Abstract
Glycine and glycine receptors (GlyRs) were analyzed immunocytochemically in the retina of the frog Rana ridibunda. Glycine was localized to somata of glycinergic amacrine and interplexiform cells. Approximately 50% of the cells in the amacrine cell layer were found to be glycinergic. GlyRs of the inner plexiform layer (IPL) were localized to brightly fluorescent puncta, probably representing postsynaptic clusters of GlyRs. GlyR clusters were not evenly distributed across the IPL but showed patterns of stratification specific for the various GlyR subunits. Clusters containing the α1 subunit formed four narrow strata within the IPL. Clusters containing the α3 subunit were more abundant and covered the whole IPL, with a band of higher density in stratum 3. Clusters of GlyRs were also observed in the outer plexiform layer. Thus, several isoforms of synaptic GlyRs involved with different synapses and inhibitory circuits are present in the frog retina.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glycine, together with γ-aminobutyric acid (GABA), is a major inhibitory neurotransmitter of the vertebrate retina (for reviews, see Marc 1985, 1989; Pourcho 1996; Kalloniatis and Tomisich 1999). Dependent on the species investigated, glycine has been localized to 30%–50% of all amacrine cells comprising many different morphological types (Pourcho and Goebel 1985; Yazulla and Studholme 1990; MacNeil and Masland 1998; Menger et al. 1998). A subpopulation of interplexiform cells appears to be glycinergic, but their numbers vary between species and depend on the methods applied for their labeling (uptake of 3H-glycine or glycine immunoreactivity; Marc and Lam 1981; Marc 1985; Yazulla and Studholme 1990). Glycine has also been localized to bipolar cells, especially in the mammalian retina. However, these cells have been shown to accumulate glycine through their gap junctions with glycinergic amacrine cells (for reviews, see Pow 2001; Vaney 2002), and it is debatable whether they release glycine as their transmitter.
The action of glycine is mediated through distinct receptors, a chloride conductance being activated upon the binding of glycine with such a receptor (for reviews, see Vannier and Triller 1997; Legendre 2001; Laube et al. 2002). The glycine receptor (GlyR) is composed of ligand-binding α subunits and structural β subunits that bind to the clustering protein gephyrin. In the adult, three copies of the α subunit and two copies of the β subunit form the pentameric receptor protein. Molecular cloning has revealed four genes coding for the α1, α2, α3, and α4 subunits, and only one gene coding for the β subunit (Matzenbach et al. 1994).
The localization of GlyRs and gephyrin in the mammalian retina has been studied by using three monoclonal antibodies (mAbs): mAb2b recognizes the α1 subunit, mAb4a the α1, α2, α3, α4, and, to a lesser extent, the β subunits, and mAb7a recognizes most isoforms of gephyrin (Pfeiffer et al. 1984; Schröder et al. 1991). Recently, a new polyclonal antibody that recognizes the α3 subunit has been raised in rabbits (Haverkamp et al. 2003). Both light and electron microscopy have shown that GlyRs are clustered at postsynaptic sites (Sassoè-Pognetto et al. 1994; Sassoè-Pognetto and Wässle 1997). Gephyrin is also clustered at postsynaptic sites (Pourcho and Owczarzak 1991a, 1991b) and acts as a scaffold protein that binds GlyRs to the cytoskeleton. It is not only essential for the synaptic aggregation of GlyRs, but also for most isoforms of GABAA receptors (Sassoè-Pognetto et al. 1995; Essrich et al. 1998; Feng et al. 1998; Kneussel et al. 1999; Fischer et al. 2000).
Relatively little is known about GlyRs in lower vertebrate retinae. The existing data concern fish and Xenopus laevis retinae, and the synaptic localization of gephyrin (mAb7a) has been described by Smiley and Yazulla (1990), Yazulla and Studholme (1991a, 1991b), and Zucker and Ehinger (1993). Because gephyrin is also involved with the clustering of GABAA receptors, it can no longer be considered as a specific marker of glycinergic synapses. The distribution of GlyRα1 expressing synapses has recently been revealed in the zebrafish retina by immunostaining with mAb2b (Yazulla and Studholme 2001).
In the present study, we have localized immunocytochemically the GlyRs in the retina of the frog Rana ridibunda, a species that we have widely used in electrophysiological experiments (Kupenova 1997; Popova et al. 1997, 2000; Vitanova et al. 1997). We have applied the antibodies mAb2b (against GlyRα1), mAb7a (against gephyrin), and mAb4a (against “all” GlyRs), and a novel antibody against GlyRα3 (Haverkamp et al. 2003).
