b (GlyRα1) which was applied at several different dilutions failed to cause any significant immunostaining in the retina. In contrast, the two polyclonal antisera against the α2 and α3 subunits of the receptor caused strong and bright immunofluorescence.
The immunostaining with the novel polyclonal antibody against the α2 subunit of the receptor (GlyRα2) is shown in Fig. 2. The antibody is raised in rabbit against the peptide CTYKIIRHEDVHKK, which comprises the C-terminal 13 amino acids of the mouse GlyR α2 subunit, with an additional N-terminal cystein residue (Haverkamp et al. 2004). It can be seen (Fig. 2a) that the GlyR α2 immunofluorescence is located mainly in the IPL. Two horizontal bands can be distinguished: an upper (distal) band which is thin and slender and a lower (proximal) one which is broader and brighter. No immunofluorescence in the OPL exists. However, few bright puncta scattered in the inner nuclear layer (INL) can be seen.
Low-power fluorescent micrograph of a vertical section through the retina immunolabeled for α2 subunit of the glycine receptor (GlyRα2). a The GlyRα2 labeling is distributed mainly in the IPL. Single puncta in the INL also exist. b Nomarski micrograph, showing the retinal layers: the indications are as in Fig. 1; scale bar 55 μm
The immunostaining with the novel polyclonal antibody against the α3 subunit of the receptor (GlyRα3) (Haverkamp et al. 2003) is represented in Fig. 3. The labels are expressed in the plexiform layers of the retina. Fine distinct puncta distributed in several horizontal bands can be seen in the IPL. Similar puncta forming a single horizontal band are located in the OPL.
Low-power fluorescent micrograph of a vertical section through the retina of X. laevis immunolabeled for α3 subunit of the glycine receptor (GlyRα3). a The GlyRα3 labeling is distributed in the OPL and the IPL. b Nomarski micrograph, showing the retinal layers: the indications are as in Fig. 1; scale bar 55 μm
In order to confirm indirectly the existence of glycine receptors in X. laevis, we stained the retina with the monoclonal antibody mAb7a (Geph) directed against the protein Gephyrin which is known to participate in the formation of the glycine receptor complex. The immunoreactivity against the Gephyrin is shown in Fig. 4. It is spread widely in the retina causing strong and bright inmunofluorescence in different layers. Two types of staining can be distinguished: staining of cell processes and staining of cell bodies. The staining of the cell processes is located in the IPL. It has a punctate character and resembles too much the immunolabeling caused by the glycine receptors antibodies (Fig. 4c). This staining may be determined as synaptic staining. In contrast, the staining of a great number of cell bodies in the nuclear layers of the retina is an example of extrasynaptic staining. It may be supposed that the antibody stains those cell bodies where Gephyrin is synthetized, packed and processed for further transportation toward the postsynaptic membranes.
Fluorescent micrograph of a vertical section through the retina of X. laevis immunolabeled for gephyrin (Geph). a Low-power micrograph, showing the punctate Geph labels in the IPL, as well as the Geph labels in the ONL, INL and GCL, where a lot of cell bodies are stained. b Nomarski micrograph, showing the retinal layers: the indications are as in Fig. 1; scale bar 55 μm. c the region from the boxed area in (a) is represented here at higher magnification in order the punctate labels are better seen; scale bar 12.5 μm
Our next aim was to discover if co-localization exists between the puncta stained with antibody mAb4a (GlyR “all”) directed against all subunits of the receptor and the puncta stained with the antibodies GlyRα2 and GlyRα3 directed against the α2 and the α3 subunits of the receptor, respectively. In order to study this, we made several double-labelings.
