Abstract
Placental C4d deposition is frequent in preeclampsia, and shallow placentation is a characteristic of both preeclampsia and miscarriage. This study was conducted to determine the relationship among placental C4d, maternal human leukocyte antigen (HLA) antibodies, and placental pathology in preeclampsia and miscarriage cases. The patient population (N = 104) included those with (1) preterm preeclampsia with fetal growth restriction (PE-FGR; n = 21), (2) preterm preeclampsia (PE; n = 20), (3) spontaneous preterm delivery (sPTD; n = 39), and (4) miscarriage (n = 24). C4d immunohistochemistry was performed, and the presence of maternal plasma HLA antibodies was examined. C4d staining of the syncytiotrophoblast was more frequent in PE-FGR patients (76.2 %) than in PE (10.0 %; p < 0.001) and sPTD (2.6 %; p < 0.001) patients. Maternal HLA antibody-positive rate was not different among the study groups. There was a significant correlation between C4d immunoreactivity and placental pathology consistent with maternal vascular underperfusion (p < 0.001) but not with maternal HLA antibody status. In miscarriages, the positive rates of C4d, HLA class I, and HLA class II antibodies were 58.3, 25.0, and 12.5 %, respectively. There was no correlation between the presence of maternal HLA class I or II antibodies and placental C4d immunoreactivity. This study confirms frequent placental C4d deposition in preeclampsia with fetal growth restriction and miscarriage. The association between placental C4d deposition and pathological findings of maternal vascular underperfusion suggests that C4d staining of the syncytiotrophoblast is a consequence of defective placentation rather than of a specific maternal immune response against fetal HLA. The study also demonstrates the usefulness of C4d as a biomarker of placentas at risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complement component 4d (C4d) is a split product of C4, and its deposition in an allograft is indicative of antibody-mediated rejection although there are some recent issues with its sensitivity [1, 2]. For example, both focal and diffuse immunoreactive C4d in peritubular capillary endothelial cells of renal allografts strongly associates with the presence of circulating donor-specific human leukocyte antigen (HLA) antibodies and poor allograft outcome [3, 4]. Studies have demonstrated C4d deposition in the syncytiotrophoblast in patients with certain placental lesions and pregnancy-related disorders, including miscarriage [5], antiphospholipid syndrome [6], systemic lupus erythematosus [7], villitis of unknown etiology (VUE; anti-fetal cellular rejection of the placenta) [8–10], placental infarct [10], and preeclampsia. A systematic analysis of different complement components by Buurma et al. has provided evidence for placental complement dysregulation in patients with preeclampsia [9]. C4d deposition in the syncytiotrophoblast was evident in half of all preeclampsia cases, while it is present in only 3 % of healthy pregnant women. C4d placental deposition was likely a consequence of classical complement activation because mannose-binding lectin staining was not observed. Although the selective localization of C4d to VUE foci indicates that C4d deposition correlates with antibody-mediated rejection [8], it is not known if placental C4d deposition in patients with preeclampsia associates with the presence of circulating maternal HLA antibodies or if it is a non-HLA-related response induced by different types of placental injuries.
Preeclampsia is an enigmatic disorder defined by the onset of maternal hypertension and proteinuria during pregnancy [11]. Preeclampsia affects approximately 5 % of all pregnancies [12] and accounts for a large proportion of both maternal and neonatal morbidities [13, 14]. Patients with preeclampsia have a systemic vascular inflammatory response [15] and imbalance between pro- and anti-angiogenic factors, such as placental growth factor, sFlt-1, and soluble endoglin [16–18]. The primary characteristics of preeclampsia are shallow placentation and defective physiological remodeling of spiral arteries at the implantation site [19, 20]. Failure of physiological remodeling of spiral arteries results in increase of placental villous damage by turbulent intervillous blood flow jet and ischemia-reperfusion injury associated with spontaneous vasoconstriction [21]. These features are also common to fetal growth restriction, and preeclampsia associates with fetal growth restriction in more than one third of all cases [22, 23]. Furthermore, severe and early forms of defective placentation and placental oxidative stress may cause miscarriage, and it has been proposed that miscarriage and preeclampsia constitute a disease spectrum rather than being isolated disorders [24, 25]. Therefore, the placenta of patients with preeclampsia and miscarriage is a good model to study defective placentation, placental ischemia-reperfusion injury, and oxidative stress.