Materials and methods
Tissue preparation
All procedures were in accordance with the Bulgarian law for animal experiments. Retinae of the frog R. ridibunda were examined. The animals were deeply anesthetized with halothane and decapitated. The eyes were removed and dissected, and the posterior eyecups with retina were immediately immersed in 4% (w/v) paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4, for 15–30 min. After fixation, the retinae were dissected from the eyecup and cryoprotected in graded sucrose solutions (10%, 20%, and 30% w/v), respectively. Cryostat sections were cut at 14 μm, mounted on slides, and stored at −20°C.
Antibodies
Three mAbs raised against purified GlyR were used; they were kindly provided by Heinrich Betz, Frankfurt. Their preparation and specificity have been described in detail previously (Pfeiffer et al. 1984; Schmitt et al. 1987; Schröder et al. 1991). Briefly, mAb2b recognizes a single band of 48 kDa in Western blots of purified spinal cord GlyR preparations (Pfeiffer et al. 1984) and has been shown to be specific for the 10 N-terminal residues of the GlyRα1 subunit (Schröder et al. 1991). MAb4a recognizes an epitope between positions 96 and 105 of the GlyRα1 subunit (Schröder et al. 1991), which is highly conserved in all α subunits and the β subunit (Grenningloh et al. 1987, 1990; Kuhse et al. 1990; Harvey et al. 2000). MAb7a is specific for the 93-kDa protein gephyrin (Pfeiffer et al. 1984) and does not cross-react with any of the other GlyR polypeptides (Schmitt et al. 1987). The antibodies were diluted 1:100.
A polyclonal goat antibody against GlyRα2 (N-18) was purchased from Santa Cruz Biotechnology (Heidelberg, Germany) and was used at a dilution of 1:200. A polyclonal rabbit antibody against the C-terminus of GlyRα3 was used at a dilution of 1:1,000. This antibody was a kind gift from R.H. Harvey (London), and its specificity is described in Haverkamp et al. (2003). A polyclonal antiserum against glycine raised in rat was a kind gift from David Pow (Brisbane) and was used at a dilution of 1:1,000 (Pow et al. 1995).
Western blot analysis
Western blots were performed on homogenates prepared from frog retina. Unfortunately, fresh tissue was not available, and frozen tissue had to be used. Tissues were homogenized in buffer containing 0.32 M sucrose, 0.04 M HEPES, and a protease inhibitor cocktail (Complete mini, Roche Diagnostics, Mannheim). After a low-speed spin (2,000g, 3 min), the supernatant was spun at 13,000g for 20 min at 4°C. The pellets were resuspended in homogenization buffer. Protein (20 μg) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 10% gel and was transferred to nitrocellulose by using standard techniques. After being blocked in 5% (w/v) powdered milk in TRIS-buffered saline (TBS: 0.01 M TRIS–HCl, pH 7.4, 0.15 M NaCl) containing 0.05% (v/v) Tween-20 (TBST) for 1 h at room temperature, the membranes were incubated in mAb4a (1:100) overnight. After three washes for 10 min each in TBST, the blots were incubated in goat anti-mouse IgG conjugated to horseradish peroxidase (New England Biolabs, 1:2,000) in TBST for 1 h. Following washes in TBST and TBS, the signal was developed in the blots by using enhanced chemiluminescence (Amersham Biosciences).
Western blots of retina showed that antibody mAb4a recognized a single band with an apparent molecular weight of ≈35 kDa (see below). This corresponds to the fragment of 35 kDa of GlyRs after proteolytic cleavage. Cleavage of GlyRs is known to be rapid (Büttner et al. 2001), and since we only had access to frozen tissue, this cleavage is to be expected.
Immunocytochemistry
The antibodies were diluted in phosphate-buffered saline (PBS), pH 7.4, containing 5% Chemiblocker (Chemicon, Hofheim) and 0.5% (w/v) Triton X-100. Immunocytochemical labeling was performed by the indirect fluorescence method. After preincubation in PBS containing 5% Chemiblocker and 0.5% (w/v) Triton X-100, the sections were incubated overnight in the primary antibodies, followed by incubation (1 h) in the secondary antibodies, which were conjugated either to Alexa TM 594 (red fluorescence) or Alexa TM 488 (green fluorescence; Molecular Probes, Eugene, Ore.). In double-labeling experiments, sections were incubated in a mixture of primary antibodies, followed by a mixture of secondary antibodies.
Light microscopy
Fluorescent specimens were viewed with a Zeiss (Oberkochen, Germany) Axiophot microscope equipped with a fluorescent filter set that was wedge-corrected, i.e., shifting from one filter to the other did not introduce spatial displacements. Black-and-white digital images were taken by using a cooled charge-coupled device camera (spot 2; Diagnostic Instruments, Sterling Heights, Mich.). Images taken with the red and green fluorescent filters were pseudocolored and superimposed by using metaview software (Universal Imaging, West Chester, Pa.). Confocal micrographs were taken on a Zeiss LSM5 Pascal confocal microscope equipped with an argon laser and a HeNe laser. High-resolution scanning was performed with a Plan-apochromat 63X/1.4 objective and with 1,024×1,024 or 2,048×2,048 pixels. Single optical sections are shown. The brightness and the contrast of the final images were adjusted by using Adobe Photoshop 5.5.