Figure 5 shows sections double-labeled for GlyRα2 and GlyR“all” subunits. The left half of a section immunolabeled for GlyRα2 is represented in Fig. 5a. The same section is immunolabeled for GlyR“all” and its right half is represented in Fig. 5b. The left and the right halves of the section are aligned one to another so that the continuity of the section “to be restored”. It can be seen that the patterns of staining of the two halves are very much alike in certain regions and differ in other regions. The thick bright band in the proximal part of the IPL can be followed in both halves of the section. However, the thin band in the IPL that is very well expressed in the GlyRα2 staining (Fig. 5a) has no counterpart in the GlyR“all” staining (Fig. 5b). At the lower panel of the same figure (Fig. 5d, e) one and the same region of another section double-labeled for GlyRα2 (Fig. 5d) and GlyR“all” (Fig. 5e) is represented at high power resolution. It can be seen that the area surrounded by boxes contains puncta which are immunopositive both for GlyRα2 and for GlyR“all” antibodies.
Double-labeling with GlyRα2 and mAb4a (GlyRs “all”) antibodies. a–c Low-power micrographs of the left (a) and right (b) halves of one and the same vertical section of the retina immunolabeled for GlyRα2 subunit (a) and for all subunits of the glycine receptor (GlyRs “all”) (b); c Nomarski micrograph of the section showing the retinal layers—the indications are as in Fig. 1; scale bar 55 μm. d, e High power micrographs of one and the same region from a vertical section of the retina immunolabeled for GlyRα2 subunit (d) and for all subunits of the glycine receptor (GlyRs “all”) (e). In each of the micrographs a group of puncta from the IPL is enclosed in a box. The comparison between the puncta enclosed shows that co-localizations between them exist. Scale bar 20 μm
Figure 6a and b shows a section double-labeled for GlyRα3 (Fig. 6a) and GlyR“all” subunits (Fig. 6b). On the figures one and the same area of the IPL is represented at high power resolution. It can be noticed that many puncta are in register. Some of them are denoted by means of two surrounding boxes.
High-power micrographs of double-labeled vertical sections of the retina. a, b Double-labeling with GlyRα3 antibody (a) and with GlyRs “all” antibody (b) of one and the same region of the IPL. c, d Double-labeling with GlyRα3 antibody (c) and with Geph antibody (d). The puncta enclosed in boxes, as well as the puncta indicated by the two sets of arrows, co-localize. Scale bar 20 μm
Next, we made double-labeling with GlyRα3 and Gephyrin (Geph) antibodies (Fig. 6c, d). On the figures one and the same area of the IPL labeled for GlyRα3 (Fig. 6c) and Geph (Fig. 6d) is represented at high power resolution. By means of surrounding boxes and two sets of arrows some co-localizations of the puncta that are immunopositive both for GlyRα3 and Geph are shown.
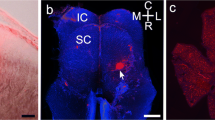
In order to clarify what is the reason for the absence of α1 subunit labels in Xenopus retina, we stained the Xenopus spinal cord with the mAb2b antibody (Fig. 7). As can be seen in Fig. 7d, no mAb2b immunofluoresence can be detected in the spinal cord. The same areas, however, stained with mAb4a showed bright immunofluorescence (Fig. 7b). Clearly, mAb4a recognizes the glycine receptors in the spinal cord. In contrast, mAb2b does not recognize GlyRα1 subunit both in the spinal cord and the retina of Xenopus.
Fluorescent micrographs of two sections through the spinal cord of X. laevis. a, b Nomarski image (a) and mAb4a immunofluorescence (b) in the same field of a spinal cord section. c, d Nomarski image of another spinal cord section (c), immunostained with mAb2b (d). No immunofluorescence can be detected in (d). Scale bar 50 μm
Discussion
In accordance with our preliminary expectations, we succeeded in revealing immunocytochemically glycine receptors in the retina of X. laevis, a species widely used in the experimental practice. Our results are in agreement with the electrophysiological data according to which glycine is involved in the surround responses of both on- and off-bipolar cells in X. laevis retina (Stone and Schütte 1991). Our results are in agreement with the immunocytochemical data of other authors who have also demonstrated glycine receptors in the lower vertebrate retinae (Smiley and Yazulla 1990; Yazulla and Studholme 1991a, b, 2001; Zucker and Ehinger 1993). In the majority of these studies the antibody mAb7a which recognizes the 93 kDa protein gephyrin has been used. However, the recent data show that gephyrin is not a subunit of the glycine receptor. Although first co-purified with the mammalian glycine receptors (Graham et al. 1985), now gephyrin is considered to be a distinct protein anchoring both the glycine and GABAA receptors toward the cytoskeleton (Sassoè-Pognetto et al. 1995; Fischer 2000; Legendre 2001). Hence, our data are the first to demonstrate glycine receptors in X. laevis retina using the monoclonal antibody mAb4a (GlyR“all”) directed against all subunits of the receptor.