Major placental pathology clusters are composed of intra-amniotic infection and inflammation, maternal vascular underperfusion, fetal vascular thrombo-occlusive disease, and chronic placental inflammation [26–29]. Studies in chronic placental inflammation (chronic chorioamnionitis, chronic deciduitis, and VUE) clearly indicate that the presence of maternal HLA antibodies is a surrogate of maternal anti-fetal antibody-mediated rejection in patients with spontaneous preterm deliveries [30]. Therefore, the aim of this study was to determine if a correlation exists among the deposition of placental C4d, the presence of maternal HLA antibodies, and pathological changes of the placenta in patients with preeclampsia and miscarriage.
Materials and methods
Patient materials
Placental tissues were obtained from the Department of Pathology, Seoul National University Hospital and the Infertility Registry of Hamchoon Women’s Clinic. Corresponding patient plasma samples were obtained at the time of delivery or miscarriage. The patient population (N = 104) included those with (1) preterm preeclampsia with fetal growth restriction (PE-FGR; n = 21), (2) preterm preeclampsia (PE; n = 20), (3) spontaneous preterm delivery (sPTD; n = 39), and (4) miscarriage (n = 24). Placental lesions were diagnosed by a pathologist (C.J.K.), who was masked to clinical findings, according to previously described criteria [27–29].
Preterm labor was diagnosed by the presence of regular uterine contractions and cervical dilation, followed by delivery prior to 37 weeks of gestation. Preterm prelabor rupture of membranes was defined by pooling of amniotic fluid in the vagina or a positive nitrazine test during the sterile speculum examination, which was conducted prior to 37 weeks of gestation in the absence of labor. Preeclampsia was defined as newly diagnosed high blood pressure (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg) with onset after 20 weeks of gestation and proteinuria (of 24 hours urine protein ≥300 mg or a dipstick ≥1+) on at least two occasions taken at least 6 hours apart [31]. We classified the preeclampsia patients into two groups: those with preeclampsia with fetal growth restriction and those without fetal growth restriction, according to the birth weight of the newborn. Neonates weighing <10th percentile of the birth weight for the gestational age were diagnosed with fetal growth restriction. The diagnosis was made based on a new fetal-infant growth chart for preterm infants [32]. Gestational age at delivery and maternal age were adjusted by matching process between PE-FGR/PE and sPTD groups. The exclusion criteria were the followings: pregnancy with multifetal pregnancy, significant fetal anomaly, severe maternal medical disease, gestational diabetes on insulin, cerclage operation, placenta previa, placental abruption, fetal death in utero, intrauterine growth restriction by unknown cause, oligohydramnios only by unknown cause, cord prolapse, spontaneous fetal distress by unknown cause, and preterm labor combined with preeclampsia. Miscarriage cases were consecutively enrolled regardless of chromosomal status, because our previous study demonstrated frequent placental C4d immunoreactivity regardless of chromosomal status.
Tissue microarray
Tissue microarrays were prepared using formalin-fixed, paraffin-embedded blocks of preterm delivery cases. Two 2-mm-thick tissue cores were obtained from donor blocks and transferred onto recipient blocks after reviewing hematoxylin- and eosin-stained sections. Three tissue microarrays were constructed from preterm delivery cases and used for immunohistochemistry. For miscarriage cases, whole paraffin-embedded blocks were used for sectioning.
