Abstract
Purpose
To evaluate the pattern of recurrence of breast cancer according to its biological subtype in a large cohort of patients treated with therapy representative of current practice.
Patients and methods
Patients treated between 2000 and 2016 with known biological subtype were eligible. Data were prospectively collected. Primary endpoint was the subtype-dependent pattern and time of recurrence. Loco-regional and distant site and time of recurrence were assessed.
Results
Median follow-up time was 80.8 months. For 12,053 (82.5%) of 14,595 patients with primary non-metastatic invasive breast cancer a subtype classification was possible. The luminal A subtype had the highest 10-year survival followed by luminal B and luminal/HER2. The worst survival demonstrated HER2 enriched and TNBC. HER2 and TNBC had the highest rate of recurrence in the first 5 years, whereas the rate of recurrence for luminal A and luminal B tumors was initially low, but remained continuously even after 10 years of follow-up. Luminal A tumors demonstrated the lowest rate of distant metastases predominantly in bone. So did luminal B tumors. HER2 enriched subtype was characterized with increased rate of loco-regional recurrence and distant metastases in bone, liver and brain. Luminal/HER2 had pattern of relapse similar to HER2 enriched tumors, with exception of loco-regional relapse and brain metastases. TNBC had higher rate of lung, bone and brain metastases as well as loco-regional relapse.
Conclusion
Breast cancer subtypes are associated with different time and pattern of recurrence and it should be considered during treatment decision.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is a very heterogenous disease. 20% of the patients will experience relapse. In the 1990s and in the beginning of the new century the hormone receptor (HR) status, overexpression of human epidermal growth factor receptor-2 (HER2), histologic grade and nodal status were used as prognostic factors (Colleoni et al. 2016; Kennecke et al. 2010; Saphner et al. 1996; Voduc et al. 2010).
Based on the knowledge, the breast cancer is very heterogeneous (Perou et al. 2000) a new definition of intrinsic subtypes has been suggested (Goldhirsch et al. 2011). Four major intrinsic breast cancer subtypes has been proposed luminal A (HR positive, HER2 negative, low proliferative activity), luminal B (HR positive, higher proliferative activity or HER2 positive), HER2 enriched (HR negative and HER2 overexpressing tumors), and triple-negative breast cancer (TNBC) (HR and HER2 negative) (Perou et al. 2000). Gene expression profiling suggested 14 to be the best cut-off point of KI67 to distinguish luminal A from luminal B breast cancer subtype (Cheang et al. 2009). A few resent studies recognized that different breast cancer subtypes are associated with different biological behavior. However, the data obtained by these trials are still not representative of the whole population of breast cancer patients. This is based on their monocentric character (Cossetti et al. 2015), the therapies used were not representative of modern treatment strategies (Kennecke et al. 2010; Metzger-Filho et al. 2013; Voduc et al. 2010), and the subtypes were defined using the receptor status only (Gong et al. 2017; Kennecke et al. 2010; Voduc et al. 2010). The data should be also interpret by caution because of the small number of patients in some studies (Gabos et al. 2010; Ribelles et al. 2013; Voduc et al. 2010) as well as the selection bias based on the inclusion criteria used (Metzger-Filho et al. 2013).
In this large population-based analysis with long follow-up, we aimed to investigate the current patterns of relapse of breast cancer patients treated with current treatment strategies. The prospectively assessed data were retrospectively analyzed.
Patients and methods
Patients
We investigated cases of female breast cancer included in the prospectively maintained regional cancer registry of Saxony-Anhalt, a federal state of Germany. The tumor registry contains prospectively collected information on diagnosis, age, tumor stage, receptor status, tumor grading, lymph node status, date of diagnosis, date of disease recurrence, site of recurrence, date of death and the treatment regimens used (Eggemann et al. 2015). We analyzed women with breast tumors diagnosed between 200 and 2016 in 10 hospitals in Saxony-Anhalt (Eggemann et al. 2015). Patients with primary metastatic breast cancer (n = 994), sarcoma (n = 24) and phylloides tumor (n = 15) were excluded (Fig. 1). The remaining 14,595 patients were assessed for eligibility.
