Abstract
Expression patterns of glycoconjugates were examined by lectin histochemistry in the nasal cavity of the Japanese red-bellied newt, Cynops pyrrhogaster. Its nasal cavity consisted of two components, a flattened chamber, which was the main nasal chamber (MNC), and a lateral diverticulum called the lateral nasal sinus (LNS), which communicated medially with the MNC. The MNC was lined with the olfactory epithelium (OE), while the diverticulum constituting the LNS was lined with the vomeronasal epithelium (VNE). Nasal glands were observed beneath the OE but not beneath the VNE. In addition, a secretory epithelium was revealed on the dorsal boundary between the MNC and the LNS, which we refer to as the boundary secretory epithelium (BSE) in this study. The BSE seemed to play an important role in the construction of the mucous composition of the VNE. Among 21 lectins used in this study, DBA, SBA and Jacalin showed different staining patterns between the OE and the VNE. DBA staining showed remarkable differences between the OE and the VNE; there was intense staining in the free border and the supporting cells of the VNE, whereas there was no staining or weak staining in the cells of the OE. SBA and Jacalin showed different stainings in the receptor neurons for the OE and the VNE. Furthermore, UEA-I and Con A showed different stainings for the nasal glands. UEA-I showed intense staining in the BSE and in the nasal glands located in the ventral wall of the MNC (VNG), whereas Con A showed intense staining in the BSE and in the nasal glands located in the dorsal and medial wall of the MNC (DMNG). The DMNG were observed to send their excretory ducts into the OE, whereas no excretory ducts were observed from the VNG to the OE or the VNE. These results suggested that the secretion by the supporting cells as well as the BSE and the DMNG establishes that there are heterogeneous mucous environments in the OE and the VNE, although both epithelia are situated in the same nasal cavity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All vertebrates possess a sensory epithelium for perceiving odoriferous molecules. This epithelium consists of receptor neurons, supporting cells and basal cells, and is often associated with nasal glands (Getchell et al 1988; Eisthen 1992; Shipley et al. 1995). Vertebrate species have two types of sensory epithelia, the olfactory epithelium (OE) and the vomeronasal epithelium (VNE) (Parsons 1967; Eisthen 1992; Shipley et al. 1995; Eisthen 1997), and each type has a different role in olfaction. The OE is observed in all vertebrate species, and is sensitive to the ordinary odorants. The VNE is not observed in fishes but appears first in urodeles in phylogeny (Bertmar 1981; Eisthen 1992, 1997), and detects the specialized odorants called pheromones (Estes 1972; Wysocki 1979; Wysocki et al. 1980). The physiological distinction between the OE and the VNE, however, remains confusing; the VNE can also respond to some kinds of general odorants (Sam et al. 2001), while in animals with destroyed VNE but with intact OE, pheromones can also induce reproductive behavior (Dorries et al. 1997) and a hormonal change (Cohen-Tannoudji et al. 1989). To research the nature of the distinction between the OE and the VNE, Cynops pyrrhogaster appears to be a suitable candidate, because its species-specific sex pheromone has been recently identified (Kikuyama et al. 1995; Kikuyama and Toyoda 1999), and, as an urodele, it has the dual olfactory system, where the OE and the VNE are anatomically separated. Nonetheless, little is known about the biochemical properties of the OE and the VNE of the newt olfactory system.
In the olfactory system, the biochemical properties of the OE and the VNE have been often studied by lectin histochemistry. Lectins are sugar-binding proteins or glycoproteins of non-immune origin (Goldstein et al. 1980). By using lectins as probes in histochemistry, it is possible to visualize the expression patterns of glycoconjugates (Lis and Sharon 1986). Furthermore, different expression of glycoconjugates may reflect the functional differences between the OE and the VNE (Key and Giorgi 1986; Hofmann and Meyer 1991; Schwarting and Crandall 1991; Franceschini and Ciani 1993; Takami et al. 1994; Shapiro et al. 1995; Nakajima et al. 1998; Franceschini et al. 2000; Saito and Taniguchi 2000). In this study, therefore, glycoconjugates in the OE and the VNE of the newt were examined by lectin histochemistry. This study revealed different mucous environments for the OE and the VNE, although they were situated in the same nasal cavity. In addition, we report the presence of a gland-like structure on the dorsal boundary between the MNC and the LNS, the boundary secretory epithelium (BSE), in C. pyrrhogaster for the first time.