Immunohistochemistry
Four micron-thick paraffin sections were used for immunostaining. A rabbit polyclonal anti-C4d antibody was the primary antibody (diluted 1:200; Cell Marque Corporation, Rocklin, CA, USA), and immunostaining was performed with the BenchMark XT automated slide preparation system (Ventana Medical Systems, Inc., Tucson, AZ, USA). The positive control tissue was a renal biopsy specimen from a patient with documented acute allograft rejection. For the isotype control, rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) was used at the same dilution. The degree of syncytiotrophoblast C4d immunoreactivity was scored as 0: none (<10 %), 1: focal (10–50 %), or 2: diffuse (>50 %) [9]. C4d scoring of placentas was performed by a pathologist (C.J.K.).
Detection of HLA antibodies
Maternal plasma samples were obtained at the time of delivery or miscarriage and stored at −80 °C until use. Screening for the presence of HLA class I, HLA class II, and MHC class I polypeptide-related sequence A (MICA) antibodies was performed with the LABScreen® Mixed Class I and II assay (One Lambda Inc., Canoga Park, CA, USA) and LABScan™ 200 flow analyzer (Luminex Corporation, Austin, TX, USA) according to the manufacturers’ instructions. For each test, 20 μl of plasma was mixed with 5 μl of beads. Data analysis was performed using HLA Fusion software (One Lambda Inc.). A normalized background ratio greater than 3 was defined as positive.
Statistical analysis
The Kolmogorov–Smirnov test was used to analyze the distributions for normality of continuous variables. If data were not normally distributed, Kruskal–Wallis analysis and the Mann–Whitney U test were used for multiple comparisons among groups. If data were normally distributed, one-way analysis of variance and an unpaired t test were used. For post-hoc analysis, the Tukey test was applied.
For categorical variables, proportions were analyzed by the Fisher’s exact test or Chi-square test. Means and medians with interquartile ranges were calculated for continuous variables, whereas frequencies and percentages were reported for categorical variables. SPSS (version 18.0, SPSS Inc., Chicago, USA) was used for all statistical analyses. All p values were two-sided, and p values <0.05 were considered statistically significant.
Results
Placental C4d immunoreactivity
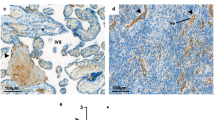
Tables 1 and 2 summarize the clinical characteristics of patients with preterm deliveries and miscarriages, respectively. The gestational ages of preeclampsia cases ranged from 25.1 and 36.7 weeks (Online resource 1). Initial clinical assessments of deliveries in the spontaneous preterm group consist of preterm premature rupture of the membrane (n = 21, 53.8 %) and preterm labor (n = 18, 46.2 %). Ureaplasma urealyticum or Mycoplasma hominis was found (by culture or PCR of amniotic fluid or umbilical cord blood) in seven out of thirty-eight patients tested (18.4 %). Placental C4d immunoreactivity was linear along the syncytiotrophoblast and cytoplasmic in villous cytotrophoblasts in patients with preterm deliveries (Fig. 1). In addition, there were significant differences in C4d immunoreactivity in patients with PE-FGR (n = 21), PE (n = 20), and sPTD (n = 39; p < 0.001; Fig. 2). C4d staining of the syncytiotrophoblast was more frequent in PE-FGR patients (76.2 %) than in PE (10 %, p < 0.001) and sPTD (2.6 %, p < 0.001) patients. The severity of preeclampsia in PE-FGR and PE cases is shown in Table 3, and C4d immunoreactivity was not associated with the severity of preeclampsia. C4d staining in the cytotrophoblast was also frequently detected; however, there were no differences in C4d staining in the cytotrophoblast among patients with PE-FGR (66.7 %), PE (90.0 %), and sPTD (89.7 %). C4d staining of the syncytiotrophoblast was also detected in 14 out of 24 patients with miscarriages (58.3 %; Fig. 2). Furthermore, C4d staining of the cytotrophoblast was not observed in all miscarriage cases in contrast to preterm deliveries. No difference was noted between preterm deliveries and miscarriages in terms of the C4d immunoreactive pattern on the syncytiotrophoblast.
