Abstract
Sarcomatoid carcinomas recently came into the spotlight through genetic profiling studies and also as a distinct model of epithelial–mesenchymal transition. The literature on sarcomatoid carcinomas of gallbladder is limited. In this study, 656 gallbladder carcinomas (GBC) were reviewed. Eleven (1.7%) with a sarcomatoid component were identified and analyzed in comparison with ordinary GBC (O-GBC). Patients included 9 females and 2 males (F/M = 4.5 vs. 3.9) with a mean age-at-diagnosis of 71 (vs. 64). The median tumor size was 4.6 cm (vs. 2.5; P = 0.01). Nine patients (84%) presented with advanced stage (pT3/4) tumor (vs. 48%). An adenocarcinoma component constituting 1–75% of the tumor was present in nine, and eight had surface dysplasia/CIS; either in situ or invasive carcinoma was present in all cases. An intracholecystic papillary-tubular neoplasm was identified in one. Seven showed pleomorphic–sarcomatoid pattern, and four showed subtle/bland elongated spindle cells. Three had an angiosarcomatoid pattern. Two had heterologous elements. One showed few osteoclast-like giant cells, only adjacent to osteoid. Immunohistochemically, vimentin, was positive in six of six; P53 expression was > 60% in six of six, keratins in six of seven, and p63 in two of six. Actin, desmin, and S100 were negative. The median Ki67 index was 40%. In the follow-up, one died peri-operatively, eight died of disease within 3 to 8 months (vs. 26 months median survival for O-GBC), and two were alive at 9 and 15 months. The behavior overall was worse than ordinary adenocarcinomas in general but was not different when grade and stage were matched. In summary, sarcomatoid component is identified in < 2% of GBC. Unlike sarcomatoid carcinomas in the remainder of pancreatobiliary tract, these are seldom of the “osteoclastic” type and patients present with large/advanced stage tumors. Limited data suggests that these tumors are aggressive with rapid mortality unlike pancreatic osteoclastic ones which often have indolent behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most gallbladder neoplasms are adenocarcinomas that are collectively regarded under the heading of “pancreatobiliary type” adenocarcinomas [1, 2]. Several rare types of carcinoma, including mucinous, squamous, adenosquamous, and poorly cohesive cell, have been identified and better characterized in the gallbladder in the past few years [3,4,5].
Sarcomatoid carcinomas have attracted significant attention in the past few years because, in most organs, they stand out as a distinct group in genetic profiling studies. They are also increasingly being considered as potential for specific targeted therapies because of the differential molecular pathways altered in these tumors [6,7,8]. Moreover, they present the epitome of epithelial–mesenchymal transition seen in cancers which has become a major focus in carcinogenesis research [9,10,11,12].
However, the information on GBCs with sarcomatoid differentiation is sparse; it is mentioned only in a few case reports in the literature [13,14,15,16,17,18,19,20,21]. There is virtually no systematic analysis regarding the frequency and clinicopathologic characteristics of GBC with sarcomatoid differentiation.
The aim of this study was to determine the frequency of sarcomatoid component in invasive GBCs, as well as to define the characteristics these tumors. We also aimed to determine their prognosis and compare them with ordinary gallbladder carcinomas (O-GBC).
Materials and methods
Cases and definition
Six hundred fifty-six cholecystectomies diagnosed with invasive GBC at Universidad de La Frontera, Temuco, Chile; Wayne State University, Detroit, MI, USA; and Emory University, Atlanta, GA, USA, and the authors’ consultation files were analyzed. All the pathology material available were reviewed for sarcomatoid changes. The presence of at least one of the sarcomatoid patterns (pleomorphic–sarcomatoid, spindle cell, and/or angiosarcomatoid) was used as the inclusion criterion. An average of 12 tumor slides per case were reviewed.
Histopathological analysis
After the sarcomatoid study group was verified based on the definition above, all tumor slides were investigated for morphological features including pleomorphic–sarcomatoid, spindle cell and/or angiosarcomatoid patterns, presence of heterologous elements, and osteoclast-like giant cells as well as high-grade dysplasia/carcinoma in situ (also called biliary intraepithelial neoplasm of grade 3) in the adenocarcinoma component.