Materials and methods
Animals
Six adult C. pyrrhogaster of both sexes were purchased from Hamamatu Biological Materials (Shizuoka, Japan) and used as materials. All procedures were in accordance with the Guide for Care and Use of Animal Experimentation at Ehime University. After anesthesia by intraperitoneal injection of 5.0×10-3 ml/g body weight of sodium pentobarbital (Abbott, North Chicago, USA) and chilling, they were sacrificed by cardiac perfusion with physiological saline followed by Bouin's solution without acetic acid. The upper jaw including the nose was cut off from the head, immersed in the same fixative for 24 h, and decalcified by immersion in 0.25 M ethylendiamine tetra-acetic acid (EDTA) in 0.1 M phosphate buffer (pH 7.4) for 2 weeks. The EDTA solution was exchanged on every third day. After 2 weeks of decalcification, specimens were routinely embedded in paraffin. Paraffin sections were cut coronally at 5 µm in thickness and processed for lectin histochemistry.
Lectin histochemistry
Lectin histochemistry was performed by use of 21 kinds of biotinylated lectins (Vector, Burlingame, USA) after the avidin–biotin peroxidase complex (ABC) method using the Vectastain ABC kit (Vector, Burlingame, USA), as described previously (Saito et al. 2000). Briefly, after deparaffinization and elimination of endogenous peroxidase, sections were incubated with 1% bovine serum albumin at 32°C for 30 min to block non-specific reaction. Sections were then rinsed with 0.02 M phosphate-buffered saline (PBS) (pH 7.4), and incubated with biotinylated lectins at 4°C for 48 h. After rinsing with PBS, sections were incubated with the Vectastain ABC reagent at 32°C for 30 min. Finally, sections were colorized with 0.05 M Tris–HCl (pH 7.6) containing 0.01% 3–3′ diaminobenzidine tetrahydrochloride and 0.003% H2O2 for 10 min. Control stainings were performed by the preabsorption of lectins with an excess amount of specific sugar residues, or by use of PBS to replace either the biotinylated lectins or the ABC reagent. No specific lectin stainings were observed in the control stainings (data not shown). Optimal concentrations and sugar specificity of lectins used are listed in Table 1.
Results
Organization of the newt nasal chamber
The nasal cavity of C. pyrrhogaster consists of a flattened single chamber, which is the main nasal chamber (MNC), and a lateral diverticulum, called the lateral nasal sinus (LNS). The OE lines the MNC and the VNE lines the LNS (Fig. 1). In the MNC, the OE was divided into several grooves by ridges of non-sensory epithelia (Fig. 1A and B). Such grooves were not observed in the LNS (Fig. 1A and C). The OE and the VNE represented the pseudostratified epithelium (Fig. 1B and C). Elongated nuclei of the supporting cells were arranged in a layer in the upper third of the epithelium. Oval nuclei of the receptor neurons were densely packed in the basal two thirds of the epithelium. Spherical nuclei of the basal cells were arranged sparsely just above the basal laminae of the epithelia. Immature cells, which were generated from the basal cells and located in a deep part of the epithelia, could not be distinguished from mature neurons in this study. The nasal glands were observed in the dorsal, medial and ventral regions of the lamina propria of the MNC (Fig. 1A). No glandular structure was observed in the lamina propria of the LNS, except in the secretory epithelium on the dorsal boundary between the MNC and the LNS (Fig. 1A). This epithelium is observed as an unbranched alveolar gland, and we refer to it as the boundary secretory epithelium (BSE) in this study.
Histological structure of the nasal cavity of C. pyrrhogaster stained with hematoxylin and eosin. A The right nasal cavity. Lateral is on the right and ventral is at the bottom. The nasal cavity was a pair of flattened single chambers, and comprised the main nasal chamber (MNC) and the lateral nasal sinus (LNS). Closed arrows indicate the grooves of the MNC representing the olfactory epithelium (OE). The arrowhead indicates the groove of the LNS representing the vomeronasal epithelium (VNE). The open arrow indicates the boundary secretory epithelium (BSE) located at the dorsal boundary between the MNC and LNS. Nasal glands (NG) were observed in the dorsal, medial and ventral walls of the MNC. Bar 200 µm. B A chosen view of the OE. The apical side of the OE consisted of the layer of the supporting cells (SC), and a large number of the receptor neurons (RN) were located under this layer, and the basal cells (BC) are located just above the basal lamina. The NG were observed under the OE. Bar 100 µm. C The VNE. The location of the layers of the SC, RN and BC was similar to the OE arrangement. No NG were observed under the VNE. Bar 100 µm
Lectin staining patterns in the OE and the VNE of the newt
Free border
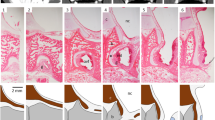
Of the 21 kinds of lectins used in this study, all except BSL-II stained the free borders of the OE and the VNE (Table 2). Among these 20 lectins, DBA showed different staining for the OE and the VNE; it stained the free border of the VNE intensely, but did not stain that of the OE (Fig. 2C and D). The remaining 19 lectins showed the same staining pattern in the OE and the VNE with varying intensities (Fig. 2A, B, E, F, G and H).
