Abstract
Purpose
This study explored the relationship of ER expression levels with HER2 staining properties and heterogeneity and discussed the differences in HER2 assessment caused by the 2018 ASCO/CAP guideline updates from that of the 2013 version.
Methods
HER2-positive breast cancer was divided into three groups of the high hormone receptor expression (LH-high) group, low expression (LH-low) group, or negative (NLH) group to (1) compare differences in the percentage of the HER2 IHC test score of 2 + based on the 2013 ASCO/CAP guideline and in the intratumor heterogeneity of HER2 expression for breast cancer with an IHC score of 3 + among these groups, (2) compare the HER2/CEP17 ratio and the average HER2 copy number, and classified ISH groupings according to the 2018 ASCO/CAP guideline algorithm.
Results
(1) Of 244 HER2-positive breast cancers, the cases with a HER2 IHC score of 2 + (n = 54, 22.1%) were significantly more common in the LH-high group (n = 45, P < 0.001). The frequency of heterogeneity was low (n = 25, 10.2%) for the HER2 score of 3 + (n = 190, 77.9%), and significantly higher in the LH-high group (n = 19, 76%, P = 0.002). (2) In a HER2 IHC score of 2 + , Group 2 which is deemed HER2 negative according to the revised 2018 ASCO/CAP guideline was observed in 17 (39.5%) out of 43 cases, of which 16 cases (94.1%) were in the LH-high group.
Conclusions
The LH-high group is a heterogeneous group largely consisting of heterogeneous cases with HER2 IHC scores of 2 + or 3 + . NLH, in contrast, is a homogenous group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common malignancy in women [1], and overexpression or genetic amplification of human epidermal growth factor receptor 2 (HER2) is observed in 15–25% of invasive breast cancer [2, 3]. HER2-positive breast cancer was previously classified as a poor prognosis group [4], but currently anti-HER2 therapy has shown remarkable effectiveness for this group of cancer and improved prognosis [3]. In the clinicopathologic definition, HER2-positive breast cancer can be divided into hormone receptor-positive breast carcinoma (luminal HER2, LH) and hormone receptor-negative breast carcinoma (non-luminal HER2, NLH) [5]. HER2-positive breast cancer can be further subdivided into five patterns by gene analysis [6], and it has been reported that there are differences in clinical response to treatment and prognosis depending on the subgroup [6, 7]. Previously, we classified HER2-positive breast cancer into LH and NLH groups for clinicopathological study and demonstrated that HER2-positive breast cancer forms diverse groups in terms of morphology and immune response [8]. In other words, this study showed the non-uniformity of HER2-positive breast cancer by its nature, not only for treatment response and prognosis, but also for clinicopathological findings.
The American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guideline is widely used for HER2 testing and assessment worldwide [9, 10]. Accurate assessment of HER2 status and accuracy control of staining are critical components as these directly relate to the clinician's decision on indication for anti-HER2 therapy [2, 9, 10], but several problems have been reported for fixation and other technical features. One of such problems includes intratumor heterogeneity of HER2 expression. Intratumor HER2 heterogeneity is estimated to be around 13–36% with a varying range of the rate among reports [11]. When HER2 protein expression is heterogeneous across the tumor, it is generally scored 2 + by the HER2 immunohistochemistry (IHC) test. HER2 IHC 2 + tumors are often equivocal both in protein expression and gene amplification [12], and it has also been reported that patients in the HER2 IHC score 2 + /in situ hybridization (ISH) + group had significantly poorer prognosis than those of the IHC 3 + group [13] and breast cancer with intratumor heterogeneity for HER2 expression did not respond to treatment well [14]. Lee et al. investigated the relationship between intratumor heterogeneity of HER2 protein expression and HER2-positive breast cancer subgroups and reported that hormone receptor (HR)- /HER2 + breast cancer showed stronger expression in IHC staining, higher average HER2 copy numbers and HER2/CEP17 ratios, and lower HER2 genetic heterogeneity, compared with HR + /HER2 + breast cancer [15]. Another report showed an inverse correlation of HER2 expression and hormone receptor expression [16]. All of these findings suggest a possible association of HER2 expression with hormone receptor expression and its expression level.