Immunohistochemical analyses for vimentin, epithelial markers (AE1/AE3, CK8/18, CK5/6, and epithelial membrane antigen, in order to investigate immunophenotypic evidence of epithelial differentiation), MUC1 (a marker frequently expressed in GBC), p63 (detected in “metaplastic” and sarcomatoid carcinomas in other organs such as the breast), actin (expressed in some sarcomas but also in sarcomatoid carcinomas), desmin (for evidence of muscular differentiation), S100 protein, Ki67, P53, MLH1, and MSH2 were performed in the six cases with tissue available for analysis.
Evaluation of clinical parameters
Information on the patients’ gender, age, clinical presentation, and follow-up were obtained through pathology databases, patients’ charts or by contacting the patients’ primary physicians or the Surveillance Epidemiology End Results (SEER) database in the USA and the Civil Registry and death certificate database in the IT Department of the Head Office of the South Araucania Health Service in Chile. Patients who died within the first 30 days of the postoperative period were excluded from the survival analysis.
Statistical analysis
Clinical outcomes were recorded and analyzed by Kaplan–Meier curves, and the differences in survival between selected groups were compared by log-rank analysis.
The association between mortality and types of carcinomas was investigated.
Results
Incidence and clinical features
Among 656 cases of GBC, sarcomatoid component was identified in 11 (1.6%). The patients were nine females and two males (F/M = 4.5 vs. 3.9 in O-GBC). The mean age was 71 years vs. 64 in O-GBC.
Pathologic findings
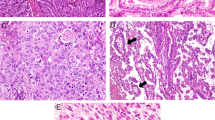
Macroscopically, the tumors had an exophytic polypoid growth in five cases (Fig. 1a), which manifested as compact growth of malignant spindle cells in the histologic sections (Fig. 1b). In one case, this polypoid growth was due to an intracholecystic papillary tubular neoplasm (i.e., preinvasive) component (Fig. 1c). In six cases, the tumor had the ulcero-vegetating or plaque-like growth of ordinary GBCs.
Macroscopic photograph of a sarcomatoid carcinoma (a). Growth pattern was illustrated in this low power photomicrograph (b). In one case, the exophytic component of the tumor was an intracholecystic papillary tubulary neoplasm composed of compact papillary proliferation of preinvasive dysplastic lesion (c)
An adenocarcinoma component, constituting 1–75% of the tumor, accompanied the sarcomatoid component in nine cases. The remaining two cases were purely sarcomatoid. Both of these pure sarcomatoid carcinomas had patterns similar to the other cases, showed mucosal oriented tumors, and did not show any findings of any conventional sarcoma patterns. Both of these also had high-grade dysplasia/CIS, and one of the two showed keratin expression.
The mean and median tumor sizes were 5.3 and 4.6 cm, compared to 2.9 and 2.5 cm in O-GBC (P = 0.01).
Seven cases exhibited a pleomorphic–sarcomatoid pattern (Fig. 2a–c), and four showed a prominent spindle cell pattern composed of subtle, bland, stromal appearing cells. One of these four was mistaken as reactive myofibroblasts on frozen section. Three cases had angiosarcomatoid pattern with prominent clefts and/or intermixed vascularity (Fig. 2d).
Different sarcomatoid patterns are identified in sarcomatoid carcinoma of the gallbladder, including pleomorphic spindle cells (a) and pleomorphic epithelioid cells (b). Infiltration of the sarcomatoid carcinoma cells into the vessel walls could be observed (c). Three cases had angiosarcomatoid pattern (d), also accompanied by inflammatory cells (e). In some areas, the tumor displayed whorl formation as well as collagenization pattern and plumb relatively uniform cells that are often seen in fibroblastic and fibrohistiocytic neoplasms (f). Eight patients had surface dysplasia/CIS in the surface epithelium; in this photomicrograph, high-grade dysplasia is depicted on the surface, with sarcomatoid carcinoma cells in the stroma (g). Osteoid formation could be seen (h), and in one case, this was accompanied by osteoclastic giant cells (i). Median Ki67 index was 40% (j). P53 expression was detected in all 6/6 of cases tested (k). CK18 was commonly retained in the sarcomatoid component (l)
Surface dysplasia/carcinoma in situ of non mass-forming type (Fig. 2g) was detected in eight cases, and an intracholecystic papillary tubular neoplasm was identified in one (Fig. 1c).
Two cases had heterologous elements (Fig. 2h, i) (one bone/cartilage, one cartilage only). Only one showed only few osteoclast-like giant cells which were adjacent to osteoid.
