Abstract
This study aimed to identify differences in genetic alterations between low- and high-grade lesions in myxofibrosarcoma (MFS) and to examine the efficacy of immune checkpoint inhibitors in 45 patients with MFS. First, genetic differences between low- and high-grade components within the same tumor were analyzed in 11 cases using next-generation sequencing. Based on the obtained data, Sanger sequencing was performed for TP53 mutations in the remaining 34 patients. Loss of heterozygosity (LOH) analysis was performed at the TP53 and RB1 loci. Immunohistochemistry was performed for FGFR3, KIT, MET, programmed death receptor ligand 1 (PD-L1), CD8, FOXP3, and mismatch repair proteins. The microsatellite instability status was also evaluated in all cases. TP53 deleterious mutations and LOH at TP53 and RB1 loci were detected significantly more frequently in high-grade than in low-grade MFS (P = 0.0423, 0.0455, and 0.0455, respectively). LOH at the RB1 locus was significantly associated with shorter recurrence-free survival in both univariate and multivariate analyses. TP53 alterations, such as mutation and LOH, were more frequently observed in low-grade areas within high-grade MFS than in pure low-grade MFS. The positive PD-L1 expression rate was 35.6% (16/45), and all these 16 cases were high-grade. A high density of both CD8+ and FOXP3+ tumor-infiltrating lymphocytes was associated with PD-L1 positivity. LOH at the RB1 locus was identified an independent adverse prognostic factor for recurrence-free survival in patients with MFS. Immune checkpoint inhibitors may be a therapeutic option for a subset of high-grade MFS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myxofibrosarcoma (MFS) is a malignant fibroblastic neoplasm with variable myxoid stroma, cellular pleomorphism, and a distinct curvilinear vascular pattern [1]. It represents approximately 5% of soft tissue sarcomas and is one of the most frequent histological types of soft tissue sarcomas in elderly patients [2]. The World Health Organization (WHO) classification of tumors morphologically categorizes MFS into three histological grades (low grade, intermediate grade, and high grade) based on cellularity, cytological atypia, and proliferative activity [1]. Histological grades correlate with distant metastases and tumor-associated mortality [3]. Low-grade neoplasms are unlikely to metastasize, whereas intermediate- and high-grade neoplasms may develop metastases in approximately 20–30% of cases [4, 5]. MFS is a tumor with a highly complex karyotype, and the number of cytogenetic aberrations has been shown to correlate with MFS tumor grade [6]. Therefore, it is crucial to identify the genetic factors that contribute to tumor progression to improve MFS prognosis.
Tumors with complex karyotypes typically emit signals that increase their immunogenicity and create immunosuppressive contextures in the tumor microenvironment [7,8,9,10]. Considering that programmed cell death 1 (PD-1)/programmed death receptor ligand 1 (PD-L1)-mediated immune evasion is associated with tumor immunosuppressive mechanisms, immune checkpoint inhibitors may be an effective therapy for an MFS subset with a highly complex karyotype.
The aim of this study was to elucidate the mechanisms of tumorigenesis and tumor progression in MFS by evaluating the differences in genetic alterations between low- and high-grade components within the same tumor. Furthermore, PD-L1 and mismatch repair (MMR) protein expressions, profiles of tumor-infiltrating lymphocytes (TILs), and microsatellite instability (MSI) status were examined to explore the possibility of applying immunotherapy.
Materials and methods
Case selection
We selected 45 MFS patients, with primary and recurrent tumors, that were surgically resected at Juntendo University Hospital between June 2007 and June 2019. In all cases, sections were prepared from the maximum cut surface of the gross section for histological evaluation, and additional samplings were performed in areas with different characteristics or where the tumor was close to the margin on gross examination. The 2020 WHO classification of soft tissue and bone tumors formed the basis of histological criteria for diagnosis and tumor grading [1]. All resected specimens were subjected to a uniform protocol for formalin-fixed, paraffin-embedded (FFPE) specimens.
Next-generation sequencing
For next-generation sequencing (NGS), we selected 11 cases with clear margins between low-grade and high-grade components within the tumor. DNA was extracted from each component by microdissection, and DNA integrity was assessed. Genomic DNA from each component and non-tumoral tissue was extracted using a QIAamp FFPE tissue kit (Qiagen, Antwerp, Belgium). NGS was performed as previously described [11], using the Ion Ampliseq Cancer Hotspot Panel v2 (Thermo Fisher Scientific, Waltham, MA, USA).
Sanger sequencing and loss of heterozygosity analysis
The NGS findings frequently detected TP53 mutations, TP53 loss, and RB1 loss in high-grade components. Hence, we examined TP53 mutations in the remaining 34 cases using Sanger sequencing. The TP53 deleterious status of single nucleotide variants (SNVs) and insertion/deletions (Indels) was determined using ClinVar report and p53 immunohistochemistry (IHC) analysis. For cases where ClinVar showed TP53 alterations with uncertain significance/conflicting interpretations of pathogenicity, samples with p53 overexpression determined by IHC were judged as deleterious and those without p53 overexpression as not deleterious. TP53 loss and RB1 loss were also assessed by loss of heterozygosity (LOH) analyses in all 45 cases. LOH analyses were performed for each low- and high-grade component of those analyzed by NGS. In the remaining 34 cases not analyzed by NGS, DNA was extracted from tumor and non-tumor tissues using the QIAamp FFPE tissue kit (Qiagen, Antwerp, Belgium) or Maxwell RSC DNA FFPE Kit – PKK Custom (Promega, Madison, WI, USA). Tumor DNA was extracted from the specimen containing the highest-grade area within the tumor.