As revealed by mAb4a, the glycine receptors in X. laevis retina are located in the IPL and the OPL. The glycine receptors distribution in the IPL is uneven and a trend for lamination of the layer can be distinguished (Fig. 1). This means that the glycine receptors in the IPL are located in several different types of synapses, and so mediate the effects of several distinct types of glycinergic neurons. Previous data about the glycine immunoreactivity in both frog (Smiley and Basinger 1989; Connaughton et al. 1999; Vitanova et al. 2003) and kindred tiger salamander retina (Watt and Florack 1993; Yang 1996; Wu and Maple 1998) allow us to suppose that these neurons are different subtypes of glycinergic amacrine cells.
As concerns the OPL, only a single band of staining is seen. The glycine receptor immunoreactivity revealed in this layer can be regarded to for the synaptic contacts which the glycinergic interplexiform cells make here, as have been described for X. laevis and fish retinae (Smiley and Basinger 1988; Wu and Maple 1998; Connaughton et al. 1999).
Our next aim was to determine the subunit composition of the glycine receptors in Xenopus retina. For this purpose we used the monoclonal antibody mAb2b directed against the α1 subunit and two new antisera directed against the α2 and α3 subunits. According to our data, the glycine receptors in X. laevis retina are composed of α2 and α3 subunits. We have not observed specific labeling with the monoclonal antibody mAb2b which causes punctate labeling in the frog retina of R. ridibunda (Vitanova et al. 2004) and zebrafish retina (Yazulla and Studholme 2001). The failure of mAb2b to label the GlyRα1 subunit in X. laevis may be due to the following reason. It is known that mAb2b is specific for the N-terminal ten residues of the α1 subunit of the glycine receptor (Schröder et al. 1991). As was recently shown by molecular cloning, the N-terminal sequence is very different amongst the different glycine receptors, as well as between the GlyRα1 subunits of different species (Gisselmann et al. 2002). It is quite likely that this is the explanation why mAb2b recognizes the GlyRα1 subunit in the R. ridibunda retina (Vitanova et al. 2004) and does not recognize it in X. laevis retina. In contrast, the monoclonal antibody mAb4a recognizes an amino-acid sequence located more toward the center of the glycine receptor, which is highly conserved in all subunits. That is why it labels the glycine receptors in both R. ridibunda and X. laevis retinas, as well as the glycine receptors in X. laevis spinal cord.
As revealed by the polyclonal GlyRα2 antibody, the α2 subunit is mainly located in the IPL of X. laevis retina (Fig. 2). The finding of GlyRα2 subunit in the adults is somewhat curious, because until recently it was a common belief that α2 subunit is an embryonic and neonatal form of the glycine receptors in the brain. The expression of this subunit decreases after birth, whereas the expression of α1 and α3 subunits increases (Singer et al. 1998). Nonetheless, α2 subunit mRNA is still present at reduced densities in the adult nervous system (Racca 1998) and in the rat retina as well (Greferath et al. 1994; Enz and Bormann 1995). Recently, GlyRα2 immunoreactivity was demonstrated in the IPL of mouse (Haverkamp et al. 2004) and monkey retina (Jusuf et al. 2005). Now we successfully revealed synaptic localization of the GlyRα2 subunit in lower vertebrate retina.