C4d immunoreactivity in the villous trophoblast. a A section of tissue microarray stained with hematoxylin and eosin. b A microscopic image of C4d immunostaining in the syncytiotrophoblast. Placental C4d immunoreactivity is linear along the syncytiotrophoblast in a case of preeclampsia with fetal growth restriction at 27 weeks of gestation. c Cytoplasmic C4d immunoreactivity in the villous cytotrophoblasts in a case of spontaneous preterm delivery at 28 weeks of gestation. d C4d immunoreactivity is linear along the syncytiotrophoblast in a case of miscarriage at 7 weeks of gestation. There is no C4d immunoreactivity in the villous cytotrophoblasts (b, c ×200; d ×100)
Syncytiotrophoblast C4d immunoreactivity in different groups. a There is a significant difference in C4d immunoreactivity in cases of preeclampsia with fetal growth restriction (PE-FGR; n = 21), preeclampsia (PE; n = 20), and spontaneous preterm delivery (sPTD; n = 39, p < 0.001). C4d staining of the syncytiotrophoblast was detected in 58.3 % of patients with miscarriages. The rate of C4d staining in PE-FGR patients was higher than that in PE and sPTD patients (p < 0.001 for each). b The distribution patterns of C4d immunostaining score in each group
Based on the C4d immunopositive patterns on the syncytiotrophoblast, we additionally immunostained the full thickness placental sections of PE-FGR cases (n = 3) to see if the intermediate trophoblasts at the implantation site and those at the basal plate were C4d negative in all cases (Online resource 2).
Maternal HLA class I, HLA class II, and MICA antibodies
The rates of PE-FGR, PE, and sPTD patients positive for maternal HLA class I antibodies were 57.1 % (12/21), 35.0 % (7/20), and 53.8 % (21/39), respectively, with no difference in rates among groups (p = 0.349; Fig. 3a). The rates of PE-FGR, PE, and sPTD patients positive for maternal HLA class II antibodies were 19.0 % (4/21), 20.0 % (4/20), and 30.8 % (12/39), respectively. Similarly, there was no difference in positive rates among groups (p = 0.564; Fig. 3b). MICA antibodies were detected in only one patient in each group. There was no correlation between the presence of maternal HLA class I or class II antibodies and C4d immunoreactivity in the placenta (Fig. 3c, d).
Maternal HLA antibody status in different study groups and its relationship with C4d staining of the syncytiotrophoblast. a, b There is no difference in rates in PE-FGR, PE, and sPTD cases with regard to the presence of maternal HLA classes I and II antibodies (p = 0.349 and p = 0.564 for each). c, d C4d staining of the syncytiotrophoblast in relation to the presence or absence of maternal HLA classes I and II antibodies in preterm delivery cases (n = 80; PE-FGR, PE, sPTD). There is no significant difference in the presence of HLA classes I and II antibodies between placental C4d-positive and -negative cases (p = 0.189 and p = 0.768, respectively). e, f There is also no significant correlation between HLA classes I and II antibody status and C4d staining of the syncytiotrophoblast in patients with miscarriages
The rates of patients with miscarriages positive for maternal HLA classes I and II antibodies were 25 % (6/24) and 12.5 % (3/24), respectively. There was no association between the status of maternal HLA antibodies and C4d immunoreactivity in the placenta of patients with miscarriages (Fig. 3e, f).
Placental pathology, C4d, and maternal HLA antibodies
Pathological changes of the placenta in patients with preterm deliveries (PE-FGR, PE, and sPTD) were classified into four categories: (1) amniotic fluid infection (acute chorioamnionitis), (2) maternal vascular underperfusion, (3) fetal vascular thrombo-occlusive disease, and (4) chronic placental inflammation (chronic chorioamnionitis, VUE, and chronic deciduitis). When C4d immunoreactivity in the syncytiotrophoblast was analyzed with pathological changes of the placenta, there was a significant correlation between C4d immunoreactivity and maternal vascular underperfusion (p < 0.001; Fig. 4), while there was no association between C4d immunoreactivity and acute chorioamnionitis, fetal vascular thrombo-occlusive disease, or chronic placental inflammation. Furthermore, there was a significant correlation between the presence of maternal HLA class I antibodies and chronic placental inflammation when the maternal HLA antibody status was compared with pathological changes of the placenta (p = 0.041; Fig. 4); however, there was no association between the maternal HLA antibody status and other pathological changes.