Pathologic assessment
Breast cancer subtypes were classified according to immunohistochemical (IHC) profile (Cheang et al. 2009; Goldhirsch et al. 2011) as follows: luminal A (HR positive, HER2 negative, Ki67 < 14%), luminal B (HR positive, HER2 negative, Ki67 ≥ 14%), luminal/HER2 (HR positive and HER2 positive), HER2 enriched (HR negative and HER2 positive), and TNBC (HR and HER2 negative). HR [estrogen (ER) or progesterone receptor (PR)] status was considered positive if staining was ≥ 1%. HER2 expression was determined by IHC. IHC of 3+ represented HER2 positive expression, whereas IHC of 0 or 1+ was considered as HER2 negative expression. Tumors with IHC of 2+ were additionally investigated by fluorescence in situ hybridization and an amplification ratio of ≥ 2 was considered as HER2 overexpression (Ignatov et al. 2016). In 2542 cases, the subtype was unknown and they were excluded (Fig. 1). A total of 12,053 cases were included.
Outcomes
The primary outcome was to compare the patterns of relapse of different breast cancer subtypes. Time and site of recurrence were evaluated. The recurrence was classified as loco-regional and distant. Loco-regional relapse included the recurrence in ipsilateral breast, chest wall or regional lymph nodes. The relapse of different subtypes was compared with the pattern of relapse of luminal A tumors. Distant recurrences consist of: distant lymph node metastases (beyond the ipsilateral axillary, infra- and/or supraclavicular, internal mammary area), bone (including bone marrow), brain, liver, lung (including pleura and lymphangitic carcinomatosis), other (including peritoneal, other organs not elsewhere classified and skin not in the breast and chest wall). Secondary outcomes were disease-free (DFS) and overall survival (OS). DFS was defined as the time from the date of diagnosis and the date of loco-regional and/or distant relapse. OS was defined as the time from the date of diagnosis and the time of death from any cause. The follow-up ended with the patient’s death, last available information or the last follow-up at 19.07.2017. The manuscript was prepared in accordance with the STROBE statement criteria (von Elm et al. 2007).
Statistical analysis
The statistical calculations were performed using SPSS Version 24.0 (SPSS, Chicago, IL, USA). Patient and tumor characteristics were compared between breast cancer subtypes using the Chi-square test or Fisher’s exact test. Association between site of relapse and tumor subtype was assessed by Chi-square test. Competing risk methodology was used to estimate the cumulative incidence curves of recurrence. DFS and OS probability distribution was studied using the Kaplan–Meier method. The equality of survival curves was tested using the log rank test. Cox proportional hazards regression analysis was used to identify significant prognostic factors and luminal A tumors were used as reference variable. The statistical analyses were two sided and p values of < 0.05 were considered statistically significant.
Results
Study population and treatment characteristics
A total of 14,595 patients with primary non-metastatic invasive breast cancer at a median follow-up of 80.8 months were analyzed and for 12,053 (82.5%) of them subtype classification was possible. Among them 35.1% were luminal A, 34.2% were luminal B, 14.3% were luminal/HER2, 5.1% were HER2 enriched, and 11.4% were TNBC (Fig. 1). Clinical, pathologic and treatment characteristic and their distribution between tumor subtypes are listed in Table 1. Women with luminal A and B tumors were significantly younger than women in the other groups compared (p < 0.0001). The difference in the tumor size was also significant (p < 0.0001). Most tumors at presentation were larger than 2 cm in luminal/HER2, HER2 enriched and TNBC groups. In opposite luminal A and B tumors were often < 2 cm. The lowest rate of lymph node involvement was observed among patients with luminal A and TNBC (p < 0.0001). The most often histologic type was ductal invasive in all tumor subtypes, whereas lobular cancer was observed at high degree in luminal A and B group. The majority grade 1 tumors were identified in luminal A group. Grade 3 tumors were significantly more often observed in luminal B, luminal/HER2, HER2 enriched and TNBC tumors (p < 0.0001).