Results
Glycinergic neurons of the frog retina
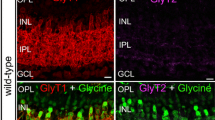
Immunocytochemical staining of sections through the frog retina with an antiserum against glycine (Fig. 1) revealed brightly fluorescent amacrine and interplexiform cell bodies located in the inner half of the inner nuclear layer (INL). The cell bodies were arrayed in two tiers in which approximately 50% of the cell bodies were labeled. We did not observe labeled displaced amacrine cells or ganglion cells. We only rarely detected interplexiform processes running toward the outer plexiform layer (OPL; arrowheads in Fig. 1B) and ramifying there (arrows). This staining pattern was in agreement with previous descriptions of glycinergic amacrine cells in lower vertebrate retinae (Yang and Yazulla 1988; Smiley and Basinger 1989; Yazulla and Studholme 1990). In contrast to the mammalian retina (see Fig. 3 in Haverkamp and Wässle 2000), no bipolar cells were found to be labeled beyond background labeling. Fine amacrine cell processes were faintly labeled throughout the inner plexiform layer (IPL; Fig. 1B), and the IPL appeared to be stratified. Following Cajal (1893), we subdivided the IPL into five strata of equal thickness.
Vertical sections through frog retina immunolabeled for glycine. A Low-power fluorescence micrograph showing the labeled perikarya in the INL. B High-power fluorescence micrograph (ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer). The labeled perikarya occupy the inner half of the INL (arrowheads, arrows interplexiform cell process ascending toward the OPL). The IPL is subdivided into five strata (1–5) of equal width, and the processes of amacrine cells are faintly labeled. Bar 100 μm (A), 35 μm (B)
Synaptic localization of GlyRs
Conventional immunohistochemistry provides little evidence for the synaptic localization of ionotropic neurotransmitter receptors, suggesting that their epitopes are not readily accessible in the postsynaptic densities (Fritschy et al. 1998). We have overcome this problem by fixing the tissue only briefly (15–30 min) by immersion into 4% paraformaldehyde. Unfortunately, tissue preservation is compromised by such brief fixation.
The antibody mAb4a is directed against an epitope that is highly conserved in all α subunits and in the β subunit (Grenningloh et al. 1987; Schröder et al. 1991) and is supposed to recognize all GlyRs. It labeled a band at approximately 35 kDa in Western blots of frog retinae (Fig. 2C). Since only frozen tissue was available, this band probably corresponded to the major fragment of GlyRs following proteolytic cleavage (Büttner et al. 2001). Immunolabeling within the IPL had a distinct punctate appearance (Fig. 2D), which suggested a synaptic localization, as demonstrated previously by electron microscopy for GlyRs and for other transmitter receptors (Brandstätter et al. 1998; Wässle et al. 1998). We, therefore, interpreted the puncta in the IPL (Fig. 2D) as clustering of GlyRs in postsynaptic densities. Low-power views (Fig. 2A) did not resolve the individual puncta but showed that their density distribution across the IPL was uneven, there being a narrow horizontal band of higher density in the lower stratum 1 and a broader band in strata 3 and 4. Label in the OPL (Fig. 2B) was also punctate, but only a low density of puncta was found. This suggested the presence of glycinergic synapses in the OPL.
Vertical section through a frog retina immunolabeled for mAb4a (thought to recognize all GlyRs). A Low-power view of mAb4a immunofluorescence (left) and the retinal layers by Nomarski optics(right; OS photoreceptor outer segments, IS photoreceptor inner segments, ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, 1–5 IPL subdivided into five strata). B OPL of the section in A at higher magnification. C Western blot analysis showing a single band labeled with an apparent molecular weight of ≈35 kDa by mAb4a. This band corresponds to GlyRs following proteolytic cleavage. D IPL from the boxed area in A at higher magnification. Bar 80 μm in (A), 45 μm (B), 20 μm (D)
The monoclonal antibody mAB2b is specific for the 10 N-terminal residues of the GlyRα1 subunit (Schröder et al. 1991). In the IPL of vertical sections immunostained with mAb2b (Fig. 3A; the corresponding area usingNomarski optics is shown in Fig. 3B), immunolabeling was punctate, suggesting that the GlyRα1 subunits were aggregated at postsynaptic sites. However, in contrast to Fig. 2, fewer puncta were found in the IPL and also their laminar distribution differed. Four bands of higher density could be distinguished (Fig. 3A): a band at the border of strata 1 and 2, a band at the border between strata 2 and 3, a band in stratum 3, and a band in stratum 4. We also counted the number of synaptic clusters in sections labeled for mAb4a (all GlyRs) and in sections labeled for mAb2b (GlyRα1). The density of mAb4a puncta was 97±24.3 (n=4, mean ± SD) per 100 μm2; that of mAb2b puncta was 59.8±4.7 (n=6, mean ± SD) per 100 μm2. This result showed that the α1 subunit was only expressed in a subpopulation of postsynaptic GlyRs of the frog retina and that there was more than one type of synaptic GlyR in the frog retina.