The GlyRα2 immunoreactivity is distributed mainly in the IPL. No labeling can be seen in the OPL of the retina (Fig. 2). The GlyRα2 immunostaining has punctate appearance which is typical for the membrane receptors staining (Brandstätter et al. 1998; Wässle et al. 1998). In double-labeling studies we demonstrated puncta which are immunopositive both for GlyRα2 and for mAb4a (GlyR“all”) antibodies (Fig. 5a, b). It is evident, however, that not all the puncta co-localize. Some of the GlyRα2 immunopositive puncta located mainly in the distal part of IPL and the nuclear layers are not stained with the mAb4a antibody. The reason that not all GlyRα2 puncta are stained with mAb4a is still unclear; may be the monoclonal antibody mAb4a does not recognize all GlyRα2 subunits, because some antigenic sites are masked within the subunits when they all assemble to form the channel.
It might be supposed that some of the GlyRα2 labels are located extrasynaptically. This is in agreement with the data about the nonsynaptic localization of great part of the α2 subunits of the glycine receptors in the central nervous system. It had been proposed that these extrasynaptically located α2 subunits might play an important role in the tonic inhibition of neurons (for review see Laube et al. 2002).
GlyRα3 immunoreactivity is distributed mainly in the IPL and the OPL of X. laevis retina (Fig. 3). Recently, we observed similar distribution of the α3 subunit in the frog retina of R. ridibunda (Vitanova et al. 2004). GlyRα3 subunit is very well studied in mammalian and primate retina as well (Haverkamp et al. 2003; Jusuf et al. 2005). Our data show that the GlyRα3 immunostaining consists of brightly fluorescent puncta. Several distinct bands in the IPL and a single band in the OPL can be followed in the retina (Fig. 3). In double-labeling studies many puncta which are immunopositive both for GlyRα3 and for mAb4a (GlyR“all”) antibodies can be seen (Fig. 6a, b). However, mAb4a does not recognize all GlyRα3 subunits and this might be explained by the same reason proposed to explain failure of full co-localization between GlyRα2 and mAb4a immunopositive puncta.
The presence of α3 subunit in the X. laevis retina was indirectly proved in double-labeling studies with Gephyrin: co-localizations between the puncta labeled for GlyRα3 and the puncta labeled for Gephyrin can be seen (Fig. 6c, d). This is in agreement with the fact that all glycine receptors in the retina depend on the gephyrin for their synaptic localization. It is known that in the gephyrin knockout mice the glycine receptors are no longer clustered at the postsynaptic membrane (Fisher et al. 2000). In the double-labeled section GlyRα3/Geph many puncta do not co-localize (Fig. 6a, b) and it can be explained by the fact that Gephyrin is an anchoring protein for all glycine receptors (not only for the receptors containing GlyRα3), and for GABAa receptors as well (Laube et al. 2002).
Taken together, two different kinds of GlyRs’ subunits in Xenopus retina were revealed: the α2 and the α3 subunits. Using the same antibodies, α1 and α3 subunits were recently revealed in the kindred frog retina of R. ridibunda (Vitanova et al. 2004). It is worth noticing that two closely related retinae (Xenopus vs Rana) differ in some of their immunocytochemical characteristics, a fact already described for other types of retinae (Yazulla and Studholme 2001). It is highly improbable that this is the reason for the absence of a certain subunit from a certain type of retina. It is more likely that slight differences in the amino-acid sequence of a certain subunit do not allow it to be revealed with the same antibody in the other type of retina (as we showed for the α1 subunit).