Staining patterns of lectins in the olfactory epithelium (OE) (A, C, E and G) and the vomeronasal epithelium (VNE) (B, D, F and H) of C. pyrrhogaster. Arrows and arrowheads indicate the lectin staining in the supporting cells and the receptor neurons, respectively. Double arrows in Figs. E, F, G and H indicate the lectin staining in the basal cells. WGA showed the same staining pattern for the OE and the VNE (A and B). DBA showed significantly different stainings in the free border and the supporting cells for the OE and the VNE (C and D). SBA showed different stainings in the receptor neurons for the OE and the VNE (E and F). DSL showed intense staining in the basal cells of both epithelia (G and H). Bar 50 µm
Supporting cells
Of the 21 kinds of lectins used in this study, all except DSL and BSL-II stained the supporting cells of the OE and the VNE (Table 2). These 19 lectins, with the exception of DBA in the VNE, mainly stained the perinuclear region of the supporting cells. DBA showed different staining patterns for the OE and the VNE. This lectin showed a faint staining in the supporting cells of the OE (Fig. 2C), but an intense and uniform staining in the cells of the VNE (Fig. 2D). The remaining 18 lectins showed the same staining pattern in the OE and the VNE with varying intensities (Fig. 2A, B, E and F).
Receptor neurons
Of the 21 kinds of lectins used, 16 lectins stained the receptor neurons of the OE and the VNE; the exceptions were DSL, BSL-II, DBA, VVA and UEA-I (Table 2). Among these lectins, SBA and Jacalin showed different stainings for the OE and the VNE. These lectins showed faint stainings in the receptor neurons of the OE (Fig. 2E), but moderate stainings in the neurons of the VNE (Fig. 2F). The remaining 14 lectins showed the same staining pattern in the OE and VNE with varying intensities (Fig. 2A and B).
Basal cells
Of the 21 kinds of lectins used, 17 lectins stained the basal cells of the OE and the VNE; the exceptions were BSL-II, DBA, VVA and UEA-I (Table 2). Among these lectins, DSL showed an intense staining in the basal cells of both epithelia, while it showed negative staining in the supporting cells and the receptor neurons of both epithelia (Fig. 2G and H). SBA and Jacalin showed moderate stainings in the basal cells of both epithelia, although it showed faint stainings in the receptor neurons of the OE (Fig. 2E and F). The remaining 14 lectins stained the basal cells with the same intensity as that observed for the receptor neurons in both epithelia, showing the same staining pattern in the OE and the VNE with varying intensities.
Nasal glands and the BSE
Of the 21 kinds of lectins used, all except BSL-II stained the nasal glands (Table 2). Among them, UEA-I and Con A showed different stainings for the nasal glands. UEA-I showed an intense staining in the nasal glands located in the ventral wall of the MNC, which we refer to as the ventral nasal glands (VNG) (Fig. 3A). Con A showed an intense staining in the nasal glands located in the dorsal and the medial wall of the MNC, which we refer to as the dorso-medial nasal glands (DMNG) (Fig. 3B). Both UEA-I and Con A also stained the BSE intensely. The remaining 18 lectins showed the same stainings in all the nasal glands and the BSE, with varying intensities (Fig. 3C). The DMNG were observed to send their excretory ducts into the OE, but ducts from the VNG were not observed in the OE or the VNE (Fig. 3B and C).