Breast conserving surgery was more common in luminal A patients, followed by TNBC, luminal B, luminal/HER2, and HER2 enriched. Patients with luminal A breast cancer were most likely to receive radiotherapy in comparison with the other tumor subtypes (p 0.002). Chemotherapy was given to 76% of patients with HER2 enriched, 75% with TNBC, 68.5% with luminal/HER2, 44.7% with luminal B and only 26.6% with luminal A subtype (p < 0.0001). The most used chemotherapy regimen was anthracycline–tamoxifen-based and was given predominantly to patients with luminal/HER2, HER2 enriched and TNBC tumors (p < 0.0001). Patients with HER2 enriched and TN-breast cancers did not receive any hormone therapy. The most administered hormone therapy was tamoxifen followed by aromatase inhibitors and combination of both. One-third of luminal A patients and one-fourth of luminal B and luminal/HER2 patients received tamoxifen, whereas aromatase inhibitors were administered in 29.8% of luminal A patients, in 30.9% of luminal B patients, and in 31.1% of luminal/HER2 patients (p < 0.0001). The longest follow-up period was in the groups of patients with luminal A tumors, followed by luminal/HER2, luminal B, HER2 enriched and TNBC (p < 0.0001).
Patient survival and outcome
Among different breast cancer subtypes the DFS and OS rates were significantly different (p < 0.0001). The luminal A subtype had the highest 10-year DFS (95.5%) compared with luminal B (90.0%), luminal/HER2 (84.9%), HER2 enriched (79.8%), and TNBC (80.9%) (p < 0.0001) (Fig. 2a). The estimated 10-year OS was 89.2% in luminal A subtype and was significantly longer than the 10-year OS for luminal B (79%), luminal/HER2 (79.6%), HER2 enriched (74.4%), and TNBC (75.8%) (p < 0.0001) (Fig. 2b).
The cumulative incidence of relapse and death was also investigated. Significant difference in occurrence of relapse and death between breast cancer subtypes was observed (Fig. 3). During the follow-up time, loco-regional and distant recurrence of breast cancer was observed in 4.8% among luminal A, 10.7% among luminal B, 16.1% among luminal/HER2, 20.2% among HER2 enriched and 19.2% among TNBC patients (p < 0.0001). The incidence of tumor relapse of HER2 enriched and TNBC was high during the first 3 years of follow-up (Fig. 3a) and the rate of relapse reached 70.2% for TNBC and 62.0% for HER2 enriched subtype at fifth year of follow-up. Luminal B and luminal/HER2 subtypes experienced also most of the relapses during the first years of follow-up; however, the patients had a smooth peak of relapse in the first 5 years with continued relapse rate after fifth year after diagnosis. Luminal A subtype tumors relapsed very slowly in the initial years with a 5-year relapse rate of 25.5% (Fig. 3a). Interestingly, the recurrence rate of luminal A patients was triplicated between year 5 and 10.
The incidence of death during the follow-up also differed between breast cancer subtypes (Fig. 3b). Again, HER2 enriched and TNBC demonstrated abrupt increase death incidence with a peak in the first 4 years, which remained relatively stable after year 5. Luminal A, luminal B and luminal/HER2 tumors demonstrated quite similar rate of death incidence with approximately two-third of death occurring in the first 5 years after diagnosis, followed by relatively smooth incidence curve in the late follow-up phase (Fig. 3b).
Pattern of recurrence according to breast cancer subtype
In patients with recurrent disease, the site of recurrence was analyzed. HER2 enriched and TNBC were the subtypes associated with increased rate of local and regional recurrence, 7.5 and 3.4% for HER2 enriched and 7.6 and 3.3% for TNBC, respectively (Table 2). Luminal A tumors were recurrent in 1.5 and 0.7% local and regional, respectively, and this difference was statistically significant. Luminal/HER2 was associated in 4.9 and in 2.2% of the cases with a local and regional relapse, respectively, whereas these rates for luminal B subtype were 2.9 and 1.5%, respectively.