Nine patients (84%) presented with advanced stage (pT3/4) tumors in comparison with 48% of O-GBC. Lymph node metastasis was present in two of five.
Immunohistochemical findings
Vimentin was positive in six of six, and epithelial markers were expressed in the sarcomatoid components of most (AE1/AE3 six of seven, CK8/18, CK5/6, and EMA in five of six) along with at least focal expression of MUC1 (five of six). p63 was expressed in two of six. Actin, desmin, and S100 protein were negative in all (zero of six). A block was available from the angiosarcomatoid areas in only one of three cases that displayed this pattern, and those areas were negative for CD34.
Median Ki67 index was 40% (range 10–50%). Ki67 was high also in the more bland-appearing areas of the tumors. P53 expression was seen in four of six cases (> 60%). MLH1 and MSH2 were retained.
Clinical course
Follow-up data were available in all of 11 cases. One patient died peri-operatively. Of the remaining ten, eight died of disease within 3 to 8 months, and two were alive at 9th and 15th months. The median survival of GBC with sarcomatoid component was shorter than that of O-GBC (8 vs. 26 months, respectively) when all adenocarcinomas were considered together but was comparable to that of poorly differentiated subset of the cases (p = 0.54). Moreover, when stage-matched comparison was performed, the difference was not statically significant (8 vs. 11 months, p = 0.34).
See Table 1 for the summary of the results.
Discussion
We reviewed 656 gallbladder adenocarcinomas for a sarcomatoid component, which, to our knowledge, represents the largest cohort to date analyzed for this purpose. Eleven cases (1.6%) had a sarcomatoid component. This largest case series allows better appreciation of the characteristics of sarcomatoid gallbladder carcinomas which are hitherto recorded in only a few case reports and reviews [13,14,15,16,17,18,19,20,21].
The mean age of patients was half-a-decade older than that of the O-GBC (71 vs. 64). The female predominance is even more pronounced than O-GBC in this cohort (4.5 vs. 3.9). The tumors are distinctively larger (mean, 5.3 vs. 2.9, P = 0.01), as previously noted in the literature [16, 19].
As in sarcomatoid carcinomas of other organs, the tumor often forms an exophytic mass with a relatively demarcated base but behaves aggressively despite that. The sarcomatoid component typically occurs in the company of ordinary adenocarcinoma, which was present in 9 out of 11 cases in this study, and an in situ carcinoma (high-grade dysplasia) was present in all cases. Heterologous elements can also be encountered, as in other types of sarcomas.
Several manifestations of the sarcomatoid pattern, including a pleomorphic–sarcomatoid pattern (n = 7), a prominent spindle cell pattern composed of subtle, bland, stromal-appearing cells (n = 4), and an angiosarcomatoid pattern with prominent clefts and/or intermixed vascularity (n = 3) can be detected in the sarcomatoid component. In fact, in isolation, selected slides from these cases can be difficult to distinguish, on the subtle end of the spectrum, from granulation tissue (and in fact, one case was misdiagnosed as “reactive” on frozen section), and in the more aggressive end of the spectrum, they can closely mimic true sarcomas. The most important clue to the “metaplastic” nature of the process in these cases is the presence of an adenocarcinoma or in situ carcinoma component which was detected in all cases in this study. Immunohistochemical profile elucidated in this study which is in accordance with what has been reported in the literature [20, 22,23,24,25,26,27,28] may also aid in this distinction by illustrating the presence of keratins (positive in all six cases in this study), although it should be kept in mind that keratin expression can be seen in a variety of sarcomas as well. Expression of vimentin is also noted uniformly in this limited study, but this is not much of diagnostic help considering all sarcomas, most melanomas and lymphomas, and even poorly differentiated carcinomas exhibit this marker.
Gallbladder carcinomas with a sarcomatoid component appear to be more aggressive tumors than ordinary GBC; however, this is probably due to the fact that they are poorly differentiated and that they also typically present in locally advanced stage, since their survival does not seem to be different than other GBCs when grade and stage-matched. Not surprisingly, their high-grade and aggressive nature is also supported by high p53 expression (60%) and a high proliferation index (median, 40%).