Staining patterns of lectins in the nasal glands and the boundary secretory epithelium (BSE). A Staining with UEA-I in the nasal chamber, counter stained with hematoxylin. The BSE (open arrow) and the ventral nasal glands (VNG), but not the dorso-medial nasal glands (DMNG), were stained intensely. Bar 200 µm. B Staining with Con A. Con A stained the DMNG intensely, but the VNG faintly. Bar 200 µm. C Staining with DBA. DBA stained the BSE, the DMNG and the VNG uniformly. Bar 200 µm
No staining difference was observed among the grooves in the MNC or between the sexes in this study.
Discussion
In this study,the expression patterns of glycoconjugates in the OE and the VNE of C. pyrrhogaster were examined by lectin histochemistry. Different expression patterns of glycoconjugates in the OE and the VNE were revealed in the free border, the supporting cells and the receptor neurons. In addition, three types of nasal gland, the BSE, the VNG and the DMNG, were identified based on their morphology and lectin histochemistry. These data revealed the heterogeneous mucous environment in the single nasal cavity of C. pyrrhogaster .
Prior to lectin histochemical examination, we observed the organization of the C. pyrrhogaster nasal chamber. The VNE appears first in urodeles in phylogeny (Bertmar 1981; Eisthen 1992, 1997), although not all urodeles have the VNE. In a study of three families of non-metamorphosing aquatic salamanders, Sirens, Amphiuma and Necturus, using a combination of light and electron microscopy, Eisthen (2000) revealed that Amphiuma has the VNE in the lateral diverticulum of the nasal cavity, whereas Necturus lacks the VNE; Sirens has the VNE in the medial diverticulum of the nasal cavity, as previously reported by Seydel (1895). In this study we confirmed that C. pyrrhogaster has the VNE in the lateral diverticulum of the nasal cavity and the C. pyrrhogaster VNE lacks the nasal gland, as previously reported by Jones (1994). Furthermore, this study is the first report about the BSE, to the best of our knowledge; the BSE seems to be C. pyrrhogaster specific, since this structure has not been reported in other vertebrate species or in other newts.
In this study, a lectin DBA revealed significant differences in the mucous environments of the OE and the VNE of C. pyrrhogaster. Both the free border and the secretory supporting cells in the VNE were intensely stained with DBA, while those in the OE were not. Such differences are thought to result from the differences in quality and/or quantity of secreted elements. The odoriferous molecules were dissolved in the mucus of the epithelium prior to reaching the cilia or microvilli of the receptor neurons (Pelosi 1996). Therefore, the mucous environment of the epithelial surface seems to influence the perireceptor event when the odoriferous molecules reach to the olfactory receptors (Getchell et al. 1984; Getchell et al. 1988; Getchell and Getchell 1992; Menco 1994). DBA-staining in this study revealed different mucous environments, and these differences may play a part in the different olfactory functions for the OE and the VNE.
The receptor neurons also showed different stainings with SBA and Jacalin for the OE and the VNE in C. pyrrhogaster . Different lectin staining patterns for the receptor neurons of the OE and the VNE have also been reported in a variety of vertebrate species (Foster et al. 1991; Takami et al. 1994; Shapiro et al. 1995; Nakajima et al. 1998; Suzuki et al. 1999; Franceschini et al. 2000). These differences may reflect the different glycoconjugate syntheses and, in turn, the different functions of the receptor neurons of the OE and the VNE. On the other hand, the lectins DSL, SBA and Jacalin showed different stainings for the receptor neurons and the basal cells. The basal cells are precursor cells of the receptor neurons (Graziadei et al. 1979; Monti Graziadei et al. 1979; Simmons et al. 1981), but their nature, especially for the cells located in the VNE, remains unclear. Although the basal cells in the OE and the VNE generate morphologically and functionally different neurons, no histochemical differences for the basal cells between the two epithelia were observed in this study. Further studies are expected to reveal histochemical and functional differences for the basal cells of the OE and the VNE.
In this study, three types of the nasal glands were identified by lectin histochemistry, that is, the BSE, the VNG and the DMNG. The VNG were labeled with UEA-I, the DMNG were labeled with Con A, and the BSE was intensely stained with both lectins. These observations indicate that the three types of nasal glands secrete different kinds of secretory products. Together with the secretory supporting cells, the BSE seems to have an important role in the construction of the mucous composition of the VNE, because no nasal gland was observed in the VNE. The dorsomedial positioning of the BSE relative to the LNS may also be advantageous for exclusively moisturizing the LNS lumen.