Despite the definition of genomic heterogeneity in the 2013 ASCO/CAP guideline stating "more than one population of tumor cells exists within the same tumor [9]," in practice, intratumor heterogeneity is divided into tumors expressing HER2 protein heterogeneously across the entire tumor (mosaic pattern) and those showing regionally attenuated protein expression (clustered/regional pattern) [17]. Breast cancers coexisted with both intraductal and invasive lesions may also present with different HER2 expression. As described above, HER2 expression in HER2-positive breast cancer is not uniform and treatment response may be influenced by whether the hormone receptor expression is positive or negative, as well as by its expression level and heterogeneity.
In response to the revision of the ASCO/CAP guideline in 2018, classification groups based on the ISH HER2/CEP17 ratio were changed. Notably, the HER2/CEP17 ratio ≥ 2.0 and average HER2 copy number < 4.0 signals per cell group (Group 2), which had resulted in positive in the 2013 guideline, were reviewed, and revised to be classified as negative if the result of repeated testing is similar.
In this study, we divided HER2-positive breast cancer into three groups: luminal breast cancer with high expression of hormone receptors (LH-high), luminal breast cancer with low expression of hormone receptors (LH-low), or the NLH group to (1) compare differences in the percentage of the HER2 IHC test score of 2 + and the intratumor heterogeneity of HER2 expression in breast cancer with an IHC score of 3 + among these subgroups, and to (2) discuss differences in HER2 assessment caused by the 2018 ASCO/CAP guideline updates from that of the 2013 version, especially focusing on Group 2 that is considered to be negative per the 2018 ASCO/CAP guideline.
Materials and methods
Subjects and clinicopathologic factors
HER2-positive invasive breast cancer was selected from invasive breast cancer cases surgically treated at JCHO Kurume General Hospital from January 2013 to December 2017. Four-μm sections were prepared from formalin-fixed, paraffin-embedded biopsy or resected specimens and subjected to hematoxylin–eosin (HE) staining and immunostaining. All specimens were observed retrospectively and re-evaluated by two pathologists (M.A. and R.Y.). Immunostaining was basically performed using biopsy specimens, and in the absence of biopsy specimens resected specimens were used, only if the patient had not been treated with preoperative adjuvant chemotherapy. Pathological records were used as a reference to collect the patient's clinical information. Tumor diameter was generally measured on surgical specimens, and in patients who received preoperative chemotherapy, the tumor diameter measured by ultrasonography at the initial visit was used.
Estrogen receptor (ER)/progesterone receptor (PgR) staining
ER and PgR staining was performed using BenchMark XT (Ventana, Tucson, AZ, USA), following the protocol procedure. Primary antibodies are as follows: ER (clone SP1, VENTANA) and PgR (clone IE2, VENTANA). For assessment, ER and PgR expression was scored using the Allred score (0–8), which takes into account the percentage and intensity of positive cells [18], with a score of ≥ 2 defined as LH. An Allred score of ≥ 4 was defined as high expression and a score of 2–3 as low expression. Accordingly, when either the ER or PgR score was ≥ 4 the specimen was categorized as LH-high, and when both ER and PgR scores were ≤ 3 or 0, it was categorized as LH-low or NLH, respectively.
HER2 IHC staining
HER2 IHC staining was performed using BenchMark XT, following the protocol procedure. HER2 (C-erbB-2) (clone 4B5, VENTANA) was used as the primary antibody. HER2 assessment was based on the ASCO/CAP guideline [9, 10]. HER2 negative was defined as an IHC score of 0 or 1 + . HER2 equivocal was defined as an IHC score of 2 + (Fig. 1b) and these cases were retested by the ISH method. HER2 positive was defined as an IHC score of 3 + (Fig. 1a) and these cases were also assessed for HER2 stainability as described below. Homogeneity was defined as the overexpression of HER2 protein in more than 90% of tumor cells, while heterogeneity was defined as the overexpression of HER2 protein in 10–90% of tumor cells [19]. In heterogeneous tumors, mosaic heterogeneity was defined as a mixture of cells with or without HER2 protein expression as confirmed by the IHC test (Fig. 1c), and clustered/regional heterogeneity was defined as the regional population of cells with weakened or loss of HER2 protein expression (Fig. 1d) [17]. When different HER2 staining properties were observed in intraductal and invasive lesions, the HER2 status was classified as in situ/invasion heterogeneity.
