Abstract
The aim of our retrospective study was to analyze patterns of subtype specific metastatic spread and to identify the time course of distant metastases. A consecutive series of 490 patients with breast cancer who underwent surgery and postoperative treatment at Semmelweis University, Hungary, and diagnosed between the years 2000 and 2007 was identified from the archives of the 2nd Department of Pathology, Hungary. Molecular subtypes were defined based on the 2011 St. Gallen recommendations. Statistical analysis was performed with SPSS Statistics for Windows, Version 22.0. Distant metastasis free survival (DMFS) was defined as the time elapsed between the first pathological diagnosis of the tumor and the first distant metastasis detection. Distant metastases were detected in 124 patients. Mean time to develop metastasis was 29 months (range 0–127 months). The longest DMFS was observed in the Luminal A (LUMA) subtype (mean 39 months) whereas the shortest was seen in the HER2-positive (HER2+) subtype (mean 21 months; p = 0.012). We confirmed that HER2+ tumors carry a higher risk for distant metastases (42.1%). LUMA-associated metastases were found to be solitary in 59% of cases, whereas HER2+ tumors showed multiple metastases in 79.2% of cases. LUMA tumors showed a preference for bone-only metastasis as compared with HER2+ and triple negative breast cancer (TNBC) cases, which exhibited a higher rate of brain metastasis. The most frequent second metastatic sites of hormone receptor (HR) positive tumors were the lung and liver, whereas the brain was the most affected organ in HR-negative (HR−) cases. Tumor subtypes differ in DMFS and in pattern of distant metastases. HER2+ tumors featured the most aggressive clinical course. Further identification of subtype-specific factors influencing prognosis might have an impact on clinical care and decision-making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is growing evidence indicating that patterns of metastasis and clinical outcome are different between breast carcinomas of different subtypes [1–5]. Patterns of metastasis as well as prognosis vary according to hormone receptor (HR) and HER2 status [6, 7]. The favored metastatic site of HR positive tumors is bone tissue, whereas this site is less frequent in case of triple-negative tumors [1, 8].
Prognosis of a metastatic breast carcinoma depends on several factors. A well-known favorable factor is a positive HR status. The first sites of distant metastases also have documented impact on survival with the best outcome for bone metastases [2]. The era of next-generation sequencing provided a new insight into the biology of different breast carcinoma subtypes and their metastases, but translation of these relatively recent data into clinical practice has not yet been accomplished [9]. Considering that approximately 30% of patients diagnosed with breast carcinoma develop distant metastatic disease, the number of studies providing reliable data on associations between conventional clinicopathological markers and metastatic pattern is relatively limited [1, 3, 5, 10]. Most studies focus on metastatic tumors, without in-depth analysis of cases without metastases. Recognizing these mentioned important gaps, the Breast International Group (BIG) launched the ambitious AURORA program in 2014 to elucidate molecular aberrations in metastatic breast cancer and analyze intra-tumor heterogeneity through genome analysis of both primary tumors and corresponding metastatic lesions [11]. Such retrospective and prospective studies will hopefully shed light on poorly understood areas of metastatic breast cancer. Against this background, we conducted a retrospective study with the aim to refine subtype-specific risk prediction models, to improve our knowledge regarding the pattern of metastasis of different breast carcinoma subtypes (identification of subtype-specific factors influencing prognosis could have an impact on clinical care and decision-making), and to identify the time course of distant metastases.
Material and methods
Breast cancer tissues
Our cohort included 490 patients with breast cancer diagnosed between the years 2000 and 2007 that underwent surgery and postoperative treatment and with known distant metastasis status and overall survival (OS) data. Clinicopathological data were obtained from the archives of Semmelweis University, 2nd Department of Pathology and of the 1st Department of Surgery, Hungary, and from the archives of Semmelweis University Dept. of Clinical Oncology. Follow-up data collection ended in June 2015. The relevant pathological data and clinical information were extracted and analyzed with permission from the Semmelweis University Regional and Institutional Committee of Science and Research Ethics (ETT-TUKEB, #185/2007), according to strict privacy standards. The recorded pathological and clinical parameters are presented in Table 1.