Tetrapods are reported to have nasal glands that send their excretory ducts into the OE and secrete mucus to the free border, and such glands are called Bowman's glands (Getchell et al. 1984; Getchell et al. 1988; Getchell and Getchell 1992). Among three nasal glands of C. pyrrhogaster described in this study, the DMNG appeared to correspond to the Bowman's glands, because the DMNG were observed to send their excretory ducts into the OE. In contrast to the DMNG, the VNG were not observed to send their ducts into the OE or the VNE. Getchell et al. (1984) performed histological and histochemical studies on the olfactory mucosa of the tiger salamander, and reported three types of the nasal glands in the ventral region of the MNC; that is, the superficial, the middle and the deep glands. The superficial glands were observed to send their excretory ducts into the OE, whereas the excretory ducts of the other two glands could not be observed. They thought that the middle and deep glands represent different segments of a single ductless glandular tube coiled within the lamina propria. Therefore, the VNG of the C. pyrrhogaster MNC may correspond to the middle and deep glands of the salamander. Such ductless glands could also be observed in the fish. In general, the fish lacks Bowman's glands, but has a ductless gland in its lamina propria of the olfactory epithelium (Getchell et al. 1992). De Beer (1924) hypothesized that the ductless gland of the fish serves as the endocrine gland. As suggested in the salamander (Getchell et al. 1984), the VNG of the C. pyrrhogaster MNC may be the evolutional vestige of fish nasal glands, and may serve as endocrine glands whose secretion can be affected by olfactory stimulation.
In summary, this study revealed the heterogeneous expression of glycoconjugates between the OE and the VNE and among the nasal glands by lectin histochemistry. These results show the different mucous environments and functional differences between the OE and the VNE, although they exist in the same nasal cavity.
References
Bertmar G (1981) Evolution of vomeronasal organs in vertebrates. Evolution 35: 359–366
Cohen-Tannoudji J, Lavenet C, Locatelli A, Tillet Y, Signoret P (1989) Non-involvement of the accessory olfactory system in LH response of anoestrous ewes to male odour. J Reprod. Fert. 86: 135–144
De Beer GR (1924) On a problematical organ in the lamprey. J Anat 59: 97–107
Dorries KM, Adkins-Regan E, Halpern BP (1997) Sensitivity and behavioral responses to the pheromone androstenone are not mediated by the vomeronasal organ in domestic pigs. Brain Behav Evol 49: 53–62
Eisthen HL (1992) Phylogeny of the vomeronasal system and of receptor cell types in the olfactory and vomeronasal epithelia of vertebrates. Microsc Res Tech 23: 1–21
Eisthen HL (1997) Evolution of vertebrate olfactory systems. Brain Behav Evol 50: 222–233
Eisthen HL (2000) Presence of the vomeronasal system in aquatic salamanders. Phil Trans R Soc Lond B 355: 1209–1213
Estes RD (1972) The role of the vomeronasal organ in mammalian reproduction. Mammalia 36: 315–341
Foster JD, Getchell ML, Getchell TV (1991) Identification of sugar residues in secretory glycoconjugates of olfactory mucosae using lectin histochemistry. Anat Rec 229: 525–544
Franceschini V, Ciani F (1993) Lectin histochemistry of cell-surface glycoconjugates in the primary olfactory projections of the newt. Cell Mol Biol 39: 651–658
Franceschini V, Lazzari M, Ciani F (2000) Lectin cytochemical localization of glycoconjugates in the olfactory system of the lizards Lacerta viridis and Podarcis sicula. Anat Embryol 202: 49–54
Getchell ML, Rafols JA, Getchell TV (1984) Histological and histochemical studies of the secretory components of the salamander olfactory mucosa: effects of isoproterenol and olfactory nerve section. Anat Rec 208: 553–565
Getchell ML, Zielinski B, Getchell TV (1988) Odorant and autonomic regulation of secretion in the olfactory mucosa. In: Margolis FL, Getchell TV (eds) Molecular neurobiology of the olfactory system. Plenum Press, New York, pp 71–98
Getchell ML, Getchell TV (1992) Fine structural aspects of secretion and extrinsic innervation in the olfactory mucosa. Microsc Res Tech 23: 111–127