IPL of a vertical section through a frog retina immunolabeled for mAb2b (recognizes the α1 subunit of the GlyR). A GlyRα1 immunolabeling is punctate and four strata of higher density of puncta can be recognized. B Retinal layers by Nomarski optics (INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, 1–5 IPL subdivided into five strata). Bar 20 μm
Recently, a polyclonal antiserum has been raised against the 13 C-terminal amino acids of the mouse GlyRα3 subunit (Haverkamp et al. 2003); its specificity has been tested in HEK 293T cells transfected with cDNAs of the various GlyR subunits. The antiserum recognizes only the GlyRα3 subunit in transfected cells. Strong specific labeling of GlyRα3 clusters and weak nonspecific labeling of Müller cells has been observed in sections through the mouse retina (Haverkamp et al. 2003). By confocal microscopy, this antiserum revealed GlyRα3-immunoreactive puncta throughout the IPL (Fig. 4A). An indication of a horizontal band of larger puncta was found in stratum 3. This became more apparent under conventional low-power fluorescence microscopy (Fig. 4C). Bands of higher density of puncta were found in stratum 1, in stratum 2, and most prominently in stratum 3. A comparison of Fig. 4A, C with Fig. 3A indicates that the GlyRα3 subunit is expressed at many more glycinergic synapses than is the GlyRα1 subunit, and that the synapses occupy different strata within the IPL. In addition to the punctate and putative synaptic labeling in the IPL, we also found labeling in horizontal cell perikarya and dendrites in the OPL (Fig. 4A).
Vertical sections through frog retina immunolabeled for the GlyRα3 subunit. A Confocal micrograph of GlyRα3 immunofluorescence. Punctate immunofluorescence is found throughout the IPL (1–5 IPL subdivided into five strata). In the OPL, a horizontal cell body (HC), horizontal cell processes, and individual puncta are labeled. B Retinal layers by Nomarski optics (ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer). C Low-power conventional fluorescence micrograph showing the laminar distribution (horizontal lines) of GlyRα3-immunolabeled puncta across the IPL. Bar 35 μm (A, B), 55 μm (C)
Next, we double-labeled sections for mAb4a and for GlyRα3 (Fig. 5A, B). A comparison of the puncta showed that many were co-localized (see boxes in Fig. 5). To study a larger sample, we superimposed two transparent sheets over the micrographs in Fig. 5A, B and traced the puncta onto the sheets. We found 528 puncta immunoreactive for mAb4a and 333 puncta expressing GlyRα3 (ratio 1.6:1). Afterwards, the two sheets were superimposed, and the number of coincident puncta counted. We found that 62% of the GlyRα3-immunoreactive puncta also expressed mAb4a. The reason that not all GlyRα3 puncta were stained with mAb4a remains a matter of speculation; perhaps mAb4a did not recognize all synaptic GlyRs because the antigenic sites within the N-terminus were masked when the subunits assembled to form the pentameric complex.
Localization of gephyrin
Gephyrin (a 93-kDa protein) is essential for the clustering of GlyRs and certain isoforms of GABAA receptors at postsynaptic sites (Essrich et al. 1998; Feng et al. 1998). In the retina of geph (−/−) mice, the clustering of GlyRs is totally abolished (Fischer et al. 2000). In sections through the IPL of frog retina immunostained for gephyrin (mAb7a), numerous gephyrin-immunoreactive puncta can be observed (Fig. 6A; the corresponding area by Nomarski optics is shown in Fig. 6B), and with the exception of a band of reduced density in stratum 4, no obvious layering of the gephyrin puncta was apparent. Gephyrin immunofluorescence was also found in most perikarya of the ONL, INL, and GCL (not shown). A comparison of Figs. 6 and 2 shows that the number of gephyrin-immunoreactive puncta is significantly higher than the number of puncta immunostained by mAb4a. This suggests that, as in the mammalian retina, gephyrin is not only expressed at glycinergic synapses, but also at GABA-ergic synapses (Sassoè-Pognetto et al. 1995; Fischer et al. 2000), and that gephyrin can no longer be considered as a specific marker of glycinergic synapses in the retina.