Immunohistochemical and FISH analysis
Molecular subtypes were defined based on the 2011 St. Gallen recommendations [12, 13] as Luminal A (LUMA), Luminal B/HER2 negative (LUMB1), Luminal B/HER2+ (LUMB2), HER2 positive (HER2+), and triple-negative breast cancer (TNBC). LUMB1 category definition was slightly modified and it included ER positive carcinomas with a Ki67 LI of >15% [14].
ER (estrogen receptor), PgR (progesterone receptor), ERBB2/HER2 (erb-b2 receptor tyrosine kinase 2) status, and Ki67 (marker of proliferation Ki67) index were evaluated by immunohistochemistry (IHC). All immunohistochemical stains were carried out at the Semmelweis University, 2nd Dept. of Pathology, Hungary, using anti-ER (clone 6F11)1:200, anti-PgR (clone 312)1:200, and anti-HER2 1:150 (clone CB11) purchased from Novocastra Laboratories Ltd. (Newcastle upon Tyne, UK), and anti-Ki67 1:100 (clone MIB1) purchased from DAKO Gmbh (Hamburg, Germany) as antibodies. Cutoff values for ER and PgR status were 1% of tumor cells with nuclear staining and defined as positive or negative. Ki67 labeling index (LI) was assessed by visually estimating the percentage of positive tumor cell nuclei [14, 15].
HER2 status was determined either as protein overexpression (3+, complete strong membrane staining) or HER2 gene amplification detected by fluorescence in situ hybridization (FISH), applying the valid ASCO/CAP guideline for the given period [16, 17].
Assessing the data related to metastatic events
DMFS was defined as the time elapsed between the initial pathology diagnosis of the tumor and diagnosis of the first distant metastasis (metastases to the bone, lung, liver, brain, pleura, and other organs excluding axillary lymph node metastases). OS was determined as the time elapsed between the first pathology diagnosis and disease specific death, with the censoring of patients who were lost to follow-up.
The following parameters were noted in all cases with metastases:
-
a.
Sites of distant metastasis [the bone, the lung, the liver, the brain, the pleura, other organs (peritoneum, adrenal, cutaneous, and one supraclavicular lymph node observed after 9 years of primary treatment)]
-
b.
Date of diagnosis of first metastasis to any organ
-
c.
Order of appearance of different organ metastases for each patient (first, second, or third in time)
All patients received systemic therapy (endocrine, targeted, cytotoxic chemotherapy or combination therapy). Of 141 patients, 85 (60.3%) with HER2+ disease received HER2-targeted therapy using trastuzumab. Of these cases, 22 (25.9%) received HER2-targeted therapy for metastatic disease and 63/85 (74.1%) as treatment of primary tumor.
Statistical analysis
Age distribution, pT and pN status, tumor grade, St. Gallen subtypes, site and pattern of metastasis, and time to metastasis were analyzed. Descriptive analysis of categorical prognostic variables was performed using SPSS software (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, USA). Differences in the distribution of characteristics between the parameters of patients with distant metastases (DM) and patients without metastases during the follow-up period were evaluated using two-sided Fisher’s exact test. Two-sided exact Mann–Whitney–Wilcoxon test was used to define age and Ki67 labeling index (LI) distribution in metastatic vs. non-metastatic cases. ROC curve estimation was also applied to define the cutoff value for Ki67 LI, but no reliable cutoff was found. Survival was investigated using Kaplan–Meier estimators. Log-rank test and multivariate Cox regression analysis (model included age, grade, pTN status, immunophenotype, and Ki67 LI) were carried out to analyze the association between different pathological parameters and DMFS or OS. Results were considered to be statistically significant if p ≤ 0.05.