HER2 ISH testing
Dual color ISH (DISH) was used for ISH testing. DISH was performed using BenchMark XT, following the protocol procedure. The ISH kit used was an INFORM HER2 Dual ISH DNA probe cocktail (VENTANA). The DISH test was additionally used to evaluate cases that had been diagnosed as an IHC score of 3 + by the prior physician and newly scored IHC 2 + (HER2 equivocal) but were unable to confirm an IHC score of 3 + by the repeated test. Initial assessment was made based on the 2013 ASCO/CAP guideline [9].
HER2 DISH assessment according to ASCO/CAP clinical practice guideline 2013 vs. 2018
After DISH was performed, HER2 status was classified as HER2 positive when the HER2/CEP17 ratio was ≥ 2.0 or the average HER2 copy number was ≥ 6.0 according to the 2013 ASCO/CAP guideline [9].
Whereas, based on the 2018 guideline [10], HER2 status was classified as HER2 positive when the HER2/CEP17 ratio was ≥ 2.0 and the average HER2 copy number was ≥ 4.0 (Group 1). HER2 status was considered as requiring a repeated test when the HER2/CEP17 ratio was ≥ 2.0 and the average HER2 copy number was < 4.0 (Group 2); when the HER2/CEP 17 ratio was < 2.0 and the average HER2 copy number was ≥ 6.0 (Group 3); or when the HER2/CEP 17 ratio was < 2.0 and the average HER2 copy number was ≥ 4.0 to < 6.0 (Group 4).
Statistical analysis
Pearson’s χ2 test or Fisher’s exact test was performed as required. A P value ≤ 0.05 was considered statistically significant. Multiplicity was adjusted by the Bonferroni method. We used Jump Pro version 13 (SAS Institute, Cary, NC) for statistical analysis.
Results
Clinicopathological characteristics and HER2 status
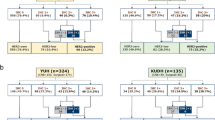
Between 2013 and 2017, 1,627 surgeries were performed for invasive breast cancer, of which 263 cases were HER2 positive (HER2 positive rate was 16.2%). A total of 244 cases were included in this study, excluding 6 cases which were diagnosed as a HER2 score of 3 + by their prior physicians but were HER2 negative as a result of additional DISH, and 13 cases which underwent preoperative chemotherapy and biopsy specimens were not available for evaluation before the chemotherapy. All subjects were women, including 158 patients (64.8%) in the LH group [128 (52.5%) in LH-high; 30 (12.3%) in LH-low] and 86 patients (35.2%) in the NLH group (Fig. 2). Table 1 shows the clinicopathological backgrounds of each group. The median age of the overall patients was 58 years and the median tumor diameter was 1.8 cm, with no significant differences in age or tumor size among the subgroups. The number of patients with a HER2 IHC score of 2 + was significantly higher in the LH-high group than in the LH-low and NLH groups (P < 0.001), while more than 80% of patients had a HER2 score of 3 + and homogeneous staining properties in the LH-low and NLH groups. Heterogeneity was less frequent in patients with a HER2 score of 3 + (n = 190, 77.9%) and observed in 25 patients (10.2%). Heterogeneity was significantly more common in the LH-high group [19 patients (76%) in LH-high; 1 patient (4%) in LH-low; 5 patients (20%) in NLH; P = 0.002). The number of patients who had clustered heterogeneity with a HER2 score of 3 + was as small as 2% overall, and there was no significant difference among the subgroups. No patients showed in situ/invasion heterogeneity in any of the subgroups.
ASCO/CAP clinical practice guideline 2013 vs. 2018
The HER2/CEP17 ratios and average HER2 copy numbers of the cases tested by DISH are shown in Table 2. Forty-three patients had a known average HER2 copy number. Twenty-six patients (60.5%) had a HER2/CEP17 ratio of ≥ 2.0 and average HER2 copy number ≥ 4.0 signals per cell (Group 1), and 17 patients (39.5%) had a HER2/CEP17 ratio of ≥ 2.0 and average HER2 copy number < 4.0 signals per cell (Group 2). There were significantly more patients in the LH-high group who had a mean copy number less than 4.0 and none in the NLH group. No patients fell under Group 3 or 4 which required a HER2/CEP17 ratio of < 2.0. The HER2 mean copy number and DISH data were unavailable from a total of 11 patients (20.4%).