Discussing the presence of the two types of α subunits in the retina of X. laevis: α2 and α3 respectively, a question arises—if these subunits are localized in one and the same or in different types of glycinergic synapses. As the antibodies against both the α2 and the α3 subunits are raised in rabbit, it is difficult to perform double-labeling and to prove or to exclude this possibility. But, let us compare the patterns of distribution of both types of immunofluorescences. They differ from one another in several aspects. While GlyRα3 immunoreactivity is present in both plexiform layers, GlyRα2 immunoreactivity is present only in the IPL and here its pattern of distribution differs from the pattern of distribution of GlyRα3 immunoreactivity (compare Figs. 2, 3). That is why it is very likely that the GlyRα2 subunit and the GlyRα3 subunit are located in different subtypes of glycine receptors. So, in X. laevis retina several distinct types of glycine receptors might exist, each of them having a specific subunits’ composition and, as a consequence, specific functional properties. The pharmacological and electrophysiological data of several authors support our notion about the diversity of the glycine receptors. It has been shown that the modulatory effects of neurosteroids on the human recombinant glycine receptors depend on the subunits’ composition: pregnenolone potentiates only the receptors containing α1 subunit, while progesterone inhibits only the receptors containing α2 subunit (Maksay et al. 2001). In addition, two distinct glycinergic currents have been demonstrated by means of whole-cell recordings: one elicited at the dendrites and another at the axon terminals of the bipolar cells (Maple and Wu 1998; Du and Yang 2002a, b). This is consistent with our notion of the presence of two different subtypes of glycine receptors in the OPL and IPL, located on the dendrites and the axons of the bipolar cells, respectively. Recently, two different subtypes of glycine receptors were localized in the somatodendritic and the axonal membrane of the supraoptic nucleus neurons as well (Deleuze et al. 2005).
It might be supposed that the GlyRs in the IPL also are not homogenous population. Since the time of Ramon y Cajal (1893) it is known that an exquisite stratification of the frog IPL exists: the bipolar cell axon terminal, the amacrine cell processes and the ganglion cell dendrites all exhibit specific stratification pattern here. In anuran retina Vigh et al. (2000) have described 21 different types of amacrine cells, at least 2 of them being glycinergic ones. That is why at least two different subtypes of glycine receptors might exist at the level of IPL, which is in agreement with our immunocytochemical studies and with our notion about the diversity of glycine receptors.
In conclusion, the presence of glycine receptors in X. laevis retina is shown immunocytochemically. They are distributed in the OPL and the IPL of the retina. The composition of the receptors includes α2 and α3 subunits. These subunits are most probably located in different synapses and are involved with different nervous circuits and functions.
References
Brandstätter JH, Koulen P, Wässle H (1998) Diversity of glutamate receptors in the mammalian retina. Vision Res 38:1385–1397
Cajal SR (1893) La retiné des vertébrés. Cellule 9:119–257
Connaughton V, Behar T, Liu W, Massey S (1999) Immunocytochemical localization of excitatory and inhibitory neurotransmitters in the zebrafish retina. Vis Neurosci 16:483–490
Deleuze C, Runquist M, Orcel H, Rabie A, Dayanithi G, Alonso G, Hussy N (2005) Structural difference between heteromeric somatic and homomeric axonal glycine receptors in the hypothalamo-neurohypophysial system. Neurosci 135(2):475–483
Du J, Yang X (2002a) Bullfrog retinal bipolar cells may express heterogeneous glycine receptors at dendrites and axon terminals. Neurosci Lett 322:177–181