Results
pTNM status and tumor grade: correlation with metastatic pattern
The majority of patients were diagnosed with a pT1 or pT2 tumor (Table 1). Distant metastatic disease evolved more frequently in higher pT groups within the follow-up period (p < 0.001, Table 2). However, we found no significant differences between pT status and different subtypes or metastasis distribution (Tables 2 and 3). Similar to pT status, pN status was also strongly associated with distant metastatic potential (p < 0.001). Metastatic disease developed more frequently in higher pN groups within the follow-up period. A higher percentage of pN3 tumors was observed in HER2+ and TNBC subtypes (p = 0.008, Table 2). As 51% of the cases with known pN status were pN0, including 22 patients (11%) with DM, we further examined the metastasis pattern in those cases. Brain metastases occurred exclusively in HR negative lesions and more often in pN0 tumors (p = 0.005). Grade 3 tumors were remarkably more frequently HR negative (HR−) (p < 0.001). Approximately one third (28%) of grade 3 tumors formed DM, whereas only 16% of grade 1 tumors developed DM during the follow-up period, but this difference was not statistically significant (p = 0.125, Table 2).
Correlation of PgR expression with distant metastases
Applying a cutoff value of 20% to distinguish between high and low PgR expression, we found that higher PgR expression was associated with better survival. Cases presenting solitary metastasis (N = 39) were more often PgR positive than those with multiple metastases (61 vs. 36%, p = 0.029). Within cases with a LUMB1 subtype, DM occurred significantly more often in PgR low than those in PgR high cases (50% (18/36) vs. 23% (8/35), p = 0.026).
Ki67 LI and distant metastasis formation
We found no significant differences in Ki67 LI between the distant metastatic and non-metastatic groups (27.5 ± 2.5 vs. 24.0 ± 1.4%, p = 0.260). However, metastases occurring in the first or second year (early metastases) had a significantly higher Ki67 LI (p = 0.025 and p = 0.041, respectively) than late metastases. Patients diagnosed with DM in the first or second year after primary diagnosis presented a mean Ki67 LI of 34.0 ± 3.8 and 33.0 ± 3.4%, whereas this was 24.0 ± 3.0 and 22.8 ± 3.2%, respectively, in cases without metastases in the first or second year.
Subtype-specific distant metastases
The bone was the most common metastatic site followed by the lung and liver (Table 1). HER2+ cases developed most often DM as 42% (24/57) of the examined HER2+ patients developed metastatic disease in the follow-up period. The lowest number of metastatic tumors was detected in the LUMA group (23% (39/170); Fig. 1). Associations between breast carcinoma subtype and the presence of any DM were statistically significant (p = 0.012). Significant differences were also revealed between subtypes regarding occurrence of solitary or multiple DM (p = 0.019; Fig. 1). Multiple metastases were most often found in HER2+ patients (79%, 19/24). Solitary metastasis formation was characteristic for LUMA (59%, 23/39), which preferentially developed bone-only metastases. Patients with a LUMA subtype developed bone metastases only in 36% (14/39); this was 19% (5/26) for patients with a LUMB1 and 30% (6/20) for patients with a LUMB2 subtype. For patients with a HER2+ or TNBC subtype, this was 4% (1/24) and 7% (1/15), respectively. Patients with the latter two subtypes showed a high incidence of organ metastasis other than the bone (46% (11/24) and 60% (9/15), respectively). Of patients with a HR+ tumor, 72% (61/81) had bone metastases while this was 49% (19/39) for patients with a HR− breast carcinoma (p = 0.016). Brain metastases alone or together with other organ metastases is most likely to occur in HER2+ (42%) and TNBC patients (26%) (p = 0.005; Fig. 1). Finally, the frequency of lung and liver metastases was not different between breast carcinoma subtypes (p = 0.328 and p = 0.803, respectively). Regarding the second metastatic site, our study revealed that the most affected organ in HR+ tumors is the lung or liver, while this was the brain in HR− cases (Fig. 1). Upon multivariate analysis including age, grade, pTN status, Ki67 LI, and the different breast carcinoma subtypes, only pN2 status was significantly associated with DMFS (Table 4).