Vertical section through a frog retina immunolabeled for gephyrin (mAb7a). A Gephyrin immunofluorescent puncta are found throughout the IPL. Amacrine cell bodies (top) are strongly labeled, whereas ganglion cell bodies (bottom) are only weakly labeled. B Retinal layers by Nomarski optics (INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, 1–5 IPL subdivided into five strata). Bar 20 μm
Discussion
Glycinergic amacrine cells
Ramón y Cajal (1893) studied the frog retina in Golgi-stained vertical sections and described the exquisite stratification of the IPL; bipolar cell axon terminals, amacrine cell processes, and ganglion cell dendrites were found in characteristic strata within the IPL and 13 different amacrine cell types were illustrated. A recent study of amacrine cells of the anuran retina, with a comparison of morphology and chemical neuroanatomy, described at least 21 different types of amacrine cells (Vigh et al. 2000). In the fish retina, 43 different types of amacrine cells have been described (Wagner and Wagner 1988) and, in the turtle retina, 37 types (Ammermüller and Kolb 1996). Modern methods, such as the photofilling technique, could possibly reveal as many as 30–50 amacrine cell types in the frog retina, comparable to a recent survey of rabbit amacrine cells (MacNeil and Masland 1998). In the mammalian retina, widefield amacrine cells are usually GABAergic, whereas narrowfield amacrine cells are glycinergic (MacNeil and Masland 1998). In the salamander retina, such a simple rule seems not to hold. Yang et al. (1991) have described that both multistratified and monostratified widefield amacrine cells and multistratified narrowfield amacrine cells express glycine immunoreactivity. At present, it is not known whether glycinergic amacrine cells of the frog retina follow the mammalian scheme and are small-field amacrine cells, or whether they are a mixed population comparable with those of the salamander retina (Vigh et al. 2000).
Diversity of GlyR
It is commonly assumed that three α and two β subunits assemble and form the chloride channel of the GlyR. The glycine-binding site is on the α subunit, whereas the structural β subunit is linked to the cytoskeleton through gephyrin (Vannier and Triller 1997; Harvey and Betz 2000; Laube et al. 2002). To date, four genes encoding the α subunits (α1, α2, α3, α4) have been found. With the exception of the α4 subunit, mRNAs for all other subunits have been revealed by in situ hybridization in the adult rodent retina (Greferath et al. 1994). Whether the α4 subunit is expressed in the mammalian central nervous system is still a matter of discussion (Matzenbach et al. 1994).
In the present study, we have successfully applied antibodies against the α1 and the α3 subunits to sections of the retina of R. ridibunda, but we have observed no specific labeling with an antiserum against the α2 subunit (not shown). The same antiserum, when applied to sections of mouse, rat, Xenopus, and turtle retinae, gives punctate label throughout the IPL (unpublished observations). The failure of the antiserum against the α2 subunit to label the retina of R. ridibunda may be caused by unknown technical reasons and may not indicate the absence of the GlyRα2 subunit from this retina.
GlyR immunoreactivity is not distributed evenly across the IPL but is aggregated in brightly fluorescent puncta that we interpret as aggregations of the receptors in postsynaptic densities. The density of the putative glycinergic synapses across the IPL is not uniform, strata of lower and higher density having been observed; these strata are characteristic for the various GlyR subunits (Figs. 3, 4). As mentioned above, the bipolar cell axon terminals, the amacrine cell processes, and the ganglion cell dendrites all exhibit specific stratification patterns across the frog IPL (Cajal 1893). Taken together, these data suggest that the different GlyR subunits are expressed in different circuits and by different neurons. Clearly, more specific markers that selectively label certain types of neurons in the frog retina are needed before this correlation can be established in more detail.
In the mammalian retina, the GlyRα1 subunit has been shown to be preferentially expressed at the synapse between AII amacrine cells and OFF-cone bipolar cells (Sassoè-Pognetto et al. 1994). Consequently, there is an aggregation of GlyRα1 puncta in the outer half of the IPL at the locular dendrites of AII cells. The distinct stratification of GlyRα1 puncta of the frog retina (Fig. 3) may therefore also follow the dendritic stratification of a subgroup of glycinergic amacrine cell. Unfortunately, these have not yet been identified.
Localization of gephyrin
Gephyrin, a 93-kDa protein, was originally purified with GlyR, and the antibody against gephyrin (mAb7a) was considered to be a specific marker of GlyRs (Graham et al. 1985). Most of the studies of the localization of GlyRs in the retina of lower vertebrates have been performed with mAb7a and thus provide information about the localization of gephyrin (Smiley and Yazulla 1990; Yazulla and Studholme 1991a, 1991b; Zucker and Ehinger 1993). Gephyrin has been shown, in the meantime, also to be co-localized with GABAA receptors (Sassoè-Pognetto et al. 1995; Essrich et al. 1998; Feng et al. 1998; Kneussel et al. 1999; Fischer et al. 2000). In agreement with this notion, we have observed the highest density of labeled synapses in the frog IPL when applying mAb7a (see Fig. 6). We have also performed double-labeling experiments with antibodies against gephyrin and GlyRα3 (not shown) and observed that only a minority of gephyrin puncta lie in register with GlyRα3 puncta. This suggests the view that, in the frog retina, as in the mammalian retina, gephyrin is involved with the clustering of GABAA and GlyRs. In a recent study of the zebrafish retina, mAb2b has been used to study the localization of the GlyRα1 subunit (Yazulla and Studholme 2001); labeling here is punctate, and the puncta are aggregated in four strata within the IPL. This is similar to the distribution of GlyRα1 in the frog retina (Fig. 3).