C4d staining of the syncytiotrophoblast, the presence of maternal HLA classes I and II antibodies, and their relationship with placental pathology. a C4d staining of the syncytiotrophoblast is significantly associated with maternal vascular underperfusion (p < 0.001) but not with other pathological conditions. b, c There is a significant correlation between the presence of maternal HLA class I antibodies and chronic placental inflammation (p = 0.041); however, no association is found with other pathological conditions and the presence of maternal HLA antibodies. ACA acute chorioamnionitis, MVU maternal vascular underperfusion, FVTOD fetal vascular thrombo-occlusive disease, CPI chronic placental inflammation
Discussion
We studied placental C4d immunoreactivity and maternal HLA antibodies in preeclampsia and miscarriage cases. The primary findings of our study are as follows: (1) placental C4d staining is linear along the syncytiotrophoblast or diffusely cytoplasmic in the cytotrophoblast; (2) C4d staining of the syncytiotrophoblast is more frequent in patients with preeclampsia and fetal grow restriction (PE-FGR) than in patients with isolated preeclampsia or spontaneous preterm delivery; (3) there is no correlation between placental C4d immunoreactivity and circulating maternal HLA class I or class II antibodies; 4) there is no difference in C4d staining in the cytotrophoblast in the different preterm groups; 5) there are significant correlations between C4d staining of the syncytiotrophoblast and maternal vascular underperfusion, as well as between the presence of maternal HLA class I antibodies and chronic placental inflammation; and 6) C4d staining of the syncytiotrophoblast is frequent in patients with miscarriages, and there is no association with maternal HLA status.
This study confirms frequent C4d deposition in the syncytiotrophoblast of preeclampsia cases. Importantly, syncytiotrophoblast C4d immunoreactivity is more frequent in cases of preeclampsia with fetal growth restriction than in isolated preeclampsia cases. Although Buurma et al. showed no correlation between isolated fetal growth restriction and placental C4d deposition [9], our observations are consistent with the patterns of C4d immunoreactivity seen in cases of pregnancy-induced hypertension (PIH) and systemic lupus erythematosus (SLE) [7]. Furthermore, Minamiguchi et al. found an inverse correlation between neonatal birth weight and placental C4d immunoreactivity in patients with PIH or SLE [7]. Girardi et al. demonstrated that complement activation leads to fetal rejection and growth restriction in a mouse model, and complement inhibition can rescue these cellular processes [33]. Therefore, there seems to be a clear relationship among C4d deposition, immunological placental injury, and growth restriction in patients with SLE, PIH, and preeclampsia. We studied preterm preeclampsia cases because spiral arteries are not remodeled adequately due to shallow trophoblast invasion in this condition. This is a primary feature of early onset preeclampsia, which occurs before 34 weeks of gestation [34, 35]. Minamiguchi et al. have also shown an association between placental C4d deposition and lower gestational age at the time of delivery [7]. Of note, previous studies have shown that the frequency of syncytiotrophoblast C4d immunoreactivity in first trimester elective abortions and term deliveries is relatively uncommon (5 % for each) [5, 8].