Discussion
We previously reported that HER2-positive breast cancer was not pathologically homogeneous by nature and could be broadly classified into LH and NLH groups by tumor morphology and immunoreactivity [8]. The present study demonstrated the non-uniformity of HER2-positive breast cancer in the HER2 staining properties among subgroups due to different hormone receptor expression levels.
In this study, the analysis using antibodies from Ventana identified a significantly higher number of cases with a HER2 IHC score of 2 + in the LH-high group, although our study did not address trends observed with other antibodies. The majority of cases that presented mosaic or clustered heterogeneity in the staining pattern with a HER2 score of 3 + was the LH-high group (n = 19, 76%). Relating to the finding that most HER2 expression was heterogeneous in the LH-high group at a moderate expression level (2 +) or higher (3 +), there is a crosstalk between ER and HER2 receptors [20], which may affect the HER2 protein expression. As it has been reported that the expression of HER2 and ER was variable and both positive and negative cases coexisted in the cancer cell subpopulation of the LH group [21], the LH-high group, which expresses both hormone and HER2 receptors simultaneously, could be considered a heterogeneous group for its expression of HER2 and hormone receptors.
The majority of cases showed homogenous staining with a HER2 score of 3 + in the LH-low and NLH groups. Compared with hormone receptor-positive groups, the hormone receptor-negative HER2-positive breast cancer could achieve a higher pathological complete response (pCR) rate with preoperative chemotherapy regimens including HER2-directed agents, according to a report [22]. Our study revealed that HER2 protein expression was relatively homogenous in the LH-low and NLH groups, suggesting their susceptibility to HER2-directed agents.
A study on in situ/invasion heterogeneity demonstrated no discrepancy in HER2 expression at the invasion site and intraductal lesion, reporting that heterogeneity rarely occurred during the course of invasion of intraductal lesions [23]. Our study also found a case where the IHC test indicated a difference in staining between invasive lesions and intraductal lesions; however, in situ/invasion heterogeneity was excluded as a result of reassessment by DISH. It is therefore unlikely that genetic mutations or genetic transformation occurs during the invasion of HER2-positive breast cancer, and if this type of staining is observed, confirmation by ISH is necessary.
The ISH test was additionally used to evaluate breast cancer cases of an IHC score of 2 + , and it was found in this study that the average HER2 copy number was significantly lower in the LH-high group. With the revision of the ASCO/CAP guideline, it is now recommended from the 2018 version to repeat testing for patients whose HER2/CEP17 ratio is ≥ 2.0 but the average HER2 copy number is < 4.0, and if the second test result is similar, the patient's HER2 status should be interpreted as negative [10]. Among the cases who were tested by additional DISH with an IHC score of 2 + and whose average HER2 copy numbers were known, 17 (39.5%) had a HER2/CEP17 ratio of ≥ 2.0 and an average HER2 copy number of < 4.0 (Group 2), and these cases could be HER2 negative if the result is confirmed by repeated testing. Since the majority of these cases (16/17, 94.1%) were in the LH-high group, they would be classified as a luminal type once tested HER2 negative. The exclusion of these types of cases from the HER2 group may lead to a clearer characterization of HER2-positive breast cancer and improve the therapeutic responsiveness of HER2-positive breast cancer to anti-HER2 treatment. Standardization of HER2 diagnosis, including fixation methods is not without controversy [2]. However, we believe it would aid in accurate HER2 diagnosis to know that many hormone receptor-positive patients with a HER2 IHC score of 2 + could ultimately be HER2 negative.
The limitation of this study includes that the number of cases tested by DISH was small because DISH was basically performed only for patients with a HER2 IHC score of 2 + , and no cases presented with a HER2/CEP17 ratio of < 2.0. Immunostaining was mostly performed on biopsy specimens and heterogeneity may have been less detected compared with that using resected specimens. In contrast, needle biopsy specimens are recommended for application to clinical diagnosis because they are well fixed and suitable for immunostaining, according to the purpose of our study designed to support clinical practice.