GlyR in the outer plexiform layer
We have found glycine-immunostained interplexiform processes (Fig. 1) and punctate GlyR immunofluorescence in the OPL (Fig. 2B) suggesting that glycinergic synapses are present in the OPL. The postsynaptic targets may be bipolar cell dendrites, horizontal cells, or even photoreceptor terminals. Glycinergic interplexiform cells have also been described in glycine high-affinity uptake experiments in Rana pipiens (Marc 1985; Smiley and Basinger 1989) and appear to be a common cell type in all vertebrate retinae (Marc 1989; Smiley and Yazulla 1990). Maple and Wu (1998) have shown, in a physiological study of the tiger salamander retina, that bipolar cells receive glycinergic synaptic input, both at their dendrites in the OPL and at their axon terminals in the IPL. Du and Yang (2002) have demonstrated, in a physiological study of the bullfrog retina, that bipolar cells express heterogeneous GlyRs at dendrites and axon terminals. The results of the present paper, revealing a diversity of GlyRs both in the OPL and in the IPL of the frog retina, are in accordance with these results.
Physiological diversity of GlyRs in the retina
Our results predict that several different isoforms of GlyRs are expressed at the synapses of the frog retina. The physiologic and pharmacologic profiles of the glycinergic currents will change depending upon the subunit composition. In artificial expression systems, the α1, α3, and α4 subunits produce channels with fast kinetics, whereas expression of the α2 subunit produces channels with slow kinetics (for reviews, see Harvey et al. 2000; Legendre 2001). Differences in kinetics of synaptic glycinergic currents have also been observed by Maple and Wu (1998) between dendritic and axonal GlyRs of tiger salamander bipolar cells. Du and Yang (2002) have observed that dendritic GlyRs of bullfrog bipolar cells have single channel conductances of 18.2 pS compared with 8.1 pS of axonal GlyRs. Pharmacological studies of glycinergic inhibition in the tiger salamander retina have revealed two types of GlyRs: one sensitive to strychnine and the other to 5,7-dichlorokynurenic acid (Han et al. 1997; Han and Slaughter 1998). They are also differentially modulated by Zn2+ (Han and Wu 1999). Taken together, these data show that GlyRs of the lower vertebrate retina are not a homogeneous population, but that different isoforms are expressed. Their major distinguishing feature seems to be the temporal scale at which they operate. Dependent on their subunit composition, GlyRs may produce tonic or phasic inhibition, and sustained and transient light responses might be the result of the expression of the different isoforms of GlyRs.
References
Ammermüller J, Kolb H (1996) Functional architecture of the turtle retina. Prog Retin Eye Res 15:393–433
Brandstätter J, Koulen P, Wässle H (1998) Diversity of glutamate receptors in the mammalian retina. Vision Res 38:1385–1397
Büttner C, Sadtler S, Leyendecker A, Laube B, Griffon N, Betz H, Schmalzing G (2001) Ubiquitination precedes internalization and proteolytic cleavage of plasma membrane-bound glycine receptors. J Biol Chem 276:42978–42985
Cajal SR (1893) La rétine des vertébrés. Cellule 9:119–257
Du J, Yang XL (2002) Bullfrog retinal bipolar cells may express heterogeneous glycine receptors at dendrites and axon terminals. Neurosci Lett 322:505–518
Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B (1998) Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci 1:563–571
Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR (1998) Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science 282:1321–1324
Fischer F, Kneussel M, Tintrup H, Haverkamp S, Rauen T, Betz H, Wässle H (2000) Reduced synaptic clustering of GABA and glycine receptors in the retina of the gephyrin null mutant mouse. J Comp Neurol 427:634–648
Fritschy JM, Weinmann O, Wenzel A, Benke D (1998) Synapse-specific localization of NMDA and GABAA receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol 390:194–210
Graham D, Pfeiffer F, Simler R, Betz H (1985) Purification and characterization of the glycine receptor of pig spinal cord. Biochemistry 24:990–994
Greferath U, Brandstätter JH, Wässle H, Kirsch H, Kuhse J, Grünert U (1994) Differential expression of glycine receptor subunits in the retina of the rat: a study using immunohistochemistry and in situ hybridization. Vis Neurosci 11:721–729