We also investigated if there is an association between maternal HLA antibody status and placental C4d deposition. Maternal HLA classes I and II antibodies were present in all groups. Although there is a relationship between C4d deposition and HLA antibody-mediated rejection in solid organ allografts [36, 37], there was no correlation between the presence of HLA classes I and II antibodies and C4d staining of the syncytiotrophoblast. Therefore, placental C4d deposition is different from the situations seen in conventional allografts. These results further support a view of Buurma et al. that placental C4d deposition is likely a consequence of ischemia-reperfusion injury [9]. They proposed that activation of the classical complement pathway in patients with preeclampsia is a nonspecific reaction to placental damage where IgM antibodies, instead of fetal antigen-specific IgG HLA antibodies, play a role. In fact, ischemia-reperfusion can induce complement activation [38], and Weiser et al. have proposed that preexisting natural antibodies are responsible for classical complement activation in a mouse hindlimb ischemia model. The hindlimbs of mice deficient in C3, C4, and serum immunoglobulin were all protected from ischemia-reperfusion injury [39]. Therefore, it is plausible that C4d immunoreactivity is closely associated with biological challenges such as oxidative stress and endoplasmic reticulum stress in placental cells as a consequence of shallow placentation and ischemia-reperfusion injury. This postulate is further supported by a significant correlation between the placental findings of maternal vascular underperfusion and C4d deposition found in this study.
An intriguing feature of placental C4d immunoreactivity is the frequent staining of the villous cytotrophoblasts in preterm delivery cases. We have also previously shown cytoplasmic C4d immunoreactivity in preterm placentas affected by cytomegaloviral villitis [10]. Lack of cytoplasmic C4d staining in the first trimester and full-term placentas strongly suggested that C4d immunoreactivity in the cytotrophoblast is a gestational age-dependent change. The trophoblast is a known source of different complement proteins, and recent studies showed C4 synthesis by human microvascular and glomerular capillary endothelial cells [40]. Therefore, C4d immunoreactivity is possibly associated with the production of C4 by cytotrophoblasts. Bulla et al. have demonstrated an increase in the expression and secretion of C4 by HTR8/SVneo trophoblast cells and primary cytotrophoblasts when treated with interferon-γ [41]. A significant association between maternal HLA class I antibody status and chronic placental inflammation is consistent with previous observations that chronic placental inflammation is a placental manifestation of maternal anti-fetal cellular rejection [29, 30].
In summary, we have investigated placental C4d deposition in major pregnancy disorders with special reference to maternal HLA status and pathological changes. C4d deposition in the syncytiotrophoblast was frequently detected in cases of preeclampsia with fetal growth restriction. It was also common in patients with miscarriages, which further confirmed our previous observation [5]. Approximately two thirds of first trimester miscarriages are the result of shallow placentation and incomplete plugging of spiral arteries, which leads to premature intervillous circulation and placental oxidative stress [42]. The results of the present study are consistent with the view that preeclampsia and miscarriage share a common pathogenesis of shallow placentation. A major limitation of the study is that it is retrospective and exploratory. A prospective study based on a larger sample size would be required, along with mechanistic investigations. Collectively, C4d seems to be a biomarker for at-risk placentas due to defective placentation during pregnancy.
References
Racusen LC et al (2004) Banff 2003 meeting report: new diagnostic insights and standards. Am J Transplant 4(10):1562–1566
Djamali A et al (2014) Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant 14(2):255–271
Kedainis RL et al (2009) Focal C4d+ in renal allografts is associated with the presence of donor-specific antibodies and decreased allograft survival. Am J Transplant 9(4):812–819
Mauiyyedi S et al (2002) Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol 13(3):779–787
Lee JY et al (2014) Placental C4d as a common feature of chromosomally normal and abnormal miscarriages. Virchows Arch 464(5):613–620
Shamonki JM et al (2007) Excessive complement activation is associated with placental injury in patients with antiphospholipid antibodies. Am J Obstet Gynecol 196(2):167 e161–165