In summary, HER2-positive breast cancer was not intrinsically uniform in terms of HER2 staining properties. The LH-high group is a heterogeneous group largely consisting of heterogeneous cases with HER2 IHC scores of 2 + or 3 +. The majority of group 2 according to the 2018 ASCO/CAP guideline were in the LH-high group. NLH, in contrast, is a homogenous group. While the LH-low group was intermediate between the LH-high and NLH groups in the analysis on tumor morphology and immune response [8], the LH-low group is considered closer to the NLH group for staining properties and heterogeneity of HER2. In view of the accuracy control of the HER2 test, the knowledge of these subgroup characteristics will be useful for breast cancer diagnosis and may also help elucidate the difference in treatment response among clinically HER2-positive breast cancer subgroups.
References
Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global cancer in women: burden and trends. Cancer Epidemiol Biomarkers Prev. 2017;26:444–57.
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43.
Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28:92–8.
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–23.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47.
Llombart-Cussac A, Cortes J, Pare L, Galvan P, Bermejo B, Martinez N, et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. Lancet Oncol. 2017;18:545–54.
Ellis MJ, Coop A, Singh B, Mauriac L, Llombert-Cussac A, Janicke F, et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol. 2001;19:3808–16.
Akashi M, Yamaguchi R, Kusano H, Obara H, Yamaguchi M, Toh U, et al. Diverse histomorphology of HER2-positive breast carcinomas based on differential ER expression. Histopathology. 2020;76:560–71.
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: american society of clinical oncology/college of american pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013.
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: american society of clinical oncology/college of american pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–22.
Seol H, Lee HJ, Choi Y, Lee HE, Kim YJ, Kim JH, et al. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol. 2012;25:938–48.
Hou Y, Nitta H, Li Z. HER2 gene protein assay is useful to determine HER2 status and evaluate HER2 heterogeneity in HER2 equivocal breast cancer. Am J Clin Pathol. 2017;147:89–95.
Tsai YF, Tseng LM, Lien PJ, Hsu CY, Lin YS, King KL, et al. HER2 immunohistochemical scores provide prognostic information for patients with HER2-type invasive breast cancer. Histopathology. 2019;74:578–86.
Hou Y, Nitta H, Wei L, Banks PM, Portier B, Parwani AV, et al. HER2 intratumoral heterogeneity is independently associated with incomplete response to anti-HER2 neoadjuvant chemotherapy in HER2-positive breast carcinoma. Breast Cancer Res Treat. 2017;166:447–57.
Lee HJ, Park IA, Park SY, Seo AN, Lim B, Chai Y, et al. Two histopathologically different diseases: hormone receptor-positive and hormone receptor-negative tumors in HER2-positive breast cancer. Breast Cancer Res Treat. 2014;145:615–23.
Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003;95:142–53.
Hanna WM, Ruschoff J, Bilous M, Coudry RA, Dowsett M, Osamura RY, et al. HER2 in situ hybridization in breast cancer: clinical implications of polysomy 17 and genetic heterogeneity. Mod Pathol. 2014;27:4–18.
Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–68.
Motoshima S, Yonemoto K, Kamei H, Morita M, Yamaguchi R. Prognostic implications of HER2 heterogeneity in gastric cancer. Oncotarget. 2018;9:9262–72.
Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29:217–33.
Masuda S, Nitta H, Kelly BD, Zhang W, Farrell M, Dennis E. Intratumoral estrogen receptor heterogeneity of expression in human epidermal growth factor receptor 2-positive breast cancer as evaluated by a brightfield multiplex assay. J Histochem Cytochem. 2019;67:563–74.
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32.
Polonia A, Oliveira G, Schmitt F. Characterization of HER2 gene amplification heterogeneity in invasive and in situ breast cancer using bright-field in situ hybridization. Virchows Arch. 2017;471:589–98.
Acknowledgements
We thank Editor Express (www.editorexpress.co.jp) for editing a draft of this manuscript.
Funding
The study was partially supported by JSPS KAKENHI grant number JP17K08707.
Author information
Authors and Affiliations
Contributions
MA and RY designed the study and wrote the manuscript. MY and MT collected surgical resection specimens. HK and JA supervised the work. TK performed the data analysis. HY and YA performed research supervision and management, as well as dissertation writing.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This retrospective study was approved by the Kurume General Hospital Ethical Committee (No. 187) and Kurume University Ethical Committee (No. 16272).
Informed consent
Informed consent was not required for this retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Akashi, M., Yamaguchi, R., Kusano, H. et al. ER staining levels affect HER2 staining and heterogeneity. Breast Cancer 28, 720–726 (2021). https://doi.org/10.1007/s12282-020-01208-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-020-01208-7