Sarcomatoid change in carcinomas has recently generated remarkable interest among cancer researchers, especially with the introduction of molecular-genetic analysis of various cancers. For example, in the breast, one of the distinct clusters identified by genomic profiling studies has proven to be those cases with sarcomatoid change [29,30,31]. Same is also true for the pancreas, where one of the four categories identified appears to represent sarcomatoid carcinomas [32]. Sarcomatoid carcinomas are believed to be the ultimate examples of “epithelial–mesenchymal transition” and stem cell regression, a phenomenon considered to be one of the most promising recent discoveries of cancer biology research [6, 11, 12]. This also underlines the importance of recognizing and analyzing sarcomatoid cancers as a distinct category to allow more focused treatment with therapies targeting the pathways altered in this group of tumors. Interestingly, of significance, both diagnostically and biologically, the sarcomatoid carcinomas of gallbladder typically retain strong keratin positivity in contrast with some of the sarcomatoid carcinomas of other sites where they not uncommonly lose their keratin [33,34,35,36]. In this regard, sarcomatoid carcinomas of gallbladder appear to be more similar to those rare sarcomatoid carcinomas that occur in the tubular gastrointestinal tract where keratin expression is retained in most cases [37,38,39,40]. In contrast, in the sarcomatoid carcinomas of the pancreas, the other main organ of pancreatobiliary tract, the keratin expression is often lost [33]. It should also be noted here that the sarcomatoid carcinomas in the gallbladder are seldom, if ever, of the “osteoclastic” type, unlike the ones in the remainder of pancreatobiliary tract [33]. This is of importance because ordinary adenocarcinomas of gallbladder and pancreas are highly similar both in their morphology, immunophenotype, and clinical behavior to an extent that they are often regarded under one heading of “pancreatobiliary type” adenocarcinomas. However, when it comes to sarcomatoid change, in the pancreas, it is almost invariably of the osteoclastic type, which often shows a surprisingly protracted clinical course [33, 41, 42]. In contrast, in the gallbladder, as this study disclosed, sarcomatoid transformation follows a different path, not at all attracting osteoclasts in any significant numbers. The fact that the sarcomatoid carcinomas in gallbladder (which are devoid of osteoclasts) behave much more aggressively than those in the pancreas (which are rich in osteoclasts) begs the question of whether osteoclasts may be acting as an inhibitory factor to the growth of the sarcomatoid carcinomas in the pancreas, or whether the differential milieu (and tumor microenvironment) in these two different compartments of the pancreatobiliary tract induce different pathways. Recently, the role of immune cells including macrophagic cells have found much attention both in the prognosis of cancers, as well as their response to therapy. The findings in this study illustrating that the osteoclast-devoid sarcomatoid carcinomas in gallbladder are much more aggressive than their osteoclast-rich counterparts in the pancreatobiliary tract is an intriguing issue that warrants further analysis.
Another interesting mechanistic question that remains unanswered is the relative impact of the ordinary adenocarcinoma component in sarcomatoid carcinomas. For the osteoclastic examples in the pancreas, in one study, the relative amount of tubular (adenocarcinoma) component was used in a grading scheme with modest prognostic value [33], and in another study, the presence of an epithelial component was associated with significantly worse outcome [42]. For sarcomatoid GBCs, it appears that the outcome is uniformly poor regardless. Since only one case had rare osteoclasts (which was next to heterologous bone), it is difficult to ascertain the significance of osteoclasts in sarcomatoid GBCs.
In summary, gallbladder carcinomas with a sarcomatoid component are extremely rare (1.6% of all GBC), discovered in slightly older patients and women, and often advanced (pT3/4) cancers at the time of diagnosis. They are virtually never of the osteoclastic type. Their prognosis appears to be even worse than ordinary gallbladder adenocarcinomas, although the difference in median survival did not reach statistical significance due to limited numbers. Further studies are needed to establish the clinical impact and to elucidate the underlying molecular mechanisms of this tumor type.
