Abstract
Placental C4d deposition is a feature of classical complement pathway activation and has been documented in various obstetrical settings. However, it is unknown whether placental C4d deposition is present in miscarriages and its frequency is different between chromosomally normal and abnormal miscarriages. This study was conducted to assess villous C4d deposition in miscarriages and to determine whether its frequency is different between chromosomally normal and abnormal miscarriages. Tissue samples (N = 58) of elective abortions (n = 20), miscarriages with normal chromosomes (n = 15), trisomy 16 (n = 13), and trisomy 22 (n = 10) were analyzed. Immunohistochemical staining for C4d and CD138 was done. Placental C4d deposition was defined as linear C4d immunoreactivity along the syncytiotrophoblast. Placental C4d immunoreactivity was detected in 73.3 % (11/15) and 56.5 % (13/23) of miscarriages with normal chromosomes and trisomy cases, respectively, while it was found in 5 % (1/20) of elective abortions (p < 0.05). Placental C4d deposition was more frequent in recurrent miscarriages (previous spontaneous abortion ≥2) than in sporadic miscarriages (76.5 vs. 30.0 %; p = 0.001). Chronic deciduitis was observed in 20.0 % (3/15) and 30.4 % (7/23) of miscarriages with normal chromosomes and trisomy cases, respectively, but not in elective abortions (p = 0.07 and 0.01, for each). The frequencies of C4d deposition (46.2 vs. 70.0 %) and chronic deciduitis (38.5 vs. 20.0 %) were not also different between trisomy 16 and trisomy 22 cases. Placental C4d deposition is a prominent feature of miscarriages regardless of their chromosomal status. The overall findings suggest that complement-mediated placental injury is a common pathology of miscarriage with diagnostic values in routine pathology practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Miscarriage affects both chromosomally normal and abnormal embryos and fetuses [1, 2]. Pathological examination of the placental and decidual compartments is essential in the evaluation of miscarriage specimens [3, 4]. Previous studies have proposed abnormal local and systemic maternal immune responses as major mechanisms of miscarriage with intact chromosomes [5]. Autoimmune disorders such as systemic lupus erythematosus and antiphospholipid syndrome [6] and the changes in the number and activation status of NK cells and T cells have been extensively studied with variable results [7, 8]. While chromosomal abnormalities of the embryo/fetus account for the major proportion of early pregnancy loss [2, 9, 10], basic mechanisms as to how chromosomal abnormalities per se lead to a miscarriage remain largely unknown although recent investigations have proposed some explanations such as gene dosage effects of trisomic regions and increased sensitivity to environmental and genetic variations [11, 12]. It also remains uncertain whether the mechanisms involved in chromosomally normal and abnormal miscarriages are similar or very different.
C4d is a split product of C4b and C4d deposition in vascular endothelial cells and is generally considered as evidence of classical complement pathway activation although there are examples of C4d-negative antibody-mediated rejection [13, 14]. Recently, classical complement activation has been shown as a mechanism of human antiphospholipid antibody-induced fetal loss with C4d deposition in the placenta [15]. A series of subsequent investigations have demonstrated the deposition of C4d in the syncytiotrophoblast in a variety of disorders. Buurma et al. have reported more frequent placental C4d deposition in preeclampsia cases than in healthy controls (50 vs. 3 %; p = 0.001) [16]. Rudzinski et al. have shown placental C4d immunoreactivity in 88 % of villitis of unknown etiology cases, while it was found in 5 % of gestational age-matched control cases (p < 0.0001). C4d deposition was also confirmed in cytomegaloviral placentitis and placental infarct [17]. Furthermore, C4d deposition was also found in fetal vessel endothelial cells, meaning that alloimmune reaction by maternally derived antibodies is present [18, 19]. These observations suggest that complement activation is a common consequence of different types of placental injuries such as ischemia-reperfusion and antigen-antibody complexes.