Goldstein IJ, Hughes RC, Monsigny M, Osawa T, Sharon N (1980) What should be called a lectin? Nature 285: 66
Graziadei PPC, Monti Graziadei GA (1979) Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J. Neurocytol. 8: 1–6
Hofmann MH, Meyer DL (1991) Functional subdivisions of the olfactory system correlate with lectin-binding properties in Xenopus. Brain Res 564: 344–347
Jones FM, Pfeiffer CJ, Asashima M (1994) Ultrastructure of the olfactory organ of the newt, Cynops pyrrhogaster. Ann Anat 176: 269–275
Key B, Giorgi PP (1986) Selective binding of soybean agglutinin to the olfactory system of Xenopus. Neuroscience 18: 507–515
Kikuyama S, Toyoda F, Ohmiya Y, Matsuda K, Tanaka S, Hayashi H (1995) Sodefrin: a female attracting peptide pheromone in newt cloacal glands. Science 267: 1643–1645
Kikuyama S, Toyoda F (1999) Sodefrin: a novel sex pheromone in a newt. Rev Reprod 4: 1–4
Lis H, Sharon N (1986) Applications of lectins. In: Liener IE, Sharon N, Goldstein IJ (eds) The lectins: properties, functions, and applications in biology and medicine. Academic Press, San Diego, pp 293–308
Menco BP (1994) Ultrastructural aspects of olfactory transduction and perireceptor events. Semin Cell Biol 5: 11–24
Monti Graziadei GA, Graziadei PPC (1979) Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J. Neurocytol. 8: 197–213
Nakajima T, Shiratori K, Ogawa K, Tanioka Y, Taniguchi K (1998) Lectin-binding patterns in the olfactory epithelium and vomeronasal organ of the common marmoset. J Vet Med Sci 60: 1005–1011
Parsons TS (1967) Evolution of the nasal structure in the lower tetrapods. Am Zoologist 7: 397–413
Pelosi P (1996) Perireceptor events in olfaction. J Neurobiol 30: 3–19
Saito S, Taniguchi K (2000) Expression patterns of glycoconjugates in the three distinctive olfactory pathways of the clawed frog, Xenopus laevis. J Vet Med Sci 62: 153–159
Sam M, Vora S, Malnic B, Ma W, Novotny MV, Buck LB (2001) Odorants may arouse instinctive behaviours. Nature 412: 142
Seydel O (1895) Über die Nasenhöle und das Jacobson'sche Organ der Amphibien: Eine vergleichend-anatomische Untersuchung. Morphol Jahrb 23: 453–543
Shapiro LS, Ee PL, Halpern M (1995) Lectin histochemical identification of carbohydrate moieties in opossum chemosensory systems during development, with special emphasis on VVA-identified subdivisions in the accessory olfactory bulb. J Morphol 224: 331–349
Shipley MT, McLean JH, Ennis M (1995) Olfactory system. In: Paxinos G (ed) The rat nervous system. 2nd edn. Academic, San Diego, pp 899–926
Simmons PA, Getchell TV (1981) Physiological activity of newly differentiated olfactory receptor neurons correlated with morphological recovery from olfactory nerve section in the salamander. J Neurophysiol 45(3): 529–549
Schwarting GA, Crandall JE (1991) Subsets of olfactory and vomeronasal sensory epithelial cells and axons revealed by monoclonal antibodies to carbohydrate antigens. Brain Res 547: 239–248
Suzuki K, Taniguchi K, Syuto B (1999) Characterization of olfactory receptor organs in Xenopus laevis Daudin. Anat Rec 255: 420–427
Takami S, Getchell ML, Getchell TV (1994) Lectin histochemical localization of galactose, N-acetylgalactosamine, and N-acetylglucosamine in glycoconjugates of the rat vomeronasal organ, with comparison to the olfactory and septal mucosae. Cell Tissue Res 277: 511–233
Wysocki CT (1979) Neurobehavioral evidence for the involvement of the vomeronasal system in mammalian reproduction. Neurosci Biobehav Rev 3: 301–341
Wysochi CT, Wellington JL, Beauchamp GK (1980) Access of urinary nonvolatiles to the mammalian vomeronasal organ. Science 207: 981–983
Acknowledgements
This work was supported in part by a grant-in-aid for encouragement of young researchers (14770904 to S.S.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saito, S., Matsui, T., Kobayashi, N. et al. Lectin histochemical study on the olfactory organ of the newt, Cynops pyrrhogaster, revealed heterogeneous mucous environments in a single nasal cavity. Anat Embryol 206, 349–356 (2003). https://doi.org/10.1007/s00429-002-0306-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-002-0306-1