References
Albores-Saavedra J, Klöppel G, Adsay N et al (2010) Carcinoma of the gallbladder and extrahepatic bile ducts, 4th edn. WHO Press, Geneva
Adsay NVKD (2009) Benign and malignant tumors of the gallbladder and extrahepatic biliary tract. In: Odze R, Goldblum J (eds) Surgical pathology of the GI tract, liver, biliary tract, and pancreas. Elsevier, Philadelphia, pp 845–875
Dursun N, Escalona OT, Roa JC, Basturk O, Bagci P, Cakir A, Cheng J, Sarmiento J, Losada H, Kong SY, Ducato L, Goodman M, Adsay NV (2012) Mucinous carcinomas of the gallbladder: clinicopathologic analysis of 15 cases identified in 606 carcinomas. Arch Pathol Lab Med 136:1347–1358. https://doi.org/10.5858/arpa.2011-0447-OA
Roa JC, Tapia O, Cakir A, Basturk O, Dursun N, Akdemir D, Saka B, Losada H, Bagci P, Adsay NV (2011) Squamous cell and adenosquamous carcinomas of the gallbladder: clinicopathological analysis of 34 cases identified in 606 carcinomas. Mod Pathol 24:1069–1078. https://doi.org/10.1038/modpathol.2011.68
Tuncel D, Roa JC, Araya JC, Bellolio E, Villaseca M, Tapia O, Jang KT, Quigley B, Saka B, Basturk O, Sarmiento J, Losada HF, Patel S, Reid MD, Memis B, Adsay V (2017) Poorly cohesive cell (diffuse-infiltrative/signet ring cell) carcinomas of the gallbladder: clinicopathological analysis of 24 cases identified in 628 gallbladder carcinomas. Hum Pathol 60:24–31. https://doi.org/10.1016/j.humpath.2016.09.008
Pang A, Carbini M, Moreira AL, Maki RG (2018) Carcinosarcomas and related cancers: tumors caught in the act of epithelial-mesenchymal transition. J Clin Oncol 36:210–216. https://doi.org/10.1200/JCO.2017.74.9523
Jones S, Stransky N, McCord CL et al (2014) Genomic analyses of gynaecologic carcinosarcomas reveal frequent mutations in chromatin remodelling genes. Nat Commun 5:5006. https://doi.org/10.1038/ncomms6006
Pécuchet N, Vieira T, Rabbe N, Antoine M, Blons H, Cadranel J, Laurent-Puig P, Wislez M (2017) Molecular classification of pulmonary sarcomatoid carcinomas suggests new therapeutic opportunities. Ann Oncol 28:1597–1604. https://doi.org/10.1093/annonc/mdx162
Cherniack AD, Shen H, Walter V, Stewart C, Murray BA, Bowlby R, Hu X, Ling S, Soslow RA, Broaddus RR, Zuna RE, Robertson G, Laird PW, Kucherlapati R, Mills GB, Weinstein JN, Zhang J, Akbani R, Levine DA, Akbani R, Ally A, Auman JT, Balasundaram M, Balu S, Baylin SB, Beroukhim R, Bodenheimer T, Bogomolniy F, Boice L, Bootwalla MS, Bowen J, Bowlby R, Broaddus R, Brooks D, Carlsen R, Cherniack AD, Cho J, Chuah E, Chudamani S, Cibulskis K, Cline M, Dao F, David M, Demchok JA, Dhalla N, Dowdy S, Felau I, Ferguson ML, Frazer S, Frick J, Gabriel S, Gastier-Foster JM, Gehlenborg N, Gerken M, Getz G, Gupta M, Haussler D, Hayes DN, Heiman DI, Hess J, Hoadley KA, Hoffmann R, Holt RA, Hoyle AP, Hu X, Huang M, Hutter CM, Jefferys SR, Jones SJM, Jones CD, Kanchi RS, Kandoth C, Kasaian K, Kerr S, Kim J, Lai PH, Laird PW, Lander E, Lawrence MS, Lee D, Leraas KM, Leshchiner I, Levine DA, Lichtenberg TM, Lin P, Ling S, Liu J, Liu W, Liu Y, Lolla L, Lu Y, Ma Y, Maglinte DT, Marra MA, Mayo M, Meng S, Meyerson M, Mieczkowski PA, Mills GB, Moore RA, Mose LE, Mungall AJ, Mungall K, Murray BA, Naresh R, Noble MS, Olvera N, Parker JS, Perou CM, Perou AH, Pihl T, Radenbaugh AJ, Ramirez NC, Rathmell WK, Roach J, Robertson AG, Sadeghi S, Saksena G, Salvesen HB, Schein JE, Schumacher SE, Shen H, Sheth M, Shi Y, Shih J, Simons JV, Sipahimalani P, Skelly T, Sofia HJ, Soloway MG, Soslow RA, Sougnez C, Stewart C, Sun C, Tam A, Tan D, Tarnuzzer R, Thiessen N, Thorne LB, Tse K, Tseng J, van den Berg DJ, Veluvolu U, Verhaak RGW, Voet D, von Bismarck A, Walter V, Wan Y, Wang Z, Wang C, Weinstein JN, Weisenberger DJ, Wilkerson MD, Winterhoff B, Wise L, Wong T, Wu Y, Yang L, Zenklusen JC, Zhang J(J), Zhang H, Zhang W, Zhu JC, Zmuda E, Zuna RE (2017) Integrated molecular characterization of uterine carcinosarcoma. Cancer Cell 31:411–423. https://doi.org/10.1016/j.ccell.2017.02.010