Distant metastasis-free patients and overall survival of patients
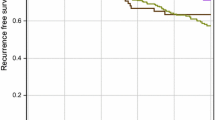
The average time to diagnosis of metastases was 29 months (range from 0 to 127 months). The longest DMFS was observed in patients with the LUMA subtype (avg. 39.0 ± 5.6 months) whereas the shortest was seen in those with the HER2+ subtype (avg. 21.0 ± 5.0 months). Second shortest DMFS was found in patients with the LUMB1 subtype (avg. 22.7 ± 4.4 months), followed by those with the TNBC (avg. 25.7 ± 5.5 months) and LUMB2 subtypes (avg. 31.8 ± 7.8 months) (Fig. 2a). We further examined whether Herceptin (trastuzumab) treatment has an effect on DM formation and DMFS, but no difference was found between treated and non-treated patients, neither in the HER2+ (76 vs. 24%, N = 58) nor in the LUMB2 subtype (54 vs. 46%, N = 74).
Overall, mean disease-specific OS was 53.4 ± 37.3 months (range 0–155). Univariate analysis of subtype-specific OS showed significant differences (p = 0.002, Fig. 2b). Patients with the LUMA subtype had the longest OS (67.2 months), followed by those with LUMB1 (53.0 months), TNBC (45.9 months), LUMB2 (41.2 months), and HER2+ (37.3 months). By multivariate analysis, HER2+ and TNBC subtypes were significant predictors of shorter OS (p = 0.036, p = 0.026, and p = 0.010, respectively) (Table 4). We found no significant difference in OS between trastuzumab-treated and non-treated patients, neither for HER2+ nor for LUMB2 subtype (p = 0.380 and p = 0.233, respectively).
Discussion
Breast-cancer-related mortality is usually due to metastatic dissemination of the primary lesion. The answer to the question when and how metastatic spread occurs is complex. Different progression models have been suggested and a variety of factors have been linked to an elevated risk of developing distant metastases [3, 4, 18–23].
We first examined associations of clinicopathological factors, including pT, pN, grade, PgR, and Ki67 LI, with the likelihood of distant relapse. In accordance with the literature, we found significant associations between high pT/pN status and the occurrence of distant metastases [24, 25] but not between tumor grade and distant metastases. Abha Soni et al. found by univariate analysis that high-histologic grade significantly correlated with central nervous system metastases, although this did not hold up in multivariate analysis [26]. Kim HJ et al. found that patients with a high-grade carcinoma more often had a shorter metastasis-free interval [24]. Aleskandarany et al. analyzed a panel of biomarkers and found a high Ki67 LI along with expression of HER2, p53, N-cadherin, P-cadherin, PIK3CA, and TOMM34 to be significantly associated with earlier development of DM [27]. We did not find a significant association between the frequency of distant metastases and Ki67 LI. However, Ki67 LI was significantly higher in patients with early metastases (in the first and second year) than in those with late metastases, which is in agreement with earlier published data [2].
Possible associations of PgR expression with the likelihood of the development of metastases are less well-documented [12]. We used a cutoff value of 20% to distinguish high and low PgR expression and found that high PgR expression is associated with better survival. Sato et al. found breast cancer-related events to be significantly lower in PgR-high Luminal B-like breast cancer, which raises the possibility that PgR status has some influence on prognosis for patients with a Luminal HER2− breast cancer [28].
We then studied in a retrospective series of 490 breast cancer patients whether breast cancer subtypes, notably different immunophenotypes, show different patterns of distant relapse. It is important to note that immunophenotype may be different between primary tumor and corresponding distant metastasis, which may affect treatment decision making [29, 30]. We found a significant correlation between breast carcinoma subtype and the occurrence of DM. Patients with a HER2+ carcinoma most often developed distant metastases and the lowest frequency was found in patients with the LUMA subtype. As previously published, we found the bone to be the most frequently involved metastatic site followed by the lung and liver [1, 31].