Du J, Yang X (2002b) Glycinergic synaptic transmission to bullfrog retinal bipolar cells is input-specific. Neuroscience 113:779–784
Enz R, Bormann J (1995) Expression of glycine receptor subunits and gephyrin in single bipolar cells of the rat retina. Vis Neurosci 12:501–507
Fischer F, Kneussel M, Tintrup H, Haverkamp S, Rauen T, Wässle H (2000) Reduced synaptic clustering of GABA and glycine receptors in the retina of the gephyrin null mutant mouse. J Comp Neurol 427:634–648
Gisselmann G, Galler A, Friedrich F, Hatt H, Bormann J (2002). Cloning and functional characterization of two glycine receptor α subunits from the perch retina. Eur J Neurosci 16(1):69–80
Graham D, Pfeiffer F, Simler R, Betz H (1985) Purification and characterization of the glycine receptor of pig spinal cord. Biochem 24:990–994
Greferath U, Brandstätter JH, Wässle H, Kirsch H, Kuhse J, Grünert U (1994) Differential expression of glycine receptor subunits in the retina of the rat: a study using immunocytochemistry and in situ hibridization. Vis Neurosci 11:721–729
Grünert U (2000) Distribution of GABA and Glycine receptors on bipolar and ganglion cells in the mammalian retina. Microsc Res Tech 50:130–140
Grünert U, Wässle H (1993) Immunocytochemical localization of glycine receptors in the mammalian retina. J Comp Neurol 335:523–537
Grünert U, Wässle H (1996) Glycine receptors in the rod pathway of the macaque monkey retina. Vis Neurosci 13:101–115
Harvey R, Schmieden V, von Holst A, Laube B, Rohrer H, Betz H (2000) Glycine receptors containing the α4 subunit in the embrionic sympathetic nervous system, spinal cord and male genital ridge. Eur J Neurosci 12:994–1001
Haverkamp S, Wässle H. (2000) Immunocytochemical analysis of the mouse retina. J Comp Neurol 424:1–23
Haverkamp S, Muller U, Harvey K, Harvey RJ, Betz H, Wässle H (2003) Diversity of glycine receptors in the mouse retina: localization of the α3 subunit. J Comp Neurol 465:524–539
Haverkamp S, Muller U, Zeilhofer HU, Harvey RJ, Wässle H (2004) Diversity of glycine receptors in the mouse retina: localization of the alpha2 subunit. J Comp Neurol 477:399–411
Jusuf P, Haverkamp S, Grünert U (2005) Localization of glycine receptors alpha subunits on bipolar and amacrine cells in primate retina. J Comp Neurol 488:113–128
Laube B, Maksay G, Schemm R, Betz H (2002) Modulation of glycine receptor function: a novel approach for therapeutic intervention at inhibitory synapses. Trends Pharmacol Sci 23:519–527
Legendre P (2001) The glycinergic inhibitory synapse. CMLS 58:760–793
Lin B, Martin PR, Solomon SG, Grunert U (2000) Distribution of glycine receptor subunits on primate retinal ganglion cells: aquantitative analysis. Eur J Neurosci 12:4155–4170
Maksay G, Laube B, Betz H (2001) Subunit-specific modulation of glycine receptors by neurosteroids. Neuropharm 41:369–376
Maple B, Wu S (1998) Glycinergic synaptic inputs to bipolar cells in the salamander retina. J Physiol 506:731–744
Matzenbach B, Maulet Y, Sefton L, Courtier B, Avner P, Guénet G-L, Betz H (1994) Structural analysis of mouse glycine receptor α subunit genes: identification and chromosomal localization of a novel variant, α4. J Biol Chem 269:2607–2612
Pfeiffer F, Simler R, Grenningloh G, Betz H (1984) Monoclonal antibodies and peptides mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc Natl Acad Sci USA 81:7224–7227
Popova E, Mitova L, Vitanova L, Kupenova P (1997) Effect of glycinergic blockade on light responses of frog retinal ganglion cells. Comp Biochem Physiol 116:255–263
Popova E, Mitova L, Vitanova L, Kupenova P (2000) Effect of 2-amino-4-phosphonobutyrate on the OFF-responses of frog retinal ganglion cells and local ERG after glycinergic blockade. Comp Biochem Physiol 126:139–151
Racca C, Gardiol A, Triller A (1998) Cell-specific dendritic localization of glycine receptor α subunit messenger RNAs. Neuroscience 84:997–1012