Papathomas TG, Duregon E, Korpershoek E, Restuccia DF, van Marion R, Cappellesso R, Sturm N, Rossi G, Coli A, Zucchini N, Stoop H, Oosterhuis W, Ventura L, Volante M, Fassina A, Dinjens WNM, Papotti M, de Krijger RR (2016) Sarcomatoid adrenocortical carcinoma: a comprehensive pathological, immunohistochemical, and targeted next-generation sequencing analysis. Hum Pathol 58:113–122. https://doi.org/10.1016/j.humpath.2016.08.006
Sung CO, Choi H, Lee K-W, Kim S-H (2013) Sarcomatoid carcinoma represents a complete phenotype with various pathways of epithelial mesenchymal transition. J Clin Pathol 66:601–606. https://doi.org/10.1136/jclinpath-2012-201271
Cates JMM, Dupont WD, Barnes JW, Edmunds HS, Fasig JH, Olson SJ, Black CC (2008) Markers of epithelial-mesenchymal transition and epithelial differentiation in sarcomatoid carcinoma: utility in the differential diagnosis with sarcoma. Appl Immunohistochem Mol Morphol 16:251–262. https://doi.org/10.1097/PAI.0b013e318156e9b4
Doval DC, Azam S, Mehta A, Pruthi A, Batra U, Choudhury KD, Kumar K (2014) A report of Sarcomatoid carcinoma of the gallbladder treated with palliative deocetaxel and gemcitabine chemotherapy. J Gastrointest Cancer 45:270–274. https://doi.org/10.1007/s12029-014-9654-3
Dong A, Dong H, Jing W, Zuo C (2016) FDG PET/CT in sarcomatoid carcinoma of the gallbladder with chondroid differentiation. Clin Nucl Med 41:638–640. https://doi.org/10.1097/RLU.0000000000001160
Kishino T, Mori T, Kawai S, Mori H, Nishikawa K, Hirano K, Matsushima S, Ohtsuka K, Ohnishi H, Watanabe T (2014) Carcinosarcoma, an atypical subset of gallbladder malignancies. J Med Ultrason 41:487–490. https://doi.org/10.1007/s10396-014-0534-z
Park HJ, Jang K-T, Choi DW, Heo JS, Choi SH (2014) Clinicopathologic analysis of undifferentiated carcinoma of the gallbladder. J Hepatobiliary Pancreat Sci 21:58–63. https://doi.org/10.1002/jhbp.3
Kataria K, Yadav R, Seenu V (2012) Sarcomatoid carcinoma of the gall bladder. J Surg Case Rep 2012:5–5. https://doi.org/10.1093/jscr/2012.2.5
Manouras A, Genetzakis M, Lagoudianakis EE, Markogiannakis H, Papadima A, Agrogiannis G, Gakiopoulou H, Kekis P, Filis K, Patsouris E (2009) Undifferentiated giant cell type carcinoma of the gallbladder with sarcomatoid dedifferentiation: a case report and review of the literature. J Med Case Rep 3:6496. https://doi.org/10.1186/1752-1947-3-6496
Zhang L, Chen Z, Fukuma M et al (2008) Prognostic significance of race and tumor size in carcinosarcoma of gallbladder: a meta-analysis of 68 cases. Int J Clin Exp Pathol 1:75–83
Kim M-J, Yu E, Ro JY (2003) Sarcomatoid carcinoma of the gallbladder with a rhabdoid tumor component. Arch Pathol Lab Med 127:e406–e408. https://doi.org/10.1043/1543-2165(2003)127<e406:SCOTGW>2.0.CO;2
Ajiki T, Nakamura T, Fujino Y, Suzuki Y, Takeyama Y, Ku Y, Kuroda Y, Ohbayashi C (2002) Carcinosarcoma of the gallbladder with chondroid differentiation. J Gastroenterol 37:966–971. https://doi.org/10.1007/s005350200162
Badmos KB, Salah Seada L, Fahad Al Rashid F, Oreiby HA (2013) Undifferentiated spindle-cell carcinoma of the gallbladder: a report of a case, an immunohistochemistry profile, and a review of the literature. Case Rep Pathol 2013:1–3. https://doi.org/10.1155/2013/267194