Immunohistochemistry
Tissue microarray (TMA) blocks, containing two foci of 2-mm cores from the highest-grade area of each case in all 45 cases, were prepared. IHC was performed in all cases using either whole sections or TMAs, depending on the primary antibodies used. When using whole sections in 11 NGS analyzed cases, we evaluated IHC in both low- and high-grade components. In contrast, in the remaining cases that were not examined by NGS, IHC was performed on the sections containing the highest-grade area. We selected IHC targets based on the targetable receptor tyrosine kinases revealed by NGS, which are frequently amplified in high-grade components. FGFR3 and c-KIT were evaluated in whole sections from all 45 cases. IHC for c-MET was performed using the TMA blocks for all 45 cases. The following antibodies were used: FGFR3 (clone B-9, sc-13121, 1:20, Santa Cruz Biotechnology, Santa Cruz, CA, USA), c-KIT (1:200, Dako, Carpinteria, CA, USA), and c-MET (clone SP44, 1:100, Ventana Medical Systems, Tucson, AZ, USA). FGFR3, c-KIT, and c-MET expressions were considered positive if at least 50% of tumor cells showed membranous and/or cytoplasmic staining. In addition, p53 and RB expressions were evaluated in whole sections in all 45 cases using antibodies against p53 (clone 1801, 1:1, BioGenex, Fremont, CA, USA) and RB (clone 13A/10, 1:50; Leica, Newcastle, UK). Overexpression of p53 was defined as strong nuclear staining in at least 10% of tumor cell nuclei; p53 complete loss was defined as complete absence of staining in tumor cell nuclei despite the physiological expression in the internal control within the slide, and for physiological expression in the internal controls, weak or strong staining in < 10% of tumor cells was considered negative as previously described [11, 12]. Complete RB loss was defined as the complete absence of staining in the tumor cell nuclei and detectible expression in the internal controls. Furthermore, PD-L1 expression on tumor cells and CD8 and FOXP3 expression in tumor-infiltrating lymphocytes (TILs) were also examined by IHC on whole sections of all 45 cases using antibodies against PD-L1 (clone 22C3, 1:50, Dako), CD8 (clone 1A5, 1:100, Leica), and FOXP3 (clone 236A/E7, 1:500, Abcam, Cambridge, UK). PD-L1 staining was performed using the Dako Autostainer Link 48 platform and an automated staining protocol validated for the PD-L1 IHC 22C3 PharmDx assay. PD-L1 positivity was defined as a combined positive score (CPS) of ≥ 1. CPS is defined as the sum of PD-L1 stained tumor cells, lymphocytes, and macrophages divided by the total number of viable tumor cells multiplied by 100 [13]. The numbers of CD8- and FOXP3-positive TILs were quantified as the average (mean) of five representative high-power fields (HPFs) (×400 magnification; field diameter = 0.5 mm). In PD-L1 positive cases, the number of TILs was quantified around the PD-L1 positive area, while in PD-L1 negative cases, the number of TILs was counted around the sites with the largest number. TIL staining was classified into two scores based on the average of five fields: low (≤ 10 per HPF) and high (> 10 per HPF). MMR protein expression was also analyzed by IHC in TMAs in all 45 cases. The primary antibodies (mouse monoclonal) used in this study were as follows: MLH1 (clone ES05, 1:10, Dako, Glostrup, Denmark), MSH2 (clone FE11, 1:20, Dako), PMS2 (dilution 1:10, clone EP51, Dako), and MSH6 (clone EP49, 1:20, Dako). Immunohistochemical staining was performed using the Ventana Benchmark XT autostainer. MMR deficiency (dMMR) was defined as the complete loss of nuclear expression of one or more MMR proteins in tumor cells. MMR proficiency (pMMR) was defined as the presence of normal staining of all MMR proteins. Lymphocytes in the germinal center of the lymph nodes and proliferative cell zones of the intestinal crypts served as positive controls for MMR staining. Negative cases of TMA were re-evaluated in whole sections.
Microsatellite instability analysis
We evaluated the MSI status for all 45 cases using the same tumoral DNA used in Sanger sequencing and LOH analyses. We examined NGS cases for both low- and high-grade components. Five markers (BAT25, BAT26, NR21, NR24, and MONO27) were used. MSI status was defined as previously described [14]. Microsatellite instability high (MSI-H) status was defined as positive for two or more microsatellite markers, while microsatellite instability low (MSI-L) status was defined as positive for only one microsatellite marker. Microsatellite stable (MSS) was defined as negative for all markers.
Survival and statistical analyses
The tumor grade was determined as the highest-grade component within the tumor with a mixture of various grades of components, regardless of the amount of the component. If the genetic alterations and IHC results differed between low- and high-grade components in the 11 NGS analyzed cases, the results of the components with genetic alterations, abnormal IHC findings, and a larger number of TILs were adopted for the cases. We categorized intermediate-grade MFS as high-grade MFS in all analyses because only three cases of intermediate-grade MFS were present in our study. Categorical and continuous variables were analyzed using the Fisher’s exact test, Student’s t-test, or Mann-Whitney U test. The surgical margin was positive when the tumor cells were exposed at the cut end. We performed Kaplan-Meier survival analysis and log-rank tests to elucidate the effects of clinicopathological factors on the prognosis of MFS. The analysis for recurrence-free survival in the chemotherapy and/or radiotherapy category excluded patients who received chemotherapy and/or radiotherapy only after local recurrence or distant metastasis. Multivariate analysis was performed using the Cox proportional hazards model for variables that were significant in each test. These statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [15], a graphical user interface for R (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was set at P < 0.05.
Results
Clinicopathological findings
Supplementary Fig. 1 shows representative cases of the three histological grades (low, intermediate, and high grade). Supplementary Table 1 summarizes the clinicopathological characteristics of 45 MFS cases with low-grade tumors and high-grade histology. Statistically significant differences were observed in age, tumor size, radiotherapy, and pStage (P = 0.0431, 0.00729, 0.0424, and 4.11 × 10−11, respectively). After surgery, 10 patients who had high-grade histology received irradiation as adjuvant therapy before local recurrence and/or distant metastasis. Among the four patients who received chemotherapy, one was treated with neoadjuvant therapy, and the remaining three were treated after local recurrence and/or distant metastasis. Seven and four out of 32 patients with high-grade tumors experienced local recurrence and distant metastasis, respectively, whereas none of the patients with pure low-grade tumors experienced local recurrence/distant metastasis. No patient presented with distant metastases at the time of surgery.