Sassoè-Pognetto M, Wässle H, Grünert U (1994) Glycinergic synapses in the rod pathway of the rat retina: cone bipolar cells express the α1 subunit of the glycine receptor. J Neurosci 14:5131–5146
Sassoè-Pognetto M, Grünert U, Greferath U, Fritschy J-M, Mohler H, Betz H, Wässle H (1995) Colocalization of gephyrin and GABAA-receptor subunits in the rat retina. J Comp Neurol 357:1–14
Schmitt B, Knaus P, Becker C-M, Betz H (1987) The M 93 000 polipeptide of the postsynaptic glycine receptor complex is a peripheral membrane protein. Biochem 26:805–811
Schröder S, Hoch W, Becker C-M, Grenningloh G, Betz H (1991) Mapping of antigenic epitopes on the α1 subunit of the inhibitory glycine receptor. Biochem 30:42–47
Singer J, Talley E, Bayliss D, Berger A (1998) Development of glycinergic synaptic transmission to rat brain stem motoneurons. J Neurophysiol 80:2608–2620
Smiley J, Basinger S (1988) Somatostatin-like immunoreactivity and glycine high-affinity uptake colocalize to an interplexiform cell of the Xenopus laevis retina. J Comp Neurol 274:608–618
Smiley J, Basinger S (1989) Glycine high-affinity uptake labels a subpopulation of somatostatin-like immunoreactive cells in the Rana pipiens retina. Brain Res 495:31–44
Smiley J, Yazulla S (1990) Glycinergic contacts in the outer plexiform layer of the Xenopus laevis retina characterized by antibodies to Glycine, GABA and Glycine receptors. J Comp Neurol 299:375–388
Stone S, Schütte M. (1991) Physiological and morphological properties of on- and off-center bipolar cells in the Xenopus retina: effects of glycine and GABA. Vis Neurosci 7:363–376
Vigh J, Banvolgyi P, Wilhelm M. (2000) Amacrine cells of the anuran retina: morphology, chemical neuroanatomy and physiology. Microsc Res Tech 50:373–383
Vitanova L, Haverkamp S, Wässle H (2003) Comparative investigation of glycine receptors in lower vertebrate retinae. Compt Rend l’Acad Bulg Sci 56:33–40
Vitanova L, Haverkamp S, Wässle H (2004) Immunocytochemical localization of glycine and glycine receptors in the retina of the frog Rana ridibunda. Cell Tissue Res 317:227–235
Wässle H, Koulen P, Brandstätter JH, Fletcher EL, Becker CM (1998) Glycine and GABA receptors in the mammalian retina. Vision Res 38:1411–1430
Watt C, Florack V (1993) Colocalization of glycine in substance P-amacrine cells of the larval tiger salamander retina. Vis Neurosci 10:899–906
Wu S, Maple B (1998) Amino acid neurotransmitters in the retina: a functional overview. Vision Res 38:1371–1384
Yang C (1996) Glutamate immunoreactivity in the tiger salamander retina differentiates between GABA-immunoreactive and glycine-immunoreactive cells. J Neurocytol 25:391–403
Yazulla S, Studholme KM (2001) Neurochemical anatomy of the zebrafish retina as determined by immunocytochemistry. J Neurocytol 30:551–592
Yazulla S, Studholme KM (1991a) Glycinergic interplexiform cells make synaptic contact with amacrine cell bodies in goldfish retina. J Comp Neurol 310:1–10
Yazulla S, Studholme KM (1991b) Glycine receptor immunoreactivity in retinal bipolar cells is postsynaptic to glycinergic and GABAergic amacrine cell synapses. J Comp Neurol 310:11–20
Zucker CL, Ehinger B (1993) Synaptic connections involving immunoreactive glycine receptors in the turtle retina. Vis Neurosci 10:907–914
Acknowledgments
I am very grateful to Heinz Wässle (MPI for Brain Research, Frankfurt/Main, Germany) for the staining of Xenopus spinal cord and to Silke Haverkamp (MPI for Brain Research, Frankfurt/Main, Germany) for her support and encouragement during my work on this paper. The work is partially supported by the Council for Medical Science in Medical University Sofia, Bulgaria (grant 3/04).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vitanova, L. Immunocytochemical study of glycine receptors in the retina of the frog Xenopus laevis . Anat Embryol 211, 237–245 (2006). https://doi.org/10.1007/s00429-005-0076-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-005-0076-7