Patterns of metastatic disease are considerably different between ER-positive and ER-negative tumors, and mechanisms involved in organotropism are being documented recently, using advanced molecular methods. Specific molecular signatures have been identified for bone and brain metastases of breast cancer [32–34]. HR-positive tumors tend to relapse later and preferentially disseminate to the bone in comparison with HR− tumors [1, 3, 26, 31, 35, 36]. We found bone metastases in approximately two-thirds of patients with an HR+ carcinoma and bone metastases only to be highly characteristic for patients with an HR+ tumor, more in particular of LUMA subtypes. Soni et al. suggested that breast cancer can be divided into those with bone-only metastases and those with the bone and another organ, with differences in biological behavior [26]. Smid et al. found that the pattern of genes of which the expression is up-regulated in HER2+ breast cancer with bone metastases is entirely different from that found in the luminal subtype [37]. Savci-Heijink et al. found that a novel 15-gene signature identified 82.4% of tumors with bone metastases, 85.2% of tumors with metastasis to the bone as the first site, and 100% of tumors with bone metastases only [38].
We did not observe statistically significant differences between breast carcinoma subtype and DM to lung and liver. Soni et al. reported that none of the subtypes metastasized only to the lung, while Smid et al. found lung metastases more often than expected in the basal subtype, but less often than expected in the luminal A subtype [26, 37]. Soni et al. also found liver metastases more frequently in the HER2+ than those in the LUMA and TNBC subtypes [26]. A similar trend was also observed in another study but this did not reach statistical significance [8].
We found brain metastases as only an event or in combination with visceral and/or bone metastases most often in HER2+ (42%) and TNBC cases (26%). Most published data agree that the LUMA subtype is less frequently associated with brain metastases than the HER2+ and TNBC subtypes [26]. Other studies have found that breast cancer patients with TNBC, HER2+, basal-like, claudin-low tumors are at higher risks of brain metastases [39–42]. Rostami et al. postulated that the incidence of brain metastasis from breast cancer increases, partly due to advances in imaging technologies, which allow earlier and better detection and the introduction of novel therapies resulting in longer survival from primary breast cancer [43].
The site of the first metastasis of different breast carcinoma subtypes is well-documented but where and when the second or third metastases occur is less clear. Our data indicate that for HR+ tumors the most frequent second metastatic sites are the lung and liver, whereas for HR− cases, the brain is the most frequently affected second organ. In breast cancer patients with HR+ subtype, with the bone as most commonly affected organ, future research might identify which characteristics are associated with elevated risks for secondary visceral metastases.
In spite of significant improvements in treatment and follow-up period of breast cancer patients in recent years, DMFS and OS are still low and remarkable differences exist between different countries. Kim HJ et al. studies a cohort of breast carcinoma cases in the same period as our cases and found a mean metastasis-free interval of 31 months (range 6–200 months). A short metastasis-free interval (<2 years) was found in 40% of patients while a minority (20%) developed metastases 5 years after initial diagnosis [24]. We found the longest OS in the LUMA subtype followed by LUMB1, TNBC, LUMB2, and HER2+ subtypes. Published data indicate that patients with a TNBC have the worst prognosis for all measured outcomes, whereas OS was better for patients with an HER2+ tumor than for those with a luminal B tumor [1]. Contrary to the data of Gerratana et al., we found the shortest DMFS and OS in patients with the HER2+ subtype. This might be due to the relatively high proportion of patients in our series who did not receive trastuzumab therapy or received trastuzumab as first line therapy. Before the introduction of targeted anti-HER2 therapies, the prognosis of HER2+ tumors was worse than that of HER2− tumors. Dawood [44] and Olson [45] maintain that HER2+ metastatic breast cancer is an incurable disease, the majority of patients dying within 5 years.
Our study has several limitations. Firstly, our results should be interpreted cautiously since our patient cohort had relatively few cases in the TNBC and HER2+ groups. In addition, less than half of the patients with HER2+ tumors received targeted anti-HER2 therapy. Secondly, cases were retrospectively selected from prospectively maintained databases. Thirdly, patients might have had undiagnosed, asymptomatic metastasis, and furthermore, approximately 8% of selected patients were lost for follow-up. Both may have biased the results. Finally, even though screening by mammography was frequently conducted in this cohort, we did not consider this information as this was rarely mentioned in patient files.