NGS analysis
As previously mentioned, 11 patients underwent NGS analysis (Fig. 1). In total, we detected eight SNVs/Indels in seven of 11 cases (63.3%). Five SNVs were observed as the same mutations throughout the low- and high-grade components within the same tumor, and three SNVs/Indels were detected only in the high-grade components: TP53 (2/11, 18.2%) and KIT (1/11, 9.1%). Furthermore, copy number variations (CNVs) detected only in high-grade components but not in low-grade components within the same tumor as lost genes were RB1 (2/11, 18.2%), APC (1/11, 9.1%), CDH1 (1/11, 9.1%), NOTCH1 (1/11, 9.1%), NPM1 (1/11, 9.1%), PTEN (1/11, 9.1%), SMARCB1 (1/11, 9.1%), and TP53 (1/11, 9.1%). Interestingly, although TP53 and RB1 losses were detected in three cases each by NGS, they were detected only in one of the two components. CNVs detected only in high-grade components but not in low-grade components within the same tumor as amplified genes were CSF1R (1/11, 9.1%), EGFR (1/11, 9.1%), EGFR-AS1 (1/11, 9.1%), ERBB2 (1/11, 9.1%), FBXW7 (1/11, 9.1%), FGFR3 (1/11, 9.1%), HRAS (1/11, 9.1%), KDR (1/11, 9.1%), KIT (1/11, 9.1%), MET (1/11, 9.1%), PDGFRA (1/11, 9.1%), and SMAD4 (1/11, 9.1%). All these amplifications were detected as triploid.
Sanger sequencing, LOH analyses, and IHC for p53 and RB
Sanger sequencing of TP53 in the remaining 34 cases not subjected to NGS was performed, and we found five additional cases with TP53 mutations in high-grade tumors. The pathogenicity of the various SNVs detected in this study is provided in Supplementary Table 2. Between the NGS and Sanger sequencing findings, TP53 mutations were identified in 10 cases. However, the TP53 missense mutation in case 2 (p.Pro85Ser) was described as of uncertain significance in ClinVar, and this case did not show p53 overexpression by IHC. Therefore, this mutation was not considered deleterious. Consequently, TP53 deleterious mutations were identified in nine cases of high-grade tumors (9/32, 28.1%) and zero cases of pure low-grade tumors (Table 1; P = 0.0423). LOH analyses of TP53 and RB1 were performed for all 45 cases, including those subjected to NGS. LOH at TP53 locus was detected in 16 cases of high-grade tumors (16/32, 50.0%) and two of pure low-grade tumors (2/13, 15.4%) (Table 1; P = 0.0455). TP53 biallelic inactivation, defined by deleterious mutation and the presence of LOH, was detected in six cases of high-grade tumors (6/32, 18.8%) and zero cases of low-grade tumors (0/13, 0%) (Table 1; P = 0.0895). TP53 alterations, such as mutation and LOH, were significantly more frequently observed in low-grade areas within high-grade MFS than in pure low-grade MFS (Supplementary Table 3: P = 0.011). In addition, LOH at RB1 locus was detected in 16 cases of high-grade tumors (16/32, 50.0%) and two cases of pure low-grade tumors (2/13, 15.4%) (Table 1, P = 0.0455). Notably, among the 11 cases with clear margins between low- and high-grade areas, the LOH status of these two gene loci was preserved between the two areas in 10 cases. In the remaining case, LOH at RB1 locus was observed only in the high-grade area. Complete RB loss by IHC was observed in seven of 18 cases with LOH at RB1 locus. Among the 27 cases without LOH at the RB1 locus, no cases revealed complete RB loss by IHC.
IHC for FGFR3, c-KIT, and c-MET
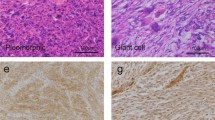
Figure 2 shows representative FGFR3 (B), c-KIT (D), and c-MET (F) IHC results. FGFR3 expression was detected in four high-grade tumors (4/32, 12.5%) and one low-grade tumor (1/13, 7.7%) (Table 1, P = 1). We detected c-KIT expression in three cases of high-grade tumors (3/32, 9.4%) but none in low-grade tumors (Table 1, P = 0.546). Two cases of high-grade tumors (2/32, 6.3%), but none of the low-grade tumors (Table 1: P = 1), displayed c-MET expression.
FGFR3, c-KIT, and c-MET expression assessed by IHC. A and B Case 11. C and D Case 36. E and F Case 22. A, C, and E Hematoxylin and eosin staining. B FGFR3, intermediate intensity, and cytoplasmic pattern. D c-KIT, weak intensity, cytoplasmic pattern. F c-MET, intermediate intensity, and membranous and cytoplasmic pattern
IHC for PD-L1, CD8, and FOXP3
Figure 3 shows representative IHC results for PD-L1 (B), CD8 (C), and FOXP3 (D). PD-L1 expression was observed in 16 cases of high-grade tumors (16/32, 50.0%) and no cases of low-grade tumors (0/13, 0%) (Table 1; P = 0.00136). A large number of CD8-positive and FOXP3-positive TILs were significantly associated with PD-L1 positivity (Table 2; P = 0.0000167 and P = 0.00366, respectively). A high score group of either CD8-positive or FOXP3-positive TILs was significantly more frequently observed in high-grade tumors than in low-grade tumors (Table 1; P = 0.0000183 and P = 0.000634, respectively). Comparing PD-L1 expression between low- and high-grade components in the 11 NGS analyzed cases, PD-L1 expression was preserved between the two areas in 10 of 11 cases; however, the remaining case (case 27) was negative in the high-grade component but positive in the low-grade component. In this case, the number of TILs was also higher in the low-grade component than in the high-grade component (CD8+ 2.4/HPF vs. 1.2/HPF, FOXP3+ 29/HPF vs. 8.6/HPF). The number of CD8+ and FOXP3+ TILs tended to be significantly larger in the high-grade component than in the low-grade component among the 11 cases analyzed by NGS (Supplementary Table 4; P = 0.0659 and 0.0708, respectively). PD-L1 positive rates and the numbers of CD8+ and FOXP3+ TILs were significantly higher in the low-grade component of high-grade MFS than those in the pure low-grade MFS (Supplementary Table 5; P = 0.000224, 0.0136, and 0.00484, respectively).
PD-L1 expression on tumor cells and CD8 and FOXP3 expressions on TILs by IHC in case 37. A Hematoxylin and eosin staining. B PD-L1 IHC. PD-L1 positivity was defined as a combined positive score (CPS) ≧1. PD-L1 stained lymphocytes and macrophages are calculated as PD-L1 positive cells in the CPS evaluation. C CD8 IHC. D FOXP3 IHC
IHC for MMR proteins and MSI analysis
TMA-based IHC revealed that one case (case 12) did not show any of the four MMR proteins and that another case (case 28) exhibited loss of MLH1 and PMS2 expression. We then re-evaluated these two cases using whole-section IHC. This analysis revealed that MLH1 and PMS2 expression was invariably lost in case 28 (Fig. 4), whereas case 12 retained both markers. As a result, only one high-grade case (case 28) was considered dMMR (1/45, 2.2%).