Minamiguchi S et al (2013) Complement split product C4d deposition in placenta in systemic lupus erythematosus and pregnancy-induced hypertension. Pathol Int 63(3):150–157
Rudzinski E et al (2013) Positive C4d immunostaining of placental villous syncytiotrophoblasts supports host-versus-graft rejection in villitis of unknown etiology. Pediatr Dev Pathol 16(1):7–13
Buurma A et al (2012) Preeclampsia is characterized by placental complement dysregulation. Hypertension 60(5):1332–1337
Lee KA et al (2013) Distinct patterns of C4d immunoreactivity in placentas with villitis of unknown etiology, cytomegaloviral placentitis, and infarct. Placenta 34(5):432–435
Milne F et al (2005) The pre-eclampsia community guideline (PRECOG): how to screen for and detect onset of pre-eclampsia in the community. BMJ 330(7491):576–580
Roberts JM, Cooper DW (2001) Pathogenesis and genetics of pre-eclampsia. Lancet 357(9249):53–56
Steegers EA et al (2010) Pre-eclampsia. Lancet 376(9741):631–644
Ghulmiyyah L, Sibai B (2012) Maternal mortality from preeclampsia/eclampsia. Semin Perinatol 36(1):56–59
Redman CW, Sargent IL (2010) Immunology of pre-eclampsia. Am J Reprod Immunol 63(6):534–543
Chaiworapongsa T et al (2013) Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. Am J Obstet Gynecol 208(4):287 e281–287 e215
Chaiworapongsa T et al (2014) Plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med 27(2):132–144
Vaisbuch E et al (2011) Circulating angiogenic and antiangiogenic factors in women with eclampsia. Am J Obstet Gynecol 204(2):152 e151–159
Brosens I et al (2011) The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 204(3):193–201
Brosens JJ et al (2002) The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol 187(5):1416–1423
Burton GJ et al (2009) Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30(6):473–482
Khong TY et al (1986) Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 93(10):1049–1059
Khong TY (2004) Placental vascular development and neonatal outcome. Semin Neonatol 9(4):255–263
Jauniaux E et al (2000) Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol 157(6):2111–2122
Burton GJ, Jauniaux E (2004) Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig 11(6):342–352
Becroft DM et al (2005) Placental villitis of unknown origin: epidemiologic associations. Am J Obstet Gynecol 192(1):264–271
Redline RW et al (2005) Placental diagnostic criteria and clinical correlation—a workshop report. Placenta 26(Suppl A):S114–S117
Redline RW (2008) Placental pathology: a systematic approach with clinical correlations. Placenta 29(Suppl A):S86–S91
Kim CJ et al (2010) The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol 23(7):1000–1011
Lee J et al (2011) A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One 6(2):e16806
(2000) Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 183(1):S1–S22
Fenton TR (2003) A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr 3:13
Girardi G et al (2006) Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med 203(9):2165–2175
Huppertz B (2008) Placental origins of preeclampsia: challenging the current hypothesis. Hypertension 51(4):970–975
Burton GJ, Yung HW (2011) Endoplasmic reticulum stress in the pathogenesis of early-onset pre-eclampsia. Pregnancy Hypertens 1(1–2):72–78
Frank R et al (2013) Correlation of circulating donor-specific anti-HLA antibodies and presence of C4d in endomyocardial biopsy with heart allograft outcomes: a single-center, retrospective study. J Heart Lung Transplant 32(4):410–417
Loupy A et al (2013) Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369(13):1215–1226
Dong J et al (1999) Strategies for targeting complement inhibitors in ischaemia/reperfusion injury. Mol Immunol 36(13–14):957–963
Weiser MR et al (1996) Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med 183(5):2343–2348
Hamer R et al (2012) Human leukocyte antigen-specific antibodies and gamma-interferon stimulate human microvascular and glomerular endothelial cells to produce complement factor C4. Transplantation 93(9):867–873
Bulla R et al (2009) Complement production by trophoblast cells at the feto-maternal interface. J Reprod Immunol 82(2):119–125
Jauniaux E, Burton GJ (2005) Pathophysiology of histological changes in early pregnancy loss. Placenta 26(2–3):114–123
Acknowledgments
This research was supported in part by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2012R1A2A2A01012368) and in part by a grant (14-521) from the Asan Institute for Life Sciences, Seoul, South Korea.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, E.N., Yoon, B.H., Lee, J.Y. et al. Placental C4d deposition is a feature of defective placentation: observations in cases of preeclampsia and miscarriage. Virchows Arch 466, 717–725 (2015). https://doi.org/10.1007/s00428-015-1759-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1759-y