Our data lead us to conclude that tumor subtypes are associated with DMFS and with patterns of distant metastases. The risk for DM is high in HER2+ tumors and low in the LUMA subtype. LUMA subtype is associated with solitary DM whereas HER2+ tumors metastasize frequently to multiple sites. A LUMA subtype characteristically develops bone-only metastases in contrast to HER2+ and TNBC subtypes, which more often develop brain metastases. HR+ tumors most frequently developed lung and liver metastases as secondary sites while this was the brain in HR− tumors. When properly performed, subtypes are predictive for preferential location of DM as well as for prognosis. Finally, early metastases are associated with high Ki67 LI whereas late metastases have a low Ki67 LI.
References
Gerratana L et al (2015) Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis 32(2):125–133
Joensuu K et al (2013) ER, PR, HER2, Ki-67 and CK5 in early and late relapsing breast cancer-reduced CK5 expression in metastases. Breast Cancer (Auckl) 7:23–34
Kast K et al (2015) Impact of breast cancer subtypes and patterns of metastasis on outcome. Breast Cancer Res Treat 150(3):621–629
Kennecke H et al (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28(20):3271–3277
Savci-Heijink CD et al (2015) Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res Treat 150(3):547–557
Aurilio G et al (2013) Discordant hormone receptor and human epidermal growth factor receptor 2 status in bone metastases compared to primary breast cancer. Acta Oncol 52(8):1649–1656
Cancello G et al (2013) Progesterone receptor loss identifies Luminal B breast cancer subgroups at higher risk of relapse. Ann Oncol 24(3):661–668
Sihto H et al (2011) Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast Cancer Res 13(5):R87
Blighe K et al (2014) Whole genome sequence analysis suggests intratumoral heterogeneity in dissemination of breast cancer to lymph nodes. PLoS One 9(12):e115346
Sant M et al (2004) Breast carcinoma survival in Europe and the United States. Cancer 100(4):715–722
Zardavas D et al (2014) The AURORA initiative for metastatic breast cancer. Br J Cancer 111(10):1881–1887
Goldhirsch A et al (2011) Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22(8):1736–1747
Goldhirsch A (2013) Personalized adjuvant therapies: lessons from the past: the opening address by the St. Gallen 2013 award recipient. Breast 22(Suppl 2):S3–S7
Cserni G et al (2014) Distribution pattern of the Ki67 labelling index in breast cancer and its implications for choosing cut-off values. Breast 23(3):259–263
Selmeci TTA, Róna Á, Molnár BÁ, Kenessey I, Székely B, Szász AM, Kulka J (2014) Prognostic role of progesteron receptor expression in HER2 negative Luminal B breast cancers. Nőgyógyászati Onkológia 19:47–49
Wolff AC et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145
Wolff AC et al (2014) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med 138(2):241–256
Ostrovnaya I et al (2010) A metastasis or a second independent cancer? Evaluating the clonal origin of tumors using array copy number data. Stat Med 29(15):1608–1621
Bueno-de-Mesquita JM et al (2009) Validation of 70-gene prognosis signature in node-negative breast cancer. Breast Cancer Res Treat 117(3):483–495
van de Vijver MJ et al (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347(25):1999–2009
Gyorffy B et al (2015) Dynamic classification using case-specific training cohorts outperforms static gene expression signatures in breast cancer. Int J Cancer 136(9):2091–2098
Sotiriou C et al (2006) Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 98(4):262–272
Brockton NT et al (2015) The Breast Cancer to Bone (B2B) Metastases Research Program: a multi-disciplinary investigation of bone metastases from breast cancer. BMC Cancer 15:512
Kim HJ et al (2015) Metastasis-free interval is closely related to tumor characteristics and has prognostic value in breast cancer patients with distant relapse. J Breast Cancer 18(4):371–377
Rugo HS (2008) The importance of distant metastases in hormone-sensitive breast cancer. Breast 17(Suppl 1):S3–S8