Grenningloh G, Rienitz A, Schmitt B, Methfessel C, Zensen M, Beyreuther K, Gundelfinger ED, Betz H (1987) The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature 328:215–220
Grenningloh G, Schmieden V, Schofield PR, Seeburg PH, Siddique T, Mohandas TK, Becker C-M, Betz H (1990) Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localisation of the corresponding genes. EMBO J 9:771–776
Han Y, Slaughter M (1998) Protein kinases modulate two glycine currents in salamander retinal ganglion cells. J Physiol (Lond) 508:681–690
Han Y, Wu W (1999) Modulation of glycine receptors in retinal ganglion cells by zinc. Proc Natl Acad Sci USA 96:3234–3238
Han Y, Zhang J, Slaughter M (1997) Partition of transient and sustained inhibitory glycinergic input to retinal ganglion cells. J Neurosci 17:3392–3400
Harvey RJ, Betz H (2000) Structure, diversity, pharmacology, and pathology of glycine receptor chloride channels. In: Endo M, Kurachi Y, Mishina M (eds) Pharmacology of ionic channel function: activators and inhibitors. Springer, Berlin Heidelberg New York, pp 479–497
Harvey RJ, Schmieden V, Holst A von, Laube B, Rohrer H, Betz H (2000) Glycine receptors containing the α4 subunit in the embryonic sympathetic nervous system, spinal cord and male genital ridge. Eur J Neurosci 12:994–1001
Haverkamp S, Wässle H (2000) Immunocytochemical analysis of the mouse retina. J Comp Neurol 424:1–23
Haverkamp S, Müller U, Harvey K, Harvey RJ, Betz H, Wässle H (2003) Diversity of glycine receptors in the mouse retina: localization of the α3 subunit. J Comp Neurol 465:524–539
Kalloniatis M, Tomisich G (1999) Amino acid neurochemistry of the vertebrate retina. Prog Retin Eye Res 18:811–866
Kneussel M, Brandstätter JH, Laube B, Stahl S, Müller U, Betz H (1999) Loss of postsynaptic GABAA receptor clustering in gephyrin-deficient mice. J Neurosci 19:9289–9297
Kuhse J, Schmieden V, Betz H (1990) Identification and functional expression of a novel ligand binding subunit of the inhibitory glycine receptor. J Biol Chem 265:22317–22320
Kupenova P, Vitanova L, Popova E, Mitova L (1997) Influence of picrotoxin and strychnine on the spectral sensitivity of the turtle ERG b- and d-wave. I. Dark adaptation. Acta Physiol Scand 159:217–225
Laube B, Maksay G, Schemm R, Betz H (2002) Modulation of glycine receptor function: a novel approach for therapeutic intervention at inhibitory synapses? Trends Pharmacol Sci 23:519–527
Legendre P (2001) The glycinergic inhibitory synapse. Cell Mol Life Sci 58:760–793
MacNeil MA, Masland RH (1998) Extreme diversity among amacrine cells: implications for function. Neuron 20:971–982
Maple BR, Wu SM (1998) Glycinergic synaptic inputs to bipolar cells in the salamander retina. J Physiol (Lond) 506:731–744
Marc RE (1985) The role of glycine in retinal circuitry. In: Morgan WW (ed) Retinal transmitters and modulators: models for the brain. CRC, Boca Raton, pp 119–158
Marc RE (1989) The role of glycine in the mammalian retina. Prog Retin Eye Res 8:67–107
Marc RE, Lam DMK (1981) Glycinergic pathways in the goldfish retina. J Neurosci 1:152–165
Matzenbach B, Maulet Y, Sefton L, Courtier B, Avner P, Guénet J-L, Betz H (1994) Structural analysis of mouse glycine receptor α subunits genes: identification and chromosomal localization of a novel variant, α4. J Biol Chem 269:2607–2612
Menger N, Pow DV, Wässle H (1998) Glycinergic amacrine cells of the rat retina. J Comp Neurol 401:34–46
Pfeiffer F, Simler R, Grenningloh G, Betz H (1984) Monoclonal antibodies and peptides mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc Natl Acad Sci USA 81:7224–7227
Popova E, Motova L, Vitanova L, Kupenova P (1997) Effect of glycinergic blockade on light responses of frog retinal ganglion cells. Comp Biochem Physiol 116:255–263
Popova E, Mitova L, Vitanova L, Kupenova P (2000) Effect of 2-amino-4-phosphonobutyrate on the OFF-responses of frog retinal ganglion cells and local ERG after glycinergic blockade. Comp Biochem Physiol 126:139–151
Pourcho RG (1996) Neurotransmitters in the retina. Curr Eye Res 15:797–803
Pourcho RG, Goebel DJ (1985) A combined Golgi and autoradiographic study of (3H)-glycine-accumulating amacrine cells in the cat retina. J Comp Neurol 233:473–480
Pourcho RG, Owczarzak MT (1991a) Connectivity of glycine immunoreactive amacrine cells in the cat retina. J Comp Neurol 307:549–561