Gao S, Huang L, Dai S et al (2015) Carcinosarcoma of the gallbladder: a case report and review of the literature. Int J Clin Exp Pathol 8:7464–7469
Kim HH, Hur YH, Jeong EH, Park EK, Koh YS, Kim JC, Kim HJ, Kim JW, Cho CK (2012) Carcinosarcoma of the gallbladder: report of two cases. Surg Today 42:670–675. https://doi.org/10.1007/s00595-012-0160-6
Kubota K, Kakuta Y, Kawamura S, Abe Y, Inamori M, Kawamura H, Kirikoshi H, Kobayashi N, Saito S, Nakajima A (2006) Undifferentiated spindle-cell carcinoma of the gallbladder: an immunohistochemical study. J Hepato-Biliary-Pancreat Surg 13:468–471. https://doi.org/10.1007/s00534-006-1100-x
Pu JJ, Wu W (2011) Gallbladder carcinosarcoma. BMJ Case Rep 2011:1–7. https://doi.org/10.1136/bcr.05.2010.3009
Takahashi Y, Fukushima JI, Fukusato T, Shiga J (2004) Sarcomatoid carcinoma with components of small cell carcinoma and undifferentiated carcinoma of the gallbladder. Pathol Int 54:866–871. https://doi.org/10.1111/j.1440-1827.2004.01771.x
Wada Y, Takami Y, Tateishi M, Ryu T, Mikagi K, Momosaki S, Saitsu H (2014) Carcinosarcoma of the gallbladder: report of a case. Clin J Gastroenterol 7:455–459. https://doi.org/10.1007/s12328-014-0522-2
Cimino-Mathews A, Verma S, Figueroa-Magalhaes MC, Jeter SC, Zhang Z, Argani P, Stearns V, Connolly RM (2016) A clinicopathologic analysis of 45 patients with metaplastic breast carcinoma. Am J Clin Pathol 145:365–372. https://doi.org/10.1093/ajcp/aqv097
Lester TR, Hunt KK, Nayeemuddin KM, Bassett RL, Gonzalez-Angulo AM, Feig BW, Huo L, Rourke LL, Davis WG, Valero V, Gilcrease MZ (2012) Metaplastic sarcomatoid carcinoma of the breast appears more aggressive than other triple receptor-negative breast cancers. Breast Cancer Res Treat 131:41–48. https://doi.org/10.1007/s10549-011-1393-6
Carter MR, Hornick JL, Lester S, Fletcher CDM (2006) Spindle cell (sarcomatoid) carcinoma of the breast. Am J Surg Pathol 30:300–309. https://doi.org/10.1097/01.pas.0000184809.27735.a1
Waddell N, Pajic M, Patch A-M et al (2015) Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518:495–501. https://doi.org/10.1038/nature14169
Muraki T, Reid MD, Basturk O, Jang KT, Bedolla G, Bagci P, Mittal P, Memis B, Katabi N, Bandyopadhyay S, Sarmiento JM, Krasinskas A, Klimstra DS, Adsay V (2016) Undifferentiated carcinoma with osteoclastic giant cells of the pancreas. Am J Surg Pathol 40:1203–1216. https://doi.org/10.1097/PAS.0000000000000689
Bansal M, Chen J, Wang X (2018) Focal anomalous expression of cytokeratin and p63 in malignant phyllodes tumor: a comparison with spindle cell metaplastic carcinoma. Appl Immunohistochem Mol Morphol 26:198–201. https://doi.org/10.1097/PAI.0000000000000453
Galera P, Khan A, Kandil D (2016) Diagnosis of metaplastic breast carcinoma: keratin OSCAR versus other cytokeratins. Appl Immunohistochem Mol Morphol 24:622–626. https://doi.org/10.1097/PAI.0000000000000230
de Brito PA, Silverberg SG, Orenstein JM (1993) Carcinosarcoma (malignant mixed müllerian (mesodermal) tumor) of the female genital tract: immunohistochemical and ultrastructural analysis of 28 cases. Hum Pathol 24:132–142
Reid-Nicholson M, Idrees M, Perino G, Hytiroglou P (2004) Sarcomatoid carcinoma of the small intestine: a case report and review of the literature. Arch Pathol Lab Med 128:918–921. https://doi.org/10.1043/1543-2165(2004)128<918:SCOTSI>2.0.CO;2
Gengler C, Tanière P, Béatrix O, Scoazec J-Y (2002) Sarcomatoid carcinoma of small intestine: diagnostic problems raised by an unusual tumor. Ann Pathol 22:43–47