Chronic endometritis has been traditionally regarded as a pathological condition closely related to pelvic inflammatory disease with microbial colonization of the uterine cavity [20, 21]. The presence of plasma cells is a histological hallmark of chronic endometritis/deciduitis [22, 23], and there is an association between chronic endometritis and miscarriage/infertility [22, 24]. Kitaya found chronic endometritis in 9.3 % of patients with recurrent miscarriages and 12.9 % of miscarriages with unknown etiology [23]. Redline et al. reported a significantly higher frequency of decidual plasma cells in the first trimester miscarriages with normal karyotype than in those with abnormal karyotype [25]. On the other hand, VUE, chronic chorioamnionitis, and chronic deciduitis with plasma cells comprise a distinct group of pathology representing the same disease process (antifetal rejection) involving different regions of the placenta [26–29]. Higher prevalence of maternal HLA panel-reactive antibodies and presence of fetal HLA-specific antibodies in these cases [30] strongly suggest that the presence of plasma cells in the endometrium/decidua is also more likely to be associated with maternal immune response to embryonic/fetal antigens than with infection.
Considering the potential role of abnormal maternal immune responses in pregnancy disorders, we hypothesized that subsets of both chromosomally normal and abnormal miscarriages may share common immunopathologies. This study was conducted to assess the frequency of placental C4d in miscarriages and to determine if the frequencies of placental C4d deposition and chronic deciduitis are different between chromosomally normal and abnormal miscarriages.
Materials and methods
Patients and tissue materials
Formalin-fixed, paraffin-embedded tissues of elective abortion and miscarriage cases were retrieved from the data registries of Asan Medical Center and Hamchoon Women’s Clinic, Seoul, Korea. The cases (N = 58) were composed of elective abortions for medical reasons (n = 20) and miscarriages with normal chromosomes (n = 15), trisomy 16 (n = 13), and trisomy 22 (n = 10). The medical reasons for elective abortions included maternal problems such cardiovascular diseases, malignancies, and drug abuse. The study was approved by the Institutional Review Boards of participating institutions.
Chromosome analysis
Chorionic villi were selected under the microscope to ensure no maternal cell contamination. The chorionic villi samples were rinsed with modified Ham’s F10 (Sigma-Aldrich, St. Louis, MO, USA) with 10 % fetal bovine serum, 1.3 % sodium bicarbonate solution, and 0.48 % antibiotic-antimycotic (Life Technologies, Grand Island, NY, USA) and minced into tiny pieces using a dissecting knife. The cell suspension was added to two types of media (BIOAMF™-2, Biological Industries, Israel; AmnioMAX-C100, Gibco, Life Technologies) in two separate T50 flasks which were then left in a humid environment at 37 °C with 5 % CO2 for long-term culture. As the colonization and mitotic activities of the cells became adequate, chromosome harvesting by conventional cytogenetic methods was performed. The number and structure of the chromosome were analyzed by conducting GTG banding. The karyotype was interpreted according to the International System for Human Cytogenetic Nomenclature (ISCN, 2005) by analyzing at least 10 metaphases structurally and 20 metaphases numerically for each sample.
Tests for antithyroid antibodies and antinuclear antibodies
Patients’ sera were used. Automated immunoassay systems were used for the determination of antimicrosome and antithyroglobulin antibodies (Roche E170, Mannheim, Germany). Assay-specific cutoffs for thyroid autoantibodies were >34.00 IU/ml (antimicrosome antibodies and antithyroid peroxidase antibodies) and >115.00 IU/ml (antithyroglobulin antibodies). Intra-assay variation was <6 % and inter-assay variation was <8 %. Antinuclear antibody (ANA) detection was performed by using an indirect immunofluorescence method based on the use of HEp-2 cells as a substrate (MBL International, Woburn, MA, USA). Samples were diluted with phosphate-buffered saline with a cutoff dilution of 1:40. Slides were examined by two observers with a fluorescence microscope at × 200 magnification. Negative and positive controls with known antibody titers were used for quality control.
Immunohistochemistry
Four-micron-thick paraffin sections were obtained and used for immunostaining for C4d and CD138. A rabbit polyclonal anti-C4d antibody (Cell Marque Corporation, Rocklin, CA, USA; dilution of 1:200) and mouse monoclonal anti-CD138 antibody (MI-15; Dako, Glostrup, Denmark; dilution of 1:100) were used as primary antibodies. Immunostaining was performed using a Ventana BenchMark XT automated slide preparation system. The positive controls for C4d and CD138 immunostaining were biopsy specimens of the kidney with acute rejection and tonsil with chronic tonsillitis, respectively. Immunostaining with corresponding isotype controls was also done using normal rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for the anti-C4d antibody and mouse IgG (Jackson ImmunoResearch Laboratories, Inc.) for the anti-CD138 antibody. C4d immunoreactivity was reviewed by one pathologist (CJK), who was masked to the clinical diagnosis.