MSI-H was not detected in any of the cases. The MSI-L and MSS rates were 3.1% (1/32) and 96.9% (31/32), respectively, in high-grade MFS and 0% (0/13) and 100% (13/13), respectively, in low-grade MFS. MSI status was not statistically different between low- and high-grade tumors (Table 1, P = 1). One MSI-L case was positive for MONO27.
Tables 1 and 3 summarize the genetic alterations, IHC expression, and MSI status in MFS. Briefly, high-grade MFS frequently presented TP53 deleterious mutations, LOH at TP53 and RB1 loci, PD-L1 expression, and a high score group of both CD8/FOXP3 positive TILs with statistical significance.
Survival analysis
The follow-up observation period ranged from 1 to 136 months, with a median duration of 22.9 months. Univariate survival analyses in all 45 cases revealed that LOH at RB1 locus (P = 0.0179), positive surgical margins (P = 0.0499), and older age (P = 0.0253) were significantly associated with shorter recurrence-free survival (Fig. 5A). However, no significant differences were observed in overall survival and metastasis-free survival for all factors (Supplementary Table 6). Multivariate analysis of recurrence-free survival of LOH at RB1 locus, surgical margin, and patient age showed that only LOH at RB1 locus was significantly correlated with shorter recurrence-free survival (P = 0.03619) (Supplementary Table 6). Univariate survival analyses in 32 cases treated by surgical resection only without chemoradiation therapy revealed that TP53 deleterious mutation (P = 0.0432), TP53 biallelic inactivation (P = 0.0329), LOH at the RB1 locus (P = 0.00059), positive surgical margin (P = 0.0000311), and older age (P = 0.0226) were significantly correlated with shorter recurrence-free survival (Fig. 5B). To further evaluate the prognostic impact of LOH at the RB1 locus, patients with RB1 LOH were divided into two groups according to RB IHC findings. Cases with RB1 LOH showed shorter recurrence-free survival regardless of RB IHC status than cases without RB1 LOH with statistical significance (P = 0.031 and 0.0402, respectively) (Fig. 5C-1, 2). Recurrence-free survival between cases with RB1 LOH/IHC (complete loss) and cases with RB1 LOH/IHC(+) was not significantly different (P = 0.73) (Fig. 5C-3).
Kaplan-Meier survival analysis according to the clinicopathological factors (recurrence-free survival). A, Analyses in all 45 cases. B, Analyses in the 32 cases treated by surgical resections only without chemoradiation therapy. C, Analyses comparing three groups: 27 cases without RB1 LOH, 7 cases with RB1 LOH(+)/IHC(complete loss), and 11 cases with RB1 LOH(+)/IHC(+)
Discussion
Previous studies have frequently detected RB1 and TP53 alterations in sarcomas with complex karyotypes, such as undifferentiated sarcoma, dedifferentiated liposarcoma, and leiomyosarcoma [16,17,18,19]. Even in MFS, these alterations have been previously identified. The Cancer Genome Atlas Research Network study described RB1, TP53, and CDKN2A deletions in 24%, 12%, and 18% of MFS cases, respectively [16]. Ogura et al. reported frequent TP53 (46%), RB1 (18%), and CDKN2A/2B (16%) mutations in MFS [20]. However, these studies did not consider the tumor grade in MFS. We attempted to detect alterations that were characteristic of the high-grade component within cases of high-grade MFS containing a low-grade component using NGS to elucidate the mechanisms associated with the acquisition of a higher malignant potential. TP53 mutation and LOH at the TP53 and RB1 loci, which were identified by NGS, were significantly more frequently detected in high-grade MFS than in pure low-grade MFS. In addition, among the 11 cases analyzed by NGS, LOH at the TP53 and RB1 loci was preserved in both low-grade and high-grade areas in 10 cases, and in the remaining case, LOH at the RB1 locus was observed only in the high-grade area. Genetic alterations in low-grade components of high-grade MFS may be somewhat different from those in pure low-grade MFS. These findings suggest that low-grade components in high-grade MFS seem to have distinct patterns for genetic alterations compared to pure low-grade MFS and that alterations in TP53 and RB1 might be associated with the acquisition of higher malignant potential in MFS. Genetic alterations in low-grade components of high-grade MFS may be somewhat different from those in pure low-grade MFS.
The molecular prognostic factors for MFS have not been fully elucidated. However, by integrating genetic analyses, Ogura et al. reported that RB1, CDKN2A, CDKN2B, and TP53 alterations were associated with poor overall survival but not with local recurrence-free survival by univariate and multivariate analyses [20]. In the present study, as tumor deaths occurred in only two cases, none of the factors was associated with overall survival. However, LOH at the RB1 locus was significantly associated with poor recurrence-free survival (P = 0.0179) in univariate analysis in all 45 cases. Even in the multivariate analysis of clinicopathological factors, only LOH at the RB1 locus was significantly correlated with poor recurrence-free survival (P = 0.03619), emphasizing that this alteration was a considerably influential prognostic factor. Complete RB loss by IHC was observed in seven of 18 RB1 LOH(+) cases. Among the 27 cases without LOH at the RB1 locus, no cases displayed complete RB loss by IHC. Furthermore, patients with RB1 LOH showed shorter recurrence-free survival regardless of RB IHC status than those without RB1 LOH with statistical significance. These findings suggest that RB1 LOH acts as a driver of MFS progression. In several previous studies, the surgical margin was a significant predictor of local recurrence in MFS [5, 21,22,23]. In the present study, 5 of 7 cases with positive surgical margins were treated with radiation therapy after surgery, and 4 of these 5 cases did not show recurrence. Both cases with positive surgical margins that were not treated with radiation therapy later showed recurrence. Univariate survival analyses in 32 cases treated with surgical resection only without chemoradiation therapy revealed that TP53 deleterious mutation, TP53 biallelic inactivation, LOH at the RB1 locus, positive surgical margin, and older age were significantly associated with shorter recurrence-free survival. However, in the univariate analysis, all 45 cases, including those who received chemoradiation therapy, were no longer significant, unlike the independent implications of RB1 LOH, positive surgical margins, and older age as adverse prognosticators. Therefore, although a positive surgical margin is also an important prognostic factor for recurrence-free survival, it is conceivable that radiation therapy could have affected the prognostic impact of positive surgical margins. In studies describing molecular markers correlating with recurrence-free survival in liposarcoma, NIP7, RPL10L, and MCM2 alterations were significantly associated with distant recurrence-free survival in multivariate analysis [24], and CDK4 and JUN amplification were associated with recurrence-free survival in univariate analysis [25]. Furthermore, in extraskeletal osteosarcoma, cases harboring simultaneous TP53 and RB1 biallelic copy number losses were associated with worse overall survival and local recurrence [26]. However, comparisons of surgical margins were not included in these studies, and no previous studies have reported any genetic alterations as stronger molecular biomarkers for recurrence-free survival than surgical margins in soft tissue sarcoma. Similarly, there are no reports showing that LOH at the RB1 locus is a prognostic factor for recurrence-free survival in soft tissue sarcomas. Here, we present the novel finding that a specific molecular biomarker has a prognostic impact exceeding the surgical margin on recurrence-free survival, although further studies are required to draw a definitive conclusion.