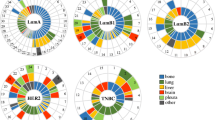
Distant metastases were also differently distributed among different subtypes. Bone metastases were the most common metastases in our study cohort (4.7%), followed by lung metastases (3.1%), liver metastases (2.7%), brain metastases (1.9%), distal lymph node metastases (1.5%), and other distant metastases (1.0%). More than 1 metastasis were significantly often observed in luminal/HER2, HER2 enriched and TNBC tumors in comparison with luminal A and luminal B tumors (Table 2). Luminal A tumors had significantly lower rate of distant metastases compared with other subtypes and the most common site of metastases was to bone (2.1%). Notably, bone metastases occurred in all subtypes of breast cancer in relatively high level. Among Luminal B breast cancer the most common metastasis was to bone (5.7%) followed by lung (2.9%) and liver (2.8%) metastases. Both HER2 subtypes revealed quite similar pattern of recurrence. Bone and liver metastases were more common. Moreover, the rate of distant nodal and other metastases was equal. The rate of lung metastases was higher in luminal/HER2 tumors (4.7 vs. 2.5%), whereas brain metastases were more common in women with HER2 enriched tumors (5.9 vs. 3.4%). TNBC was characterized by high level of lung metastases (7.0%), followed by bone metastases (5.5%), brain metastases (4.9%), distant lymph node metastases (3.8%), liver metastases (3.4%), and metastases with other localization (1.3%).
To identify the prognostic significance of different tumor subtypes cox proportional hazards regression analysis was used and luminal A tumors were used as reference variable. TNBC were associated with the highest risk of loco-regional recurrence [odds ratio (OR) = 5.6; 95% CI 4.2–7.4], nodal metastases (OR = 11.0; 95% CI 6.2–19.7), and lung metastases (OR = 6.8; 95% CI 4.8–9.7) (Table 3). HER2 enriched tumors had significantly higher rate of liver (OR = 5.4; 95% CI 3.4–6.5) and brain (OR = 21.9; 95% CI 11.3–42.3) metastases compared with luminal A tumors. Luminal/HER2 subtype had the highest risk of bone recurrence (OR = 3.9.0; 95% CI 3.0–5.1) compared with luminal A tumors. Once again, luminal/HER2 and HER2 enriched tumors demonstrated similar pattern of recurrence.
Discussion
This large study with a median follow-up of 80.8 months demonstrated that patients with different breast cancer subtypes have specific pattern and time of recurrence.
The survival outcome of patients included in our study confirmed the general knowledge about patient survival of breast cancer patients (Colleoni et al. 2016; Cossetti et al. 2015; Kennecke et al. 2010). Patient outcome is variable and dependent on tumor subtype. Luminal A tumors were statistically significant associated with favorable DFS and OS compared with the other subtypes. In opposite, HR-negative subtypes demonstrated a significantly worst survival supported by the finding of others (Cossetti et al. 2015; Kennecke et al. 2010; Metzger-Filho et al. 2013; Saphner et al. 1996). Notably, the patient survival in our study cohort is significantly improved compared with earlier studies (Early Breast Cancer Trialists’ and Collaborative 2005). This observation is more possibly associated with the fact, that in our study most of the patients were treated modern treatment strategies according to the current practice pattern. Based on the treatment modalities outcomes have improved for all breast cancer subtypes (Cossetti et al. 2015). We observed a significant reduction of breast cancer recurrence between patients treated before and after 2005, where 19.6 and 8.8% of the patients were relapsed. Thus, previously observed rates of 20–30% of relapse in breast cancer patients is not actual (Early Breast Cancer Trialists’ and Collaborative 2005; Kennecke et al. 2010; Saphner et al. 1996) and should be considered by the physicians in regard with treatment decision.