Statistical analysis
Medians and ranges for continuous variables and frequencies and percentages for categorical variables were reported. The continuous variables were compared using Mann-Whitney U test, and the categorical variables were compared using χ 2 test or Fisher’s exact test as appropriate. Statistical analyses were performed using SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
C4d immunoreactivity in the placenta
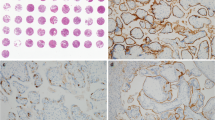
The demographics of the patients are summarized in Table 1. There was no difference in the median gestational age among elective abortions (median 6.6, range 5 ~ 11 weeks) and chromosomally normal (median 7.0, range 6 ~ 9 weeks) and abnormal cases (median 7.0, range 6 ~ 9 weeks). Distinct and linear C4d immunoreactivity along the syncytiotrophoblast was found in 73.3 % (11/15) and 56.5 % (13/23) of chromosomally normal and abnormal miscarriages, respectively. On the other hand, very focal and limited villous C4d deposition was found in only 5 % (1/20) of elective abortions. Placental C4d deposition was significantly more common in both chromosomally normal and abnormal miscarriages compared to elective abortions (p < 0.001 for each). C4d deposition was found only in 1 out of 20 elective abortion cases (5 %), and the patient was a primigravid woman who received renal transplantation. The positive signals were detected as a thin linear immunoreactivity along the syncytiotrophoblast surface or also as diffuse cytoplasmic immunoreactivity. However, C4d immunoreactivity was not found in villous cytotrophoblasts or extravillous trophoblasts. Among the C4d-positive, chromosomally normal miscarriages (n = 11), three cases showed very strong immunoreactivity in several clusters of chorionic villi (Fig. 1), while focal immunoreactivity restricted to a few villi was found in the remaining eight cases. One of the cases with strong C4d immunoreactivity was a case of chronic intervillositis, and C4d immunoreactivity was prominent along the periphery of the ischemic chorionic villi affected by inflammation (Fig. 2). Chromosomally abnormal miscarriages also showed similar C4d immunoreactive pattern with rather strong immunoreactivity in three cases and focal immunoreactivity in ten cases (Fig. 3). There was no significant difference in C4d-positive rates between chromosomally normal and abnormal cases (73.3 vs. 56.5 %; p = 0.294) and also between trisomy 16 and trisomy 22 cases (46.2 vs. 70.0 %; Fig. 4a, b). C4d-positive rate was higher in recurrent miscarriages (previous spontaneous abortion ≥2) compared to sporadic miscarriages (76.5 vs. 30.0 %, p = 0.001; Fig. 4c). Of note, four out of six cases with strong C4d immunoreactivity were cases of recurrent miscarriages with adverse clinical courses regardless of chromosomal status, and two of them were positive for antinuclear antibodies. One woman had a history of preterm preeclampsia in her previous pregnancy. Overall, antinuclear antibody-positive rates in chromosomally normal and abnormal miscarriages were 6.7 % (1/15) and 8.7 % (2/23), respectively. Antithyroid antibody was positive in 13.0 % (3/23) of chromosomally abnormal miscarriages, but not in chromosomally normal miscarriages (Table 1). One patient was positive for both antinuclear antibody and antithyroid antibody.