FGFR3, c-KIT, and c-MET expressions as measured by IHC were not observed more frequently in high-grade MFS than in pure low-grade MFS and were not significantly associated with prognosis. Previous studies have identified mutations and amplifications of FGFR3 and KIT in MFS [27, 28], but a detailed analysis of these genes has not been performed. In contrast, MET overexpression by IHC has been reported to positively correlate with higher grades (FNCLCC grades 2–3) in MFS [29, 30], and independently correlates with worse metastasis-free and overall survival with statistical significance [30]. However, these findings conflict with those of our study, which revealed that MET overexpression showed a trend toward better prognosis.
Several studies have assessed PD-L1 expression by IHC in various sarcoma subtypes, but several meta-analyses and mini-reviews have reported that the positive rates show variability [31,32,33,34]. These results might be due to the variety of PD-L1 antibodies used for IHC, differing cut-off values, and the evaluation methods for PD-L1 positivity. In addition, the small number of each sarcoma subtype in those studies might have degraded the significance of the results. In MFS, Kosemehmetoglu et al. reported the PD-L1 positivity rate of neoplastic cells by IHC as 0% (0/4) using the E1L3N (Cell Signaling) antibody [35] and Vargas et al. reported the positive rate of tumor cells on TMAs as 18% (17/97) using the SP263 (VENTANA) antibody [36]. In the present study, the PD-L1 positivity rate assessed by CPS on whole sections was 35.6% (16/45) using the 22C3 (Dako) antibody. This frequency is slightly higher than the previously reported values. The different scoring methods may have affected this variation.
CPS was combined with a positive score, including not only tumor cells but also lymphocytes and macrophages stained with PD-L1 in positive cells. Phase Ib trials for pembrolizumab to treat recurrent or metastatic squamous cell carcinoma of the head and neck and advanced gastric cancer suggested that the assessment of PD-L1 expression in tumor and immune cells might be correlated with the response rate to pembrolizumab [37, 38]. Furthermore, phase II and III trials used CPS to effectively evaluate PD-L1 expression with using pembrolizumab as a therapy for recurrent or metastatic squamous cell carcinoma of the head and neck and advanced gastric or gastroesophageal junction cancer [39,40,41]. PD-L1 CPS positivity was correlated with a high density of both CD8+ and FOXP3+ TILs in head and neck squamous cell carcinomas [42, 43] and both densities were associated with better disease-specific survival [42]. In addition, a high density of CD8+ and FOXP3+ TILs was significantly correlated with PD-L1 expression in various sarcomas [34, 44], and a high density of CD8+ TILs was an independent prognostic factor for a favorable prognosis [44]. In the present study, immune cells such as lymphocytes and macrophages were variably accompanied by PD-L1 expression in all cases. A large number of CD8+ and FOXP3+ TILs were significantly associated with PD-L1 CPS positivity. As in previous studies, we found that the profile of TILs was closely related to PD-L1 positivity but did not affect the prognosis of patients with MFS. CPS is a simple evaluation method for PD-L1 expression using IHC because it does not need to distinguish PD-L1 positive tumor cells from lymphocytes and macrophages [13]; however, previous studies have generally not used it for sarcomas. Further analyses are required to establish a standard method, including consideration of CPS, to evaluate PD-L1 expression in sarcomas.
All PD-L1 positive cases in this study were high-grade MFS, and the high score group of CD8+ and FOXP3+ TILs was observed more frequently in high-grade MFS than in pure low-grade MFS. This suggests that the PD-L1 positivity rate and the profile of TILs vary according to tumor grade in MFS. However, comparing PD-L1 expression between low- and high-grade components revealed that only one out of 11 cases analyzed by NGS had PD-L1 expression that was negative in the high-grade component but positive in the low-grade component. In this case, the number of TILs was also higher in the low-grade component than in the high-grade component. Compared with pure low-grade MFS, PD-L1 positive rates and the numbers of CD8+ and FOXP3+ TILs were higher in low-grade components of high-grade MFS. These findings suggest that not only the genetic alterations but also the tumor microenvironment may differ between low-grade component within high-grade MFS and pure low-grade MFS. In addition to tumor grade, the number of TILs may also be important for PD-L1 expression.
In our study, PD-L1 expression was associated with poor prognosis in recurrence-free survival, although it did not reach statistical significance (P = 0.186). Wang et al. showed that PD-L1 expression correlated with poor overall survival (HR 1.45, 95% CI 1.11–1.90, P < 0.01), metastasis-free survival (HR 1.58, 95% CI 1.14–2.19, P < 0.01), and event-free survival (HR 2.82, 95% CI 1.69–4.71, P < 0.01) in different sarcomas (HR; hazard ratio, 95% CI) [34]. In a multicenter, two-cohort, open-label, phase II study of pembrolizumab monotherapy for safety and activity in patients with advanced soft tissue sarcoma or bone sarcoma (SARC028), seven (18%) of 40 patients with soft tissue sarcoma had an objective response, including four (40%) of 10 patients with undifferentiated pleomorphic sarcoma, two (20%) of 10 patients with liposarcoma, and one (10%) of 10 patients with synovial sarcoma [45]. Thus, immune checkpoint inhibitors could be effective in a subset of patients with MFS, especially those with PD-L1 expression.