Fukuda T, Kamishima T, Ohnishi Y, Suzuki T (1996) Sarcomatoid carcinoma of the small intestine: histologic, immunohistochemical and ultrastructural features of three cases and its differential diagnosis. Pathol Int 46:682–688
Chen P, Chen Q, Ni X et al (2012) Clinicopathological features and prognostic analysis of esophageal sarcomatoid carcinoma. Zhonghua Zhong Liu Za Zhi 34:287–290. https://doi.org/10.3760/cma.j.issn.0253-3766.2012.04.011
Reid MD, Muraki T, HooKim K, Memis B, Graham RP, Allende D, Shi J, Schaeffer DF, Singh R, Basturk O, Adsay V (2017) Cytologic features and clinical implications of undifferentiated carcinoma with osteoclastic giant cells of the pancreas: an analysis of 15 cases. Cancer Cytopathol 125:563–575. https://doi.org/10.1002/cncy.21859
Luchini C, Pea A, Lionheart G, Mafficini A, Nottegar A, Veronese N, Chianchiano P, Brosens LAA, Noë M, Offerhaus GJA, Yonescu R, Ning Y, Malleo G, Riva G, Piccoli P, Cataldo I, Capelli P, Zamboni G, Scarpa A, Wood LD (2017) Pancreatic undifferentiated carcinoma with osteoclast-like giant cells is genetically similar to, but clinically distinct from, conventional ductal adenocarcinoma. J Pathol 243:148–154. https://doi.org/10.1002/path.4941
Acknowledgments
This study has been presented in the USCAP Annual Meeting in 2015, March 21–27 at Boston, MA, USA.
Author information
Authors and Affiliations
Contributions
• Orhun Cig Taskin: Data curation, investigation, validation, writing original draft, and writing review and editing.
• Gizem Akkas: Data curation, investigation, validation, writing original draft, and writing review and editing.
• Bahar Memis: Data curation, investigation, writing original draft, and writing review and editing.
• Ipek Erbarut Seven: Data curation, investigation, and writing review and editing.
• Olca Basturk: Project administration, supervision, writing original draft, and writing review and editing.
• Kee-Taek Jang: Supervision and writing review and editing.
• Juan C Roa: Conceptualization, data curation, investigation, methodology, project administration, and writing review and editing.
• Juan Carlos Araya: Data curation, investigation, and writing review and editing.
• Enrique Bellolio: Data curation, investigation, and writing review and editing.
• Hector Losada: Data curation, investigation, and writing review and editing.
• Juan Sarmiento: Data curation, investigation, and writing review and editing.
• Serdar Balci: Data curation, formal analysis, methodology, and validation.
• Burcin Pehlivanoglu: Data curation, investigation, validation, and writing review and editing.
• Michelle Reid: Writing original draft and writing review and editing.
• Jill Koshiol: Data curation, formal analysis, methodology, and validation.
• Volkan Adsay: Conceptualization, data curation, investigation, methodology, project administration, resources, supervision, writing original draft, and writing review and editing.
Corresponding author
Ethics declarations
The Helsinki principles were respected in the making of this study. Moreover, patients’ confidentiality data was kept via their guidelines. The studies were accomplished in agreement with the institutional review board requirements.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Orhun Cig Taskin and Gizem Akkas are co-contributing first authors.
Electronic supplementary material
ESM 1
(DOCX 58 kb)
Rights and permissions
About this article
Cite this article
Taskin, O.C., Akkas, G., Memis, B. et al. Sarcomatoid carcinomas of the gallbladder: clinicopathologic characteristics. Virchows Arch 475, 59–66 (2019). https://doi.org/10.1007/s00428-019-02583-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-019-02583-z