Pourcho RG, Owczarzak MT (1991b) Glycine receptor immunoreactivity is localized at amacrine synapses in cat retina. Vis Neurosci 7:611–618
Pow DV (2001) Amino acids and their transporters in the retina. Neurochem Int 38:463–484
Pow DV, Wright LL, Vaney DI (1995) Immunocytochemical detection of amino acid neurotransmitters in paraformaldehyde-fixed tissues. J Neurosci Methods 56:115–123
Sassoè-Pognetto M, Wässle H (1997) Synaptogenesis in the rat retina: subcellular localization of glycine receptors, GABAA receptors, and the anchoring protein gephyrin. J Comp Neurol 381:158–174
Sassoè-Pognetto M, Wässle H, Grünert U (1994) Glycinergic synapses in the rod pathway of the rat retina: cone bipolar cells express the α1 subunit of the glycine receptor. J Neurosci 14:5131–5146
Sassoè-Pognetto M, Kirsch J, Grünert U, Greferath U, Fritschy J-M, Möhler H, Betz H, Wässle H (1995) Colocalization of gephyrin and GABAA-receptor subunits in the rat retina. J Comp Neurol 357:1–14
Schmitt B, Knaus P, Becker C-M, Betz H (1987) The M 93000 polypeptide of the postsynaptic glycine receptor complex is a peripheral membrane protein. Biochemistry 26:805–811
Schröder S, Hoch W, Becker C-M, Grenningloh G, Betz H (1991) Mapping of antigenic epitopes on the α1 subunit of the inhibitory glycine receptor. Biochemistry 30:42–47
Smiley JF, Basinger SF (1989) Glycine high-affinity uptake labels a subpopulation of somatostatin-like immunoreactive cells in the Rana pipiens retina. Brain Res 495:31–44
Smiley JF, Yazulla S (1990) Glycinergic contacts in the outer plexiform layer of the Xenopus laevis retina characterized by antibodies to glycine, GABA and glycine receptors. J Comp Neurol 299:375–388
Vaney DI (2002) Retinal neurons: cell types and coupled networks. Perspect Anal Philos 136:239–254
Vannier C, Triller A (1997) Biology of the postsynaptic glycine receptor. Int Rev Cytol 176:201–244
Vigh J, Banvölgyi T, Wilhelm M (2000) Amacrine cells of the anuran retina: morphology, chemical neuroanatomy, and physiology. Microsc Res Tech 50:373–383
Vitanova L, Kupenova P, Popova E, Mitova L (1997) Influence of picrotoxin and strychnine on the spectral sensitivity of the turtle ERG b- and d-wave. II. Light adaptation. Acta Physiol Scand 159:227–235
Wagner HJ, Wagner E (1988) Amacrine cells in the retina of a teleost fish, the roach (Rutilus rutilus): a Golgi study on differentiation and layering. Philos Trans R Soc Lond Biol 321:263–324
Wässle H, Koulen P, Brandstätter JH, Fletcher EL, Becker C-M (1998) Glycine and GABA receptors in the mammalian retina. Vision Res 38:1411–1430
Yang C-Y, Yazulla S (1988) Light microscopic localization of putative glycinergic neurons in the larval tiger salamander retina by immunocytochemical and autoradiographical methods. J Comp Neurol 272:343–357
Yang C-Y, Lukasiewicz P, Maguire G, Werblin FS, Yazulla S (1991) Amacrine cells in the tiger salamander retina: morphology, physiology, and neurotransmitter identification. J Comp Neurol 312:19–32
Yazulla S, Studholme KM (1990) Multiple subtypes of glycine-immunoreactive neurons in the goldfish retina: single- and double-label studies. Vis Neurosci 4:299–309
Yazulla S, Studholme KM (1991a) Glycinergic interplexiform cells make synaptic contact with amacrine cell bodies in goldfish retina. J Comp Neurol 310:1–10
Yazulla S, Studholme KM (1991b) Glycine-receptor immunoreactivity in retinal bipolar cells is postsynaptic to glycinergic and GABAergic amacrine cell synapses. J Comp Neurol 310:11–20
Yazulla S, Studholme KM (2001) Neurochemical anatomy of the zebrafish retina as determined by immunocytochemistry. J Neurocytol 30:551–592
Zucker CL, Ehinger B (1993) Synaptic connections involving immunoreactive glycine receptors in the turtle retina. Vis Neurosci 10:907–914
Acknowledgments
We are grateful to F. Boij for technical assistance, to A. Staab for performing the Western blots, and to I. Odenthal for typing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Deutsche Forschungsgemeinschaft SFB269/B4
Rights and permissions
About this article
Cite this article
Vitanova, L., Haverkamp, S. & Wässle, H. Immunocytochemical localization of glycine and glycine receptors in the retina of the frog Rana ridibunda . Cell Tissue Res 317, 227–235 (2004). https://doi.org/10.1007/s00441-004-0914-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-0914-6