MSI-H was not identified in any of the cases in this study, but MSI-L was detected in one high-grade MFS case, which was determined to be pMMR by IHC. In contrast, we identified dMMR in one high-grade tumor. However, this case was MSS and represented an MLH1 altered pattern. Previous studies have noted a high concordance rate between MSI analysis and MMR protein expression in more than 90% of colorectal cancers [46]. However, this rate may be lower in solid cancers other than colorectal cancers [47]. A recent study examining MSI status using targeted NGS in 15,045 patients with cancer (in more than 50 cancer types) identified 5.7% (45/785) of soft tissue sarcomas with MSI-H/indeterminate [48]. In other studies, all 50 samples of various types of soft tissue and bone sarcomas examined by NGS, and all 71 soft tissue sarcomas examined by PCR were MSS [49, 50]. In the latter case, all 71 soft-tissue sarcomas had pMMR. Furthermore, another report detected dMMR in seven of 304 cases of various types of soft tissue sarcomas (7/304, 2.3%) (four unclassified sarcomas, one uterine leiomyosarcoma, one PEComa, and one rhabdomyosarcoma), the most common being unclassified sarcomas (4/40, 10%), with zero occurrence of MFS cases (0/6) [51]. NGS analysis formed the basis of this report, but IHC for MMR proteins showed protein expression patterns corresponding to molecular abnormalities in all dMMR cases. Furthermore, compared to 70 dMMR carcinomas, seven dMMR sarcomas showed a lower tumor mutation burden (TMB) (median 28 vs. 16, P = 0.006) [51]. It has also been reported that the TMB is low in MFS [16, 20]. MSI-H tumors are thought to be TMB-high (TMB-H), with a high probability of colorectal and gastric cancers [52, 53]. However, it is less likely that the dMMR MFS in this study showed TMB-H.
Hyperploid cancer cells become immunogenic because of a constitutive endoplasmic reticulum stress response, resulting in aberrant cell surface exposure to calreticulin, which initiates protective immune responses mainly involving cytotoxic T lymphocytes (CTLs) [7]. Similarly, cells with complex karyotypes exhibit features of senescence and produce proinflammatory signal characteristic of a senescence-associated secretory phenotype and high expression of NKG2D and DNAM1 ligands on their surface, stimulating natural killer cell-mediated cytotoxic responses [8, 9]. Tumors with complex karyotypes appear to have mechanisms, apart from neoantigens, which increase their immunogenicity. Conversely, tumors with high levels of somatic copy number alterations (SCNAs) create an immunosuppressive microenvironment, defined by a paucity of antitumor immune cells such as CD8+ T cells and an increased population of pro-tumorigenic immune cells such as M2 macrophages [10]. The expression of cytotoxic functions such as granzymes and the IFN-γ pathway is significantly lower in tumors with high degrees of aneuploidy than in those with low degrees of aneuploidy [10]. Karyotypes and aneuploidy were not examined in this study, although they generally exhibit a complex karyotype. LOH at RB1 and TP53 loci was more frequently observed in high-grade tumors than in pure low-grade tumors. However, all copy numbers of genes revealed as amplified by NGS were threefold higher in this study; thus, SCNAs were not considered high in MFS. Therefore, it is reasonable that a large number of TILs infiltrate high-grade MFS, especially in PD-L1-positive cases.
Finally, genomic alteration data in this study might be diluted by admixed inflammatory cells, because MFS usually contain admixed neoplastic fibroblastic cells and inflammatory cells. This cannot be denied, especially for the CNV data, because all genes were amplified only threefold. Thus, genes with true significance in cancer biology may not have been identified by this study.
In conclusion, LOH at the RB1 locus was found to be a prognostic factor for adverse recurrence-free survival. TP53 mutations and LOH at TP53 and RB1 loci may be associated with MFS tumor progression. Immune checkpoint inhibitors may be a therapeutic option for the MFS subset.
References
Huang HY, Mentzel TDW, Shibata T (2020) Myxofibrosarcoma. In: WHO Classification of Tumours Editorial Board, ed. World Health Organization classification of soft tissue and bone tumours, 5th edn. IARC Press, Lyon, pp 124–126
Roland CL, Wang WL, Lazar AJ, Torres KE (2016) Myxofibrosarcoma. Surg Oncol Clin N Am. 25(4):775–788. https://doi.org/10.1016/j.soc.2016.05.008
Sanfilippo R, Miceli R, Grosso F, Fiore M, Puma E, Pennacchioli E et al (2011) Myxofibrosarcoma: prognostic factors and survival in a series of patients treated at a single institution. Ann Surg Oncol. 18(3):720–725. https://doi.org/10.1245/s10434-010-1341-4
Mentzel T, Calonje E, Wadden C, Camplejohn RS, Beham A, Smith MA et al (1996) Myxofibrosarcoma. Clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Pathol. 20(4):391–405. https://doi.org/10.1097/00000478-199604000-00001
Look Hong NJ, Hornicek FJ, Raskin KA, Yoon SS, Szymonifka J, Yeap B et al (2013) Prognostic factors and outcomes of patients with myxofibrosarcoma. Ann Surg Oncol. 20(1):80–86. https://doi.org/10.1245/s10434-012-2572-3
Willems SM, Debiec-Rychter M, Szuhai K, Hogendoorn PC, Sciot R (2006) Local recurrence of myxofibrosarcoma is associated with increase in tumour grade and cytogenetic aberrations, suggesting a multistep tumour progression model. Mod Pathol. 19(3):407–416. https://doi.org/10.1038/modpathol.3800550
Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M et al (2012) An immunosurveillance mechanism controls cancer cell ploidy. Science. 337(6102):1678–1684. https://doi.org/10.1126/science.1224922
Lopez-Soto A, Gonzalez S, Lopez-Larrea C, Kroemer G (2017) Immunosurveillance of malignant cells with complex karyotypes. Trends Cell Biol. 27(12):880–884. https://doi.org/10.1016/j.tcb.2017.09.001
Santaguida S, Richardson A, Iyer DR, M’Saad O, Zasadil L, Knouse KA et al (2017) Chromosome mis-segregation generates cell-cycle-arrested cells with complex karyotypes that are eliminated by the immune system. Dev Cell. 41(6):638–51 e5. https://doi.org/10.1016/j.devcel.2017.05.022
Davoli T, Uno H, Wooten EC, Elledge SJ (2017) Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science. 355(6322). https://doi.org/10.1126/science.aaf8399
Akazawa Y, Saito T, Hayashi T, Yanai Y, Tsuyama S, Akaike K et al (2018) Next-generation sequencing analysis for gastric adenocarcinoma with enteroblastic differentiation: emphasis on the relationship with hepatoid adenocarcinoma. Hum Pathol. 78:79–88. https://doi.org/10.1016/j.humpath.2018.04.022
Yanai Y, Hayashi T, Tsuyama S, Nasu M, Hashimoto T, Kajiyama Y et al (2022) Clinicopathological and mutational analysis of esophageal basaloid squamous cell carcinoma. Virchows Arch. https://doi.org/10.1007/s00428-022-03350-3
Kulangara K, Zhang N, Corigliano E, Guerrero L, Waldroup S, Jaiswal D et al (2019) clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 143(3):330–337. https://doi.org/10.5858/arpa.2018-0043-OA
Patil DT, Bronner MP, Portier BP, Fraser CR, Plesec TP, Liu X (2012) A five-marker panel in a multiplex PCR accurately detects microsatellite instability-high colorectal tumors without control DNA. Diagn Mol Pathol. 21(3):127–133. https://doi.org/10.1097/PDM.0b013e3182461cc3
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Cancer Genome Atlas Research Network. Electronic address edsc, Cancer Genome Atlas Research N. Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell. 2017;171(4):950-965 e28. https://doi.org/10.1016/j.cell.2017.10.014.