Previous studies demonstrated significant differences for rate as well as for pattern and timing of relapse depend on breast cancer subtype. Notably, some of these studies evaluated the recurrence signature depend on HR and HER2 status. ER-negative tumors tend to relapse early in the first 3–5 years with negligible risk of recurrence after this period (Colleoni et al. 2016; Cossetti et al. 2015; Gabos et al. 2010; Kennecke et al. 2010; Metzger-Filho et al. 2013; Saphner et al. 1996; Voduc et al. 2010). In opposite, ER-positive tumors are associated with persistent risk of relapse even after 5 years. The classification of breast cancer according to molecular markers (Perou et al. 2000), requires a new outcome characterization of breast cancer patients based on this new molecular subtypes. In our study, HER2 enriched and TNBC patients were characterized by relatively rapid increase of incidence of relapse and death and is supported by older studies (Cossetti et al. 2015; Kennecke et al. 2010; Park et al. 2010; Ribelles et al. 2013). Once again, the absolute risk of recurrence in these cancer subtypes was significantly lower as already reported due to the improved chemotherapy and targeted therapy (Cossetti et al. 2015). Very recently, we were able to demonstrate that the effect of trastuzumab treatment is independent of the HR-status and should be administered to all HER positive breast cancer patients (Ignatov et al. 2016). Luminal subtypes presented by smooth peak in the initial years after diagnosis and retained the relapse risk even after 10 years of disease. This important phenomenon is supported further by the literature and is valid not only for distant but also for loco-regional relapse (Cossetti et al. 2015; Gabos et al. 2010; Kennecke et al. 2010; Metzger-Filho et al. 2013; Voduc et al. 2010), suggestive for individual follow-up and treatment strategy of breast cancer patients in accordance with tumor subtype. Although and improved survival is observed in the last decade, the early peaks of HER2 enriched and TNBC and late relapse attitude of HR-positive breast cancer is still detectable.
The follow-up and treatment could be affected by the specific site of recurrence found by us. Bone has been previously described as the most common site of metastases among HR-positive breast cancer (Gong et al. 2017; Kennecke et al. 2010; Metzger-Filho et al. 2013; Smid et al. 2008) and was confirmed by our observation. HER2-positive tumors were associated with liver metastases, 5.4 and 5.2% for HER2 enriched and luminal/HER2, respectively. This is consistent with previous results describing liver metastasis to be characteristic for HER2-positive breast cancer and from the same order of magnitude (Gong et al. 2017; Kennecke et al. 2010; Wu et al. 2016). Lung metastasis signature was observed for triple negative breast cancer (Gong et al. 2017; Kennecke et al. 2010; Metzger-Filho et al. 2013; Minn et al. 2005; Wu et al. 2016) and was confirmed in our cohort, where the most of TNBC-metastases occurred. The hazard risk of lung metastases of TNBC is almost sevenfold increased compared with luminal A breast cancer. Brain metastases were more likely to occur in patients with TNBC and HER2-enriched tumors and is in agreement with other observations (Gong et al. 2017; Kennecke et al. 2010; Park et al. 2010; Wu et al. 2016). The risk of brain metastases of HER2 enriched and TNBC is 21.9- and 18.0-fold increased compared with luminal A subtype. Interestingly, luminal/HER2 and HER2 enriched subtype showed similar pattern of relapse with exception of the rate of brain metastases, which was higher in the group of HER2 enriched tumors. In accordance with recent results (Gabos et al. 2010; Voduc et al. 2010), HER2 enriched and TNBC demonstrated similar pattern and the highest rate of loco-regional relapse.
To the best of our knowledge, this study represents the largest analysis regarding the recurrence pattern of breast cancer in accordance on its intrinsic subtypes. The major strength of our study is that the study was undertaken under real clinical conditions (e.g., multi-centric, the study population was similar to the general population and the exclusion criteria were kept to a minimum). Thus, our study possesses high external quality and actually the intent to treat population of patients with breast cancer was investigated. The population-based character of the cohort ruled out referral bias. In addition, our cohort was treated with modern hormonal therapy and chemotherapy regimens. More than 70% of HR-positive women received hormonal therapy and the most used chemotherapy was anthracycline and/or taxane-based. Moreover, approximately 55% of HER2 positive breast cancer patients received trastuzumab as targeted therapy. This treatment was performed in a standardized manner in all centres, thus excluding performance bias. Limitation of this study is the lack of central pathological review regarding HR- and HER2 status as well as pathologic grade and Ki67. The subtypes were classified based on IHC-surrogates and not gnomically, which slightly reduced the accuracy of the subtype determination. For example, TNBC is a very heterogeneous group with different biological behavior and in our study we characterized it as one group based only on HR- and HER2-status. Nevertheless, the IHC-based determination is used in daily routine and is practice orientated allowing the physicians to interpret our data in regard with their praxis.