Soni A et al (2015) Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol 143(4):471–478
Aleskandarany MA et al (2015) Markers of progression in early-stage invasive breast cancer: a predictive immunohistochemical panel algorithm for distant recurrence risk stratification. Breast Cancer Res Treat 151(2):325–333
Sato K et al (2016) Prognostic significance of the progesterone receptor status in Ki67-high and -low Luminal B-like HER2-negative breast cancers. Breast Cancer 23(2):310–317
Desmedt C, Yates L, Kulka J (2016) Catalog of genetic progression of human cancers: breast cancer. Cancer Metastasis Rev 35(1):49–62
Kulka J et al (2016) Comparison of predictive immunohistochemical marker expression of primary breast cancer and paired distant metastasis using surgical material: a practice-based study. J Histochem Cytochem 64(4):256–267
Kimbung S, Loman N, Hedenfalk I (2015) Clinical and molecular complexity of breast cancer metastases. Semin Cancer Biol 35:85–95
Bahrami T et al (2016) The molecular signature of breast cancer metastasis to bone. Anti-Cancer Drugs 27(9):824–831
Lee JY et al (2016) Gene expression profiling of breast cancer brain metastasis. Sci Rep 6:28623
Weidle UH et al (2016) Dissection of the process of brain metastasis reveals targets and mechanisms for molecular-based intervention. Cancer Genomics Proteomics 13(4):245–258
Kimbung S et al (2016) Transcriptional profiling of breast cancer metastases identifies liver metastasis-selective genes associated with adverse outcome in Luminal A primary breast cancer. Clin Cancer Res 22(1):146–157
Kimbung S et al (2015) Contrasting breast cancer molecular subtypes across serial tumor progression stages: biological and prognostic implications. Oncotarget 6(32):33306–33318
Smid M et al (2008) Subtypes of breast cancer show preferential site of relapse. Cancer Res 68(9):3108–3114
Savci-Heijink CD et al (2016) A novel gene expression signature for bone metastasis in breast carcinomas. Breast Cancer Res Treat 156(2):249–259
Harrell JC et al (2012) Genomic analysis identifies unique signatures predictive of brain, lung, and liver relapse. Breast Cancer Res Treat 132(2):523–535
Lin NU et al (2012) Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 118(22):5463–5472
Pestalozzi BC et al (2006) Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol 17(6):935–944
Saunus JM et al (2015) Integrated genomic and transcriptomic analysis of human brain metastases identifies alterations of potential clinical significance. J Pathol 237(3):363–378
Rostami R et al (2016) Brain metastasis in breast cancer: a comprehensive literature review. J Neuro-Oncol 127(3):407–414
Olson EM et al (2013) Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast 22(4):525–531
Dawood S et al (2010) Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol 28(1):92–98
Acknowledgments
The authors thank Rigóné Káli Elvira for the careful reading of the manuscript and the valuable comments.
Authors’ contributions
AMT, LV, BAM, IAM, JK, and AMSz conceived the study. BAM, IAM, JM, KF, JT, CSz, BSz, and PD carefully selected the patients’ clinical/oncological and pathological data and provided the patients’ follow-up data. LV, AB, and SVK performed data/statistical analysis. All authors were involved in data interpretation and in writing the paper and had final approval of the submitted and published manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by MKOT 2014-2016, SE-OTKA 2014-2015, KTIA_NAP_13-2014-0021, and OTKA grant no. K116151. AMSz was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences.
Additional information
István Artúr Molnár and Béla Ákos Molnár equally contributed.
Rights and permissions
About this article
Cite this article
Molnár, I.A., Molnár, B.Á., Vízkeleti, L. et al. Breast carcinoma subtypes show different patterns of metastatic behavior. Virchows Arch 470, 275–283 (2017). https://doi.org/10.1007/s00428-017-2065-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-017-2065-7