Villous C4d deposition in chromosomally normal miscarriages. Immunohistological findings of missed abortion at 6 weeks. A few CD138+ plasma cells are found in the decidua (a), and C4d immunoreactivity is observed along the syncytiotrophoblast surface (b). The patient was antinuclear antibody positive. Insets in a and b are negative isotype control immunostaining results using mouse IgG and rabbit IgG, respectively. Blighted ovum at 7 weeks showing CD138+ plasma cells in the decidua (c) and linear C4d deposition along the syncytiotrophoblast (d). An image from C4d-positive control immunostaining using a paraffin section obtained from a renal allograft with antibody-mediated rejection (e). Isotype negative control staining for C4d using rabbit IgG (f). a–f × 200 magnification
Villous C4d deposition in chromosomally abnormal miscarriages. Immunohistological findings of missed abortion with trisomy 16 at 6 weeks. Many CD138+ plasma cells are found in the decidua (a), and C4d immunoreactivity is observed along the syncytiotrophoblast surface (b). The patient was antithyroid antibody and antinuclear antibody positive. Insets in a and b are negative isotype control immunostaining results using mouse IgG and rabbit IgG, respectively. Missed abortion with trisomy 22 at 6 weeks showing CD138+ plasma cells in the decidua (c) and linear C4d deposition along the syncytiotrophoblast (d). a–d × 200 magnification
Comparisons of C4d immunopositive rates according to different parameters. a There is no difference in the C4d-positive rate between chromosomally normal and abnormal miscarriages. Villous C4d deposition is more common in miscarriages compared to elective abortions (63.2 vs. 5.0 %; p < 0.001). b The C4d-positive rate is not different between trisomy 16 and trisomy 22 cases. c C4d-positive rate is significantly higher in recurrent miscarriages compared to sporadic miscarriages (p < 0.05). d C4d-positive rate in cases with and without chronic deciduitis documented by the presence of CD138+ plasma cells
Chronic deciduitis in chromosomally normal and abnormal miscarriages
CD138-positive plasma cells in the decidua were found in 20.0 % (3/15) of chromosomally normal and in 30.4 % (7/23) of chromosomally abnormal miscarriages. Chronic deciduitis was not found in elective abortions. Therefore, chronic deciduitis was significantly more frequent in chromosomally abnormal miscarriages compared to elective abortions (p = 0.01). There was no difference in the frequency of chronic deciduitis between chromosomally normal and abnormal cases (20.0 vs. 30.4 %; p = 0.709) and also between trisomy 16 and trisomy 22 cases (38.5 vs. 20.0 %; p = 0.405). Strong CD138 immunoreactivity was also detected in the basolateral portion of the endometrial glands and villous syncytiotrophoblast, which served as internal controls.
The overall C4d-positive rates in chromosomally normal and abnormal miscarriages are shown in Fig. 4. C4d-positive rates of chronic deciduitis cases with CD138+ plasma cells and those without chronic deciduitis were 70.0 % (7/10) and 37.5 % (18/48), respectively. The C4d-positive rate tended to be higher in the cases with chronic deciduitis than in those without (p = 0.083; Fig. 4d).
Discussion
The primary and novel finding in the present study is that the evidence of classical complement pathway activation (C4d) is commonly found in chromosomally normal and abnormal miscarriages. Chronic deciduitis is also relatively frequent in both categories of miscarriage. Complement activation has been proposed as a cause of fetal rejection and intrauterine growth restriction, coupled to dysregulation of angiogenic factors. Girardi et al. have shown in a mouse model of miscarriage or intrauterine growth restriction that complement activation induced elevation of sFlt-1 as a mechanism of fetal loss [31]. In antiphospholipid syndrome, antiphospholipid antibody-induced complement activation is thought to be a cause of endothelial activation, thrombosis, and subsequent fetal loss [32]. Interestingly, mice deficient in C3 and C5 are protected from endothelial activation and thrombosis by the passive transfer of IgG antiphospholipid antibodies. It is also well known that trophoblasts express complements and complement regulatory proteins [33, 34], and membrane attack complex C5b-9 was localized to the foci of villous trophoblast injury [35].
While chromosomal abnormalities account for the major proportion of sporadic and recurrent miscarriages [2], the mechanism for this phenomenon is largely elusive. We have confirmed rather frequent placental C4d deposition in miscarriages and have done a comparative analysis of miscarriage according to the chromosomal status. Trisomy 16 and trisomy 22 cases were selected because these two types represent the most common trisomies identified in miscarriages [36] and also to minimize the complexity of chromosomal anomalies. Our observations strongly suggest that complement activation is a common mechanism of placental and fetal injuries regardless of chromosomal integrity. A significantly higher C4d-positive rate in recurrent miscarriage cases than in sporadic miscarriages suggests a possibility that C4d deposition in recurrent miscarriages is partially related to repeated maternal exposure to fetal antigens. Syncytiotrophoblast becomes an integral component of maternal intervillous circulation during pregnancy and biologically functions as the maternal endothelium [37]. Early placental development is characterized by histiotrophic nutrition from endometrial glandular secretions until the end of the first trimester when a drastic switch to hemotrophic nutrition occurs following the establishment of intervillous circulation. Endometrial secretions contain carbohydrates, lipids, and various growth factors and support the growth of the embryo and the placenta [38]. Jauniaux and Burton have proposed that the premature onset of maternal intervillous circulation due to the incomplete plugging of spiral arteries by defective trophoblast invasion and subsequent mechanical and biochemical stresses (e.g., oxidative stress) is a major pathogenetic mechanism of miscarriage [39]. In this context, it is highly possible that abnormal complement activation and C4d deposition in miscarriages are associated with the premature onset of intervillous circulation as fetal trophoblasts will be directly exposed to maternal blood [40].