Perot G, Chibon F, Montero A, Lagarde P, de The H, Terrier P et al (2010) Constant p53 pathway inactivation in a large series of soft tissue sarcomas with complex genetics. Am J Pathol. 177(4):2080–2090. https://doi.org/10.2353/ajpath.2010.100104
Steele CD, Tarabichi M, Oukrif D, Webster AP, Ye H, Fittall M et al (2019) Undifferentiated sarcomas develop through distinct evolutionary pathways. Cancer Cell. 35(3):441–56 e8. https://doi.org/10.1016/j.ccell.2019.02.002
Takahira T, Oda Y, Tamiya S, Yamamoto H, Kobayashi C, Izumi T et al (2005) Alterations of the RB1 gene in dedifferentiated liposarcoma. Mod Pathol. 18(11):1461–1470. https://doi.org/10.1038/modpathol.3800447
Ogura K, Hosoda F, Arai Y, Nakamura H, Hama N, Totoki Y et al (2018) Integrated genetic and epigenetic analysis of myxofibrosarcoma. Nat Commun. 9(1):2765. https://doi.org/10.1038/s41467-018-03891-9
Gilg MM, Sunitsch S, Leitner L, Bergovec M, Szkandera J, Leithner A et al (2020) Tumor-associated mortality and prognostic factors in myxofibrosarcoma - a retrospective review of 109 patients. Orthop Traumatol Surg Res. 106(6):1059–1065. https://doi.org/10.1016/j.otsr.2020.04.017
Liu H, Zhang X, Zhang S, Yu S (2021) Analysis of prognostic factors in 171 patients with myxofibrosarcoma of the trunk and extremities: a cohort study. Ann Transl Med. 9(16):1322. https://doi.org/10.21037/atm-21-3587
Kikuta K, Kubota D, Yoshida A, Suzuki Y, Morioka H, Toyama Y et al (2013) An analysis of factors related to recurrence of myxofibrosarcoma. Jpn J Clin Oncol. 43(11):1093–1104. https://doi.org/10.1093/jjco/hyt119
Liu J, Li R, Liao X, Jiang W. Comprehensive bioinformatic analysis genes associated to the prognosis of liposarcoma. Med Sci Monit. 2018;24:7329-39. https://doi.org/10.12659/MSM.913043.
Saada-Bouzid E, Burel-Vandenbos F, Ranchere-Vince D, Birtwisle-Peyrottes I, Chetaille B, Bouvier C et al (2015) Prognostic value of HMGA2, CDK4, and JUN amplification in well-differentiated and dedifferentiated liposarcomas. Mod Pathol. 28(11):1404–1414. https://doi.org/10.1038/modpathol.2015.96
Jour G, Wang L, Middha S, Zehir A, Chen W, Sadowska J et al (2016) The molecular landscape of extraskeletal osteosarcoma: a clinicopathological and molecular biomarker study. J Pathol Clin Res. 2(1):9–20. https://doi.org/10.1002/cjp2.29
Heitzer E, Sunitsch S, Gilg MM, Lohberger B, Rinner B, Kashofer K et al (2017) Expanded molecular profiling of myxofibrosarcoma reveals potentially actionable targets. Mod Pathol. 30(12):1698–1709. https://doi.org/10.1038/modpathol.2017.94
Lohberger B, Stuendl N, Leithner A, Rinner B, Sauer S, Kashofer K et al (2017) Establishment of a novel cellular model for myxofibrosarcoma heterogeneity. Sci Rep. 7:44700. https://doi.org/10.1038/srep44700
Ma S, Fan L, Liu Y, Wang Y, Yu K, Wang L et al (2018) MET-overexpressing myxofibrosarcoma frequently exhibit polysomy of chromosome 7 but not MET amplification, especially in high-grade cases: clinical and pathological review of 30 myxofibrosarcoma cases. Diagn Pathol. 13(1):56. https://doi.org/10.1186/s13000-018-0733-9
Lee JC, Li CF, Fang FM, Wang JW, Jeng YM, Yu SC et al (2010) Prognostic implication of MET overexpression in myxofibrosarcomas: an integrative array comparative genomic hybridization, real-time quantitative PCR, immunoblotting, and immunohistochemical analysis. Mod Pathol. 23(10):1379–1392. https://doi.org/10.1038/modpathol.2010.128
Zhu Z, Jin Z, Zhang M, Tang Y, Yang G, Yuan X et al (2017) Prognostic value of programmed death-ligand 1 in sarcoma: a meta-analysis. Oncotarget. 8(35):59570–59580. https://doi.org/10.18632/oncotarget.19168
Zheng C, You W, Wan P, Jiang X, Chen J, Zheng Y et al (2018) Clinicopathological and prognostic significance of PD-L1 expression in sarcoma: a systematic review and meta-analysis. Medicine (Baltimore). 97(25):e11004. https://doi.org/10.1097/MD.0000000000011004
Veenstra R, Kostine M, Cleton-Jansen AM, de Miranda NF, Bovee JV (2018) Immune checkpoint inhibitors in sarcomas: in quest of predictive biomarkers. Lab Invest. 98(1):41–50. https://doi.org/10.1038/labinvest.2017.128
Wang F, Yu T, Ma C, Yuan H, Zhang H, Zhang Z (2020) Prognostic value of programmed cell death 1 ligand-1 in patients with bone and soft tissue sarcomas: a systemic and comprehensive meta-analysis based on 3,680 patients. Front Oncol. 10:749. https://doi.org/10.3389/fonc.2020.00749
Kosemehmetoglu K, Ozogul E, Babaoglu B, Tezel GG, Gedikoglu G (2017) Programmed death ligand 1 (PD-L1) expression in malignant mesenchymal tumors. Turk Patoloji Derg. 1(1):192–197. https://doi.org/10.5146/tjpath.2017.01395
Vargas AC, Maclean FM, Sioson L, Tran D, Bonar F, Mahar A et al (2020) Prevalence of PD-L1 expression in matched recurrent and/or metastatic sarcoma samples and in a range of selected sarcomas subtypes. PLoS One. 15(4):e0222551. https://doi.org/10.1371/journal.pone.0222551
Chow LQM, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M et al (2016) Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 34(32):3838–3845. https://doi.org/10.1200/JCO.2016.68.1478
Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S et al (2016) Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. The Lancet Oncology. 17(6):717–726. https://doi.org/10.1016/s1470-2045(16)00175-3
Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G et al (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. The Lancet. 394(10212):1915–1928. https://doi.org/10.1016/s0140-6736(19)32591-7
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M et al (2018) Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 4(5):e180013. https://doi.org/10.1001/jamaoncol.2018.0013
Shitara K, Özgüroğlu M, Bang Y-J, Di Bartolomeo M, Mandalà M, Ryu M-H et al (2018) Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. The Lancet. 392(10142):123–133. https://doi.org/10.1016/s0140-6736(18)31257-1
Sanchez-Canteli M, Granda-Diaz R, Del Rio-Ibisate N, Allonca E, Lopez-Alvarez F, Agorreta J et al (2020) PD-L1 expression correlates with tumor-infiltrating lymphocytes and better prognosis in patients with HPV-negative head and neck squamous cell carcinomas. Cancer Immunol Immunother. 69(10):2089–2100. https://doi.org/10.1007/s00262-020-02604-w
Sanchez-Canteli M, Juesas L, Redin E, Calvo A, Lopez F, Astudillo A et al (2021) Immune cell infiltrates and neutrophil-to-lymphocyte ratio in relation to response to chemotherapy and prognosis in laryngeal and hypopharyngeal squamous cell carcinomas. Cancers (Basel). 13(9). https://doi.org/10.3390/cancers13092079
Boxberg M, Steiger K, Lenze U, Rechl H, von Eisenhart-Rothe R, Wortler K et al (2018) PD-L1 and PD-1 and characterization of tumor-infiltrating lymphocytes in high grade sarcomas of soft tissue - prognostic implications and rationale for immunotherapy. Oncoimmunology. 7(3):e1389366. https://doi.org/10.1080/2162402X.2017.1389366
Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J et al (2017) Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. The Lancet Oncology. 18(11):1493–1501. https://doi.org/10.1016/s1470-2045(17)30624-1
Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P et al (2005) Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 352(18):1851–1860. https://doi.org/10.1056/NEJMoa043146
Wang Y, Shi C, Eisenberg R, Vnencak-Jones CL (2017) Differences in microsatellite instability profiles between endometrioid and colorectal cancers: a potential cause for false-negative results? J Mol Diagn. 19(1):57–64. https://doi.org/10.1016/j.jmoldx.2016.07.008
Latham A, Srinivasan P, Kemel Y, Shia J, Bandlamudi C, Mandelker D et al (2019) Microsatellite instability is associated with the presence of Lynch syndrome pan-cancer. J Clin Oncol. 37(4):286–295. https://doi.org/10.1200/JCO.18.00283
Cote GM, He J, Choy E (2018) Next-generation sequencing for patients with sarcoma: a single center experience. Oncologist. 23(2):234–242. https://doi.org/10.1634/theoncologist.2017-0290
Campanella NC, Penna V, Ribeiro G, Abrahao-Machado LF, Scapulatempo-Neto C, Reis RM (2015) Absence of microsatellite instability in soft tissue sarcomas. Pathobiology. 82(1):36–42. https://doi.org/10.1159/000369906
Doyle LA, Nowak JA, Nathenson MJ, Thornton K, Wagner AJ, Johnson JM et al (2019) Characteristics of mismatch repair deficiency in sarcomas. Mod Pathol. 32(7):977–987. https://doi.org/10.1038/s41379-019-0202-3
(2012) Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 487(7407):330–337. https://doi.org/10.1038/nature11252
(2014) Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 513(7517):202–209. https://doi.org/10.1038/nature13480
Funding
This study was supported by Grants-in-Aid from the Japan Society for the Promotion of Science (JSPS) KAKENHI (JSPS: Grant Number # 19H03789, # 19K22694 to Y.S.; # 20K07415, # 17K08730 to T.S.).
Author information
Authors and Affiliations
Contributions
Conceptualization: Yoshiyuki Suehara, Takuo Hayashi, Tsuyoshi Saito
Methodology: Yoshiyuki Suehara, Takuo Hayashi, Keita Sasa, Tsuyoshi Saito
Formal analysis and investigation: Atsushi Yamashita, Yoshiyuki Suehara, Takuo Hayashi, Keita Sasa, Tsuyoshi Saito
Writing—original draft preparation: Atsushi Yamashita
Writing—review and editing: Tsuyoshi Saito
Funding acquisition: Yoshiyuki Suehara, Tsuyoshi Saito
Resources: Yoshiyuki Suehara, Tatsuya Takagi, Daisuke Kubota, Keita Sasa, Nobuhiko Hasegawa, Muneaki Ishijima
Supervision: Takashi Yao, Tsuyoshi Saito
Corresponding author
Ethics declarations
Ethics approval
This study was reviewed and approved by Juntendo University School of Medicine Institutional Review Board (#2021-079).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yamashita, A., Suehara, Y., Hayashi, T. et al. Molecular and clinicopathological analysis revealed an immuno-checkpoint inhibitor as a potential therapeutic target in a subset of high-grade myxofibrosarcoma. Virchows Arch 481, 1–17 (2022). https://doi.org/10.1007/s00428-022-03358-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-022-03358-9