The information obtained by our study could be successfully used for managing patients with breast cancer and to carve out individual follow-up and treatment strategies. The follow-up for TNBC and HER2-enriched cancer might be more intensive in the first 3 years after diagnosis followed by extended interval of 1 year thereafter. A follow-up beyond the fifth year is questionable. In opposite, the follow-up of luminal breast cancer patients should be performed continuously even after 5 years postoperative to detect the typical late recurrences. Performance of an appropriate physical examination and/or imaging during the follow-up should be also based on the subtype-specific site of metastases. Thus, an X-ray of the lung should be offered more often to patients with HER2-enriched and TNBC, whereas patients with luminal tumors should be extensively examined for locoregional recurrence. Nevertheless, future prospective surveillance trials should be performed to determine the optimal strategy to follow-up patients with different subtypes of breast cancer.
References
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101(10):736–750
Colleoni M, Sun Z, Price KN, Karlsson P, Forbes JF, Thurlimann B, Gianni L, Castiglione M, Gelber RD, Coates AS, Goldhirsch A (2016) Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the international breast cancer study group trials I to V. J Clin Oncol 34(9):927–935
Cossetti RJ, Tyldesley SK, Speers CH, Zheng Y, Gelmon KA (2015) Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J Clin Oncol 33(1):65–73
Early Breast Cancer Trialists’ Collaborative G (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472): 1687–1717
Eggemann H, Ignatov T, Burger E, Kantelhardt EJ, Fettke F, Thomssen C, Costa SD, Ignatov A (2015) Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr Relat Cancer 22(5):725–733
Gabos Z, Thoms J, Ghosh S, Hanson J, Deschenes J, Sabri S, Abdulkarim B (2010) The association between biological subtype and locoregional recurrence in newly diagnosed breast cancer. Breast Cancer Res Treat 124(1):187–194
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, Panel m (2011) Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22(8):1736–1747
Gong Y, Liu YR, Ji P, Hu X, Shao ZM (2017) Impact of molecular subtypes on metastatic breast cancer patients: a SEER population-based study. Sci Rep 7:45411
Ignatov T, Eggemann H, Burger E, Costa SD, Ignatov A (2016) Hormone receptor status does not alter the effect of trastuzumab in breast cancer. Endocr Relat Cancer 23(5):349–355
Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28(20):3271–3277
Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, Gelber RD, Castiglione-Gertsch M, Coates AS, Goldhirsch A, Cardoso F (2013) Patterns of recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol 31(25):3083–3090
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J (2005) Genes that mediate breast cancer metastasis to lung. Nature 436(7050):518–524
Park YH, Lee S, Cho EY, Choi YL, Lee JE, Nam SJ, Yang JH, Ahn JS, Im YH (2010) Patterns of relapse and metastatic spread in HER2-overexpressing breast cancer according to estrogen receptor status. Cancer Chemother Pharmacol 66(3):507–516
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752
Ribelles N, Perez-Villa L, Jerez JM, Pajares B, Vicioso L, Jimenez B, de Luque V, Franco L, Gallego E, Marquez A, Alvarez M, Sanchez-Munoz A, Perez-Rivas L, Alba E (2013) Pattern of recurrence of early breast cancer is different according to intrinsic subtype and proliferation index. Breast Cancer Res 15(5):R98
Saphner T, Tormey DC, Gray R (1996) Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 14(10):2738–2746
Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW (2008) Subtypes of breast cancer show preferential site of relapse. Cancer Res 68(9):3108–3114
Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H (2010) Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol 28(10):1684–1691
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370(9596):1453–1457
Wu X, Baig A, Kasymjanova G, Kafi K, Holcroft C, Mekouar H, Carbonneau A, Bahoric B, Sultanem K, Muanza T (2016) Pattern of local recurrence and distant metastasis in breast cancer by molecular subtype. Cureus 8(12):e924
Funding
This study was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors. Research and Ethical Committee, Otto-von-Guericke University, Magdeburg, Germany proved the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all patients before treatment. An additional individual consent for this analysis was not needed. Before analysis, patient data underwent a pesudonymisation.
Rights and permissions
About this article
Cite this article
Ignatov, A., Eggemann, H., Burger, E. et al. Patterns of breast cancer relapse in accordance to biological subtype. J Cancer Res Clin Oncol 144, 1347–1355 (2018). https://doi.org/10.1007/s00432-018-2644-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2644-2