Another unexpected finding in this study was that chronic deciduitis with CD138+ plasma cells was commonly found in chromosomally normal and abnormal miscarriages. Chronic deciduitis may be associated with maternal antifetal immune response and not with pelvic inflammatory disease as described earlier. Indeed, the density of plasma cells found in our study is much lower than that observed in proven infectious endometritis [41]; even limitations of CD138 immunostaining in the identification of pelvic inflammatory disease-associated histologic endometritis have been proposed. Viceti Miguel et al. could detect CD138+ plasma cells in 30 % of endometrial biopsies from women with low risk of developing pelvic inflammatory disease, demonstrating the nonspecific nature of CD138+ plasma cells in the diagnosis of pelvic inflammatory disease [42]. We were very much interested in the relationship between C4d deposition and the presence of decidual plasma cells, and the C4d-positive rate in cases with chronic deciduitis tended to be higher than that in cases without chronic deciduitis (p = 0.083).
A major limitation of this study is that serological evaluation for maternal antibodies, particularly for antibodies to fetal HLA antigens, could not be done. Systematic profiling of maternal antibodies against different antigens in the context of different types of miscarriages clearly seems to be an important venue for further research. Another limitation is that both chromosomally normal (6.7 %, 1/15) and abnormal miscarriage cases (17.4 %, 4/23) included patients with antithyroid antibodies or antinuclear antibodies. The significance of these findings in the context of C4d or chronic deciduitis could not be specifically assessed.
In conclusion, we report novel evidence supporting a possibility that chromosomally normal and abnormal miscarriages share a common mechanism of placental injury: classical complement pathway activation. Classical pathway activation is not exclusively by immune complexes. Previous studies have shown frequent villous C4d immunoreactivity in preeclampsia and placental infarcts [16, 17]. This suggests that defective placentation and ischemia-reperfusion injury of the placenta significantly contribute to the pathogenesis of both certain subsets of miscarriage and preeclampsia. Elucidation of the mechanisms of chromosomally normal, idiopathic miscarriages has been a very challenging subject to investigators. Considering the similar features between chromosomally normal and abnormal miscarriages in this study, it is possible that chromosomally abnormal miscarriage, which is far more common, is a decent window through which we can look at the pathophysiology of miscarriage. Furthermore, C4d immunohistochemistry would be very helpful in the histopathological assessment of miscarriage specimens.
References
Saravelos SH, Li TC (2012) Unexplained recurrent miscarriage: how can we explain it? Hum Reprod 27:1882–1886
Grande M, Borrell A, Garcia-Posada R, Borobio V, Munoz M, Creus M, Soler A, Sanchez A, Balasch J (2012) The effect of maternal age on chromosomal anomaly rate and spectrum in recurrent miscarriage. Hum Reprod 27:3109–3117
Grinschgl I, Mannweiler S, Holzapfel-Bauer M, Pferschy U, Hoefler G, Guertl B (2013) The role of morphology in combination with ploidy analysis in characterizing early gestational abortion. Virchows Arch 462:175–182
Musizzano Y, Fulcheri E (2010) Decidual vascular patterns in first-trimester abortions. Virchows Arch 456:543–560
Laird SM, Tuckerman EM, Cork BA, Linjawi S, Blakemore AI, Li TC (2003) A review of immune cells and molecules in women with recurrent miscarriage. Hum Reprod Update 9:163–174
Carp HJ, Selmi C, Shoenfeld Y (2012) The autoimmune bases of infertility and pregnancy loss. J Autoimmun 38:J266–J274
Beaman KD, Ntrivalas E, Mallers TM, Jaiswal MK, Kwak-Kim J, Gilman-Sachs A (2012) Immune etiology of recurrent pregnancy loss and its diagnosis. Am J Reprod Immunol 67:319–325
Yoo JH, Kwak-Kim J, Han AR, Ahn H, Cha SH, Koong MK, Kang IS, Yang KM (2012) Peripheral blood NK cell cytotoxicities are negatively correlated with CD8(+) T cells in fertile women but not in women with a history of recurrent pregnancy loss. Am J Reprod Immunol 68:38–46
Sierra S, Stephenson M (2006) Genetics of recurrent pregnancy loss. Semin Reprod Med 24:17–24
Sugiura-Ogasawara M, Ozaki Y, Katano K, Suzumori N, Kitaori T, Mizutani E (2012) Abnormal embryonic karyotype is the most frequent cause of recurrent miscarriage. Hum Reprod 27:2297–2303
Amano K, Sago H, Uchikawa C, Suzuki T, Kotliarova SE, Nukina N, Epstein CJ, Yamakawa K (2004) Dosage-dependent over-expression of genes in the trisomic region of Ts1Cje mouse model for Down syndrome. Hum Mol Genet 13:1333–1340
Yong PJ, McFadden DE, Robinson WP (2012) Protein kinase profiling in miscarriage: implications for the pathogenesis of trisomic pregnancy. J Obstet Gynaecol Can 34:1141–1148
Cohen D, Colvin RB, Daha MR, Drachenberg CB, Haas M, Nickeleit V, Salmon JE, Sis B, Zhao MH, Bruijn JA, Bajema IM (2012) Pros and cons for C4d as a biomarker. Kidney Int 81:628–639
Takeda A, Otsuka Y, Horike K, Inaguma D, Hiramitsu T, Yamamoto T, Nanmoku K, Goto N, Watarai Y, Uchida K, Morozumi K, Kobayashi T (2012) Significance of C4d deposition in antibody-mediated rejection. Clin Transplant 26(Suppl 24):43–48
Cohen D, Buurma A, Goemaere NN, Girardi G, le Cessie S, Scherjon S, Bloemenkamp KW, de Heer E, Bruijn JA, Bajema IM (2011) Classical complement activation as a footprint for murine and human antiphospholipid antibody-induced fetal loss. J Pathol 225:502–511
Buurma A, Cohen D, Veraar K, Schonkeren D, Claas FH, Bruijn JA, Bloemenkamp KW, Baelde HJ (2012) Preeclampsia is characterized by placental complement dysregulation. Hypertension 60:1332–1337
Lee KA, Kim YW, Shim JY, Won HS, Lee PR, Kim A, Kim CJ (2013) Distinct patterns of C4d immunoreactivity in placentas with villitis of unknown etiology, cytomegaloviral placentitis, and infarct. Placenta 34:432–435
Lee J, Romero R, Xu Y, Kim JS, Topping V, Yoo W, Kusanovic JP, Chaiworapongsa T, Hassan SS, Yoon BH, Kim CJ (2011) A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PloS One 6:e16806
Rudzinski E, Gilroy M, Newbill C, Morgan T (2013) Positive C4d immunostaining of placental villous syncytiotrophoblasts supports host-versus-graft rejection in villitis of unknown etiology. Pediatr Dev Pathol 16:7–13
Winkler B, Reumann W, Mitao M, Gallo L, Richart RM, Crum CP (1984) Chlamydial endometritis. A histological and immunohistochemical analysis. Am J Surg Pathol 8:771–778
Haggerty CL, Ness RB, Amortegui A, Hendrix SL, Hillier SL, Holley RL, Peipert J, Randall H, Sondheimer SJ, Soper DE, Sweet RL, Trucco G (2003) Endometritis does not predict reproductive morbidity after pelvic inflammatory disease. Am J Obstet Gynecol 188:141–148
Kasius JC, Broekmans FJ, Sie-Go DM, Bourgain C, Eijkemans MJ, Fauser BC, Devroey P, Fatemi HM (2012) The reliability of the histological diagnosis of endometritis in asymptomatic IVF cases: a multicenter observer study. Hum Reprod 27:153–158
Kitaya K, Yasuo T (2011) Immunohistochemistrical and clinicopathological characterization of chronic endometritis. Am J Reprod Immunol 66:410–415
Kitaya K, Yasuo T (2010) Aberrant expression of selectin E, CXCL1, and CXCL13 in chronic endometritis. Mod Pathol 23:1136–1146
Redline RW, Zaragoza M, Hassold T (1999) Prevalence of developmental and inflammatory lesions in nonmolar first-trimester spontaneous abortions. Hum Pathol 30:93–100
Becroft DM, Thompson JM, Mitchell EA (2005) Placental villitis of unknown origin: epidemiologic associations. Am J Obstet Gynecol 192:264–271
Jacques SM, Qureshi F (1998) Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Hum Pathol 29:1457–1461
Gersell DJ, Phillips NJ, Beckerman K (1991) Chronic chorioamnionitis: a clinicopathologic study of 17 cases. Int J Gynecol Pathol 10:217–229
Khong TY, Bendon RW, Qureshi F, Redline RW, Gould S, Stallmach T, Lipsett J, Staples A (2000) Chronic deciduitis in the placental basal plate: definition and interobserver reliability. Hum Pathol 31:292–295
Lee J, Romero R, Xu Y, Kim JS, Park JY, Kusanovic JP, Chaiworapongsa T, Hassan SS, Kim CJ (2011) Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol 66:510–526
Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE (2006) Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med 203:2165–2175
Pierangeli SS, Vega-Ostertag M, Liu X, Girardi G (2005) Complement activation: a novel pathogenic mechanism in the antiphospholipid syndrome. Ann N Y Acad Sci 1051:413–420
Bulla R, Bossi F, Agostinis C, Radillo O, Colombo F, De Seta F, Tedesco F (2009) Complement production by trophoblast cells at the feto-maternal interface. J Reprod Immunol 82:119–125
Tedesco F, Narchi G, Radillo O, Meri S, Ferrone S, Betterle C (1993) Susceptibility of human trophoblast to killing by human complement and the role of the complement regulatory proteins. J Immunol 151:1562–1570
Rampersad R, Barton A, Sadovsky Y, Nelson DM (2008) The C5b-9 membrane attack complex of complement activation localizes to villous trophoblast injury in vivo and modulates human trophoblast function in vitro. Placenta 29:855–861
Nagaishi M, Yamamoto T, Iinuma K, Shimomura K, Berend SA, Knops J (2004) Chromosome abnormalities identified in 347 spontaneous abortions collected in Japan. J Obstet Gynaecol Res 30:237–241
Richani K, Romero R, Soto E, Nien JK, Cushenberry E, Kim YM, Espinoza J, Kim CJ (2007) Genetic origin and proportion of basal plate surface-lining cells in normal and abnormal pregnancies. Hum Pathol 38:269–275
Burton GJ, Jauniaux E, Charnock-Jones DS (2007) Human early placental development: potential roles of the endometrial glands. Placenta 28(Suppl A):S64–S69
Jauniaux E, Burton GJ (2005) Pathophysiology of histological changes in early pregnancy loss. Placenta 26:114–123
Girardi G, Bulla R, Salmon JE, Tedesco F (2006) The complement system in the pathophysiology of pregnancy. Mol Immunol 43:68–77
Reighard SD, Sweet RL, Vicetti Miguel C, Vicetti Miguel RD, Chivukula M, Krishnamurti U, Cherpes TL (2011) Endometrial leukocyte subpopulations associated with Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis genital tract infection. Am J Obstet Gynecol 205(324):e321–e327
Vicetti Miguel RD, Chivukula M, Krishnamurti U, Amortegui AJ, Kant JA, Sweet RL, Wiesenfeld HC, Phillips JM, Cherpes TL (2011) Limitations of the criteria used to diagnose histologic endometritis in epidemiologic pelvic inflammatory disease research. Pathol Res Pract 207:680–685
Acknowledgments
We would like thank Ms. Yeo Won Kim for her help with the editing of our manuscript. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2012R1A2A2A01012368).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental table 1
(DOC 55 kb)
Rights and permissions
About this article
Cite this article
Lee, J.Y., Hong, JS., Kim, E.N. et al. Placental C4d as a common feature of chromosomally normal and abnormal miscarriages. Virchows Arch 464, 613–620 (2014). https://doi.org/10.1007/s00428-014